A sensitive lateral flow test strip based on silica nanoparticle/CdTe quantum dot composite reporter probes†
Yalong
Bai
ab,
Chunyuan
Tian
c,
Xinlin
Wei
b,
Yuanfeng
Wang
*b,
Dapeng
Wang
a and
Xianming
Shi
*a
aMOST-USDA Joint Research Center for Food Safety, Shanghai Jiao Tong University, 800 Dongchuan Rd, Shanghai, 200240, China. E-mail: xmshi@sjtu.edu.cn; Tel: +86 21 3420 6616
bInstitute of Food Engineering, College of Life & Environment Science, Shanghai Normal University, 100 Guilin Rd, Shanghai, 200234, China. E-mail: foodlab2010@yahoo.com.cn; Tel: +86-013-524670799; Fax: +86-021-64322933
cKey Laboratory of Analytical Chemistry for Life Science, Ministry of Education, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093, China
First published on 19th January 2012
Abstract
Novel silica nanoparticle/CdTe quantum dot (QD) composite probes were prepared and used as reporters in fluorescent lateral flow test strips. When α-fetoprotein (AFP) was used as a model analyte, the results indicated that the fluorescent test strips were at least 10 times more sensitive than conventional gold-based test strips.
Immunochromatographic lateral flow test strips are one of most common devices for the rapid detection of a number of analytes and have been applied in various fields, such as clinical diagnostics,1,2food analysis,3,4 environmental monitoring,5,6 and forensic diagnostics.7 In comparison to other conventional detection methods, especially highly sensitive instrumental analyses, lateral flow test strips provide a low-cost, one-step analysis that is user-friendly, very rapid, and does not require a skilled technician to prepare the sample, run the test, or interpret the results.8 Additionally, the test strips can be used on site (such as a patient's beside, physician's office, and home) and provide real-time results, avoiding costly sample transportation and lengthy waiting times for results.9 Thus, test strips fill an important niche in providing a rapid on-site screening method to complement the traditional quantitative analytical detection methods. The conventional test strips mainly employ colloidal gold,10–12dyes,13 or latex beads14 as reporters to generate visual signals. Colloidal gold-based test strips, in particular, have been produced commercially in large amounts for a variety of applications. However, the low sensitivity cannot keep up with new challenges, such as the early diagnosis of some vital diseases and the rapid detection of pesticide residues and veterinary drug residues, because these targets must be detected at very low concentrations. Thus, new reporters need to be explored to improve the sensitivity of immunochromatographic lateral flow test strips.
Fluorescent immunoassays are a promising alternative to conventional colorimetric detection methods due to their increased sensitivity. In the past decade, fluorescent immunodetection has been further promoted by the development of QDs. QDs have attracted much interest due to their unique spectral properties, such as excellent brightness, narrow and precise tunable emission, negligible photobleaching, fairly high quantum yields, and good chemical stability.15–17 Several investigations about water soluble CdTe QDs utilized as reporters in chemical and biological analysis have been published.3,4,18–20 Though a prototype of immunochromatographic test strips using CdTe QDs was proposed by Liet al.,21 very limited research on QD-based lateral flow test strips has been reported. The primary reason for the limited number of reports may be due to the poor sensitivity of the lateral flow test strips that employ individual CdTe QDs as labels, which can be attributed to the small size (1 to 4 nm) of the CdTe QDs.4 In preliminary experiments we similarly observed poor sensitivity using individual CdTe QDs. We report here a simple and effective alternative approach that involves binding a large number of individual CdTe QDs onto larger silica nanoparticles to obtain composite QD probes of higher fluorescence intensity (a schematic diagram can be seen in Fig. S1, ESI†). Based on this concept, a sensitive lateral flow immunofluorescent assay was developed to detect α-fetoprotein (AFP), a liver cancer-associated tumor marker, as the model analyte. The sensitivity of the silica/CdTe QD composite probe-based immunofluorescent lateral flow test strips was compared with traditional gold-based lateral flow test strips. Experimental results demonstrated that this novel immunofluorescent method has superior sensitivity.
A schematic diagram of the complete procedure for the synthesis of the composite probes is given in Scheme 1. At first, amino-modified silica nanoparticles and water-soluble CdTe QDs were prepared, respectively. Then, EDC and NHS were used to conjugate a small amount of mouse anti-AFP mAb1 and a large amount of CdTe QDs onto the surface of the amino-modified silica nanoparticles in sequence.
 | ||
| Scheme 1 Schematic diagram of the procedure for the synthesis of the fluorescent silica nanoparticle/CdTe QD composite reporter probes. | ||
Fig. 1B shows that the silica nanoparticles prepared by the inverse microemulsion method are ideally dispersed and the mean diameter of the nanoparticles is 38.5 nm (characterized by transmission electron microscopy, TEM, Hitachi H-600). In addition, the particles are almost perfectly spherical and have a smooth surface. The silica nanoparticles are easily modified with various functional groups. In this study, amino-modified silica nanoparticles were prepared by covalently bonding silane onto the surface via the coupling reagent 3-aminopropyltriethoxysilane (APTES). The result of amino-modification was confirmed by Fourier transform infrared (FT-IR) spectroscopy (Fig. S2, ESI†).
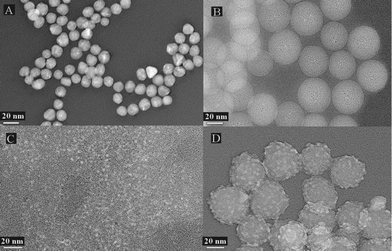 | ||
| Fig. 1 TEM images of (A) the gold nanoparticles, (B) silica nanoparticles, (C) Red-CdTe QDs and (D) silica nanoparticle/CdTe QD composite reporter probes. | ||
In our previous studies,22,23 it was found that the mean diameter of CdTe QDs increased as the reflux time increased, resulting in the luminous color of CdTe QDs transitioning from green, to yellow, to orange, and then to red. Given that the naked eye is more sensitive to red color and the Red-CdTe QDs have higher chemical stability, the Red-CdTe QDs were selected. The maximum fluorescent emission wavelength of the Red-CdTe QDs is 654 nm (Fig. S3, ESI†) and the mean diameter is 3.8 nm (Fig. 1C).
EDC and NHS are frequently used to conjugate nanoparticles with biomacromolecules.24–26 Herein, EDC and NHS were used to functionalize silica nanoparticles with both mouse anti-AFP mAb1 and CdTe QDs. The fluorescent immunoprobes were synthesized in two conjugation stages. First, a small amount of crosslinker was added to bind the amino-modified silica nanoparticles with mouse anti-AFP mAb1, then, a greater amount of crosslinker was added to bind the amino-modified silica nanoparticles with excessive amounts of MPA-capped CdTe QDs. Because the amount of mouse anti-AFP mAb1 on the probes was low (well below saturation), there were residual primary amine groups on the surfaces of the anti-AFP mAb1-conjugated silica nanoparticles available to crosslink with the MPA-capped Red-QDs. In fact, in preliminary experiments amino-modified silica nanoparticles were coupled with the Red-QDs before coupling with mouse anti-AFP mAb1, but the probes prepared by this strategy had no detectable bioactivity. The most probable reason was that the Red-QDs need to be added abundantly in order to ensure the strong fluorescence intensity of the probe; thus, there were few residual amine groups to bind with the mouse anti-AFP mAb1. When we changed the order of conjugation, this problem was resolved. Another advantage to the change was that, due to the secondary addition of the QDs, the QDs could bind with not only the amino-modified silica nanoparticles but also the mouse anti-AFP mAb1. In short, a single silica nanoparticle would combine covalently with more QDs resulting in a much brighter composite reporter probe. Fig. S3 (ESI†) shows the fluorescent emission spectrum of the composite probes. Compared with the unconjugated Red-CdTe QDs, the maximum emission peak of reporter probe red-shifted from 654 nm to 678 nm. This was caused by the conjugation with the silica nanoparticles and mouse anti-AFP mAb1. With regard to the decrease of the fluorescence intensity, this may have been due to a reduction in the total number of QDs present (i.e., not all QDs were covalently bound to the silica nanoparticles) and, at least in part, fluorescence quenching. It is conceivable that the fluorescence intensity of the individual composite probes had been increased greatly in comparison to probes prepared by conjugating a single QD with antibody directly, because each composite probe was composed of a large number of QDs. Furthermore, in order to provide direct evidence that the synthesis procedure was carried out according to the scheme, the composite probes were inspected by TEM (Fig. 1D). Compared with the silica nanoparticles (Fig. 1B), the mean diameter of the probes increased by 4.5 nm, and the surface became extremely rough. Most importantly, large numbers of small particles, which were as big as the size of the Red-QDs (Fig. 1C), were visible on the surface of the silica nanoparticles. All of the above provide strong evidence that the composite probes were prepared successfully.
For immunoassays, the quality of the antibodies and antigens is the key of the sensitivity. There is no point in comparing the sensitivity of different methods using different reagents. Therefore, we also prepared the gold-based strips using the same antibodies and antigens to verify the ultra-sensitivity of the fluorescent lateral flow test strips based on the silica nanoparticle/CdTe QDs composite probes. The gold nanoparticles were synthesized using the method of Frens.27,28 The mean diameter was 15 nm (Fig. 1A). After determining the optimum pH (8.5) and the minimum amount of mouse anti-AFP mAb1 for stabilization of the colloidal gold solution (23 μg mAb1 per 1 ml colloidal gold solution), the colloidal gold-mAb1 probes were prepared and purified.
After the silica nanoparticle/CdTe QDs composite probes and the colloidal gold-mAb1 probes were prepared, they were used as reporters to construct the lateral flow test strips, respectively. As shown in Fig. S4 (ESI†), the test strip consisted of a nitrocellulose membrane containing an anti-AFP mAb2 test line and an anti-mouse IgG control line, a conjugate pad containing the anti-AFP mAb1:silica nanoparticle/CdTe composite QD reporter probe (or colloidal gold probes), a sample pad, and an absorbent pad. All of these parts were pasted on a backing plate. Then the whole assembled test strip was cut into 4 mm strips and stored under dry conditions at room temperature.
In the present work, the detection principle of the lateral flow test strips is based on the formation of a “reporter probe:AFP:mouse anti-AFP mAb2” sandwich immuno-complex. The mouse anti-AFP mAb2, a monoclonal antibody that binds specifically to an epitope of AFP other than that bound by the anti-AFP mAb1 conjugated to the reporter probe, was immobilized in the nitrocellulose membrane (test line, T-line). In addition, a goat anti-mouse IgG was also immobilized in the membrane as the control line (C-line). After the sample was dropped into the sample pad for several minutes, the positive or negative results obtained by the lateral flow test strips rely on red-QD fluorescence under a UV lamp (365 nm; for the fluorescent test strips) or a pink color under natural light (for the gold-based test strips) in the T-line and C-line. If the AFP concentration is lower than the detection limit, only a colored C-line appeared, and if the AFP concentration is higher than the detection limit, both lines are colored.
Generally, the analytical performance of the strips can be affected by the different pore size, hydrophilicity or hydrophobicity and other properties of the membrane materials. After a series of contrast experiments, glass fiber 8964, spunbonded polyester 6613 and nitrocellulose membrane M135 were selected as sample pad, conjugate reagent pad and lateral flow pad, respectively. To obtain higher sensitivity, the amount of anti-AFP mAb2 (1 mg ml−1) and the goat anti-mouse IgG (0.25 mg ml−1) were all chosen to be 0.8 μl cm−1. In addition, the fluorescent probes were diluted 20 times with the buffer solution before dispensing onto the spunbonded polyester membrane, and the gold-probes were diluted 10 times. Although the nitrocellulose membrane was selected, some silica/CdTe composite probes absorbed nonspecifically to the membrane. To eliminate background signals caused by nonspecific absorption, the membrane was blocked with 1% BSA after the test line and control line were dispensed. Even so, there were still residual background signals on the membrane, but it did not significantly affect the observation of the test or control lines. In addition, it was also important to block the composite probes completely with BSA in the buffer in order to avoid nonspecific absorption on the test and control lines.
AFP was diluted in buffer solution to determine the sensitivity of the test strips using the colloidal-gold or silica/CdTe composite QD probes (n = 4). As shown in Fig. 2, the detection limit of the conventional gold-based strips was 20 ng ml−1AFP (Fig. 2A), while the detection limit of the fluorescent strips with silica/CdTe composite probes was 2 ng ml−1AFP (Fig. 2B), 10 times lower than that of the gold-based strips. The ultra-sensitivity stems from, not only the strong fluorescence intensity of CdTe QDs, but also the composite structure of the fluorescent probes. As a matter of fact, the composite structure is the main contribution to the sensitivity. In preliminary experiments using CdTe QD-antibody conjugates as fluorescent probes in the lateral flow test strips, the sensitivity of the strips was very low, well below even that of the gold-based strips.
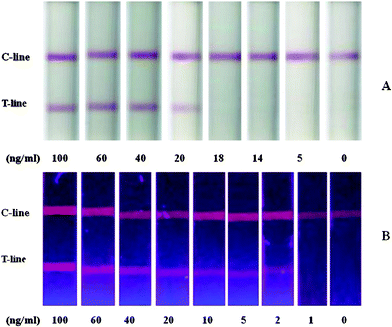 | ||
| Fig. 2 Lateral-flow test strips using different reporters: (A) colloidal gold and (B) silica/CdTe composite nanoparticles. | ||
In this study, the key to increased sensitivity was to combine a large amount of the CdTe QDs into one larger and brighter reporter probe. In previous reports,29–31 a similar approach was proposed, but the studies mainly focused on the encapsulation of QDs within silica nanospheres to yield silica-coated QDs. Although such coating brings many advantages, including an increase of the chemical stability and reduction of the biological toxicity, the encapsulation of the QDs results in low fluorescence intensity of the composite nanoparticles, reducing their efficacy as reporter probes for analyte detection. Therefore, we alternatively combined a large number of CdTe QDs directly onto the surface of the silica nanoparticles to obtain strongly fluorescent composite probes. The 10-fold increase in the sensitivity of detection over standard colorimetric detection using colloidal gold demonstrated the effectiveness of this new method using silica nanoparticle/CdTe QD composite probes.
To examine the cross-reactivity of the fluorescent test strips, carcino-embryonic antigen, prostate-specific antigen, α-L-fucosidase, ferritin, human serum albumin and human immunoglobulin G were tested. No cross-reactive band was revealed in the test line when 1 μg ml−1 of each protein was used. The results were the same as for the colloidal gold-based strips. The reproducibility of the fluorescent test strips was investigated for both within-run precision and between-run precision. The between-run precision was estimated by determining the response to standard serum with 20 ng ml−1 of AFP with three batches of fluorescent test strips prepared independently. The within-run precision and the between-run precision (n = 10) showed good reproducibility.
In conclusion, a sensitive lateral flow test strip was successfully developed in this study based on the use of silica nanoparticle/CdTe QD composite reporter probes to detect human AFP. The test strips were capable of detecting a minimum 2.0 ng ml−1AFP standard analyte in 15 min. Compared with the traditional gold-based lateral flow test strips prepared with the same reagents, these fluorescent test strips were 10 times more sensitive. There was no cross-reactivity with several tumor markers and blood proteins. In this work, AFP was used as the model analyte. The method could be used for almost any target for which antibodies are available with applications in the areas of clinical diagnostics, environmental monitoring, food analysis, forensic diagnostics and numerous other areas where rapid and sensitive analyte detection is desired. Moreover, the use of a fluorescence test strip reader would allow the new method to be quantitative. In addition, it is possible to detect multiple analytes simultaneously on a single test strip employing multiple silica/CdTe QD composite reporter probes generated using different antibodies and different size QDs with different fluorescent emissions.
This work was jointly supported by grants No. SS2012AA101001 and No. 2011DFA31220 from the Ministry of Science and Technology of China, No. 31171690, No. 30972485 and No. U1031003 from the National Natural Science Foundation of China, and grants, No. 10142201300 and No. 10DZ0503500 from the Science and Technology Commission of Shanghai Municipality.
References
- K. Kerman, T. Endo, M. Tsukamoto, M. Chikae, Y. Takamura and E. Tamiya, Talanta, 2007, 71, 1494–1499 CrossRef CAS.
- Q. Zeng, Y. Zhang, K. Song, X. Kong, M. Aalders and H. Zhang, Talanta, 2009, 80, 307–312 CrossRef CAS.
- J. Chen, F. Xu, H. Jiang, Y. Hou, Q. Rao, P. Guo and S. Ding, Food Chem., 2009, 113, 1197–1201 CrossRef CAS.
- Y. C. Kuo, Q. Wang, C. Ruengruglikit, H. Yu and Q. Huang, J. Phys. Chem. C, 2008, 112, 4818–4824 CAS.
- H. Li and F. Qu, Chem. Mater., 2007, 19, 4148–4154 CrossRef CAS.
- A. Vinayaka, S. Basheer and M. Thakur, Biosens. Bioelectron., 2009, 24, 1615–1620 CrossRef CAS.
- S. Pathak, S. Choi, N. Arnheim and M. Thompson, J. Am. Chem. Soc., 2001, 123, 4103–4104 CrossRef CAS.
- Z. Li, Y. Wang, J. Wang, Z. Tang, J. Pounds and Y. Lin, Anal. Chem., 2010, 2106–2114 Search PubMed.
- Z. Zou, D. Du, J. Wang, J. Smith, C. Timchalk, Y. Li and Y. Lin, Anal. Chem., 2010, 82, 5125–5133 CrossRef CAS.
- R. Tanaka, T. Yuhi, N. Nagatani, T. Endo, K. Kerman, Y. Takamura and E. Tamiya, Anal. Bioanal. Chem., 2006, 385, 1414–1420 CrossRef CAS.
- R. Shyu, H. Shyu, H. Liu and S. Tang, Toxicon, 2002, 40, 255–258 CrossRef CAS.
- J. Dominguez, N. Gali, L. Matas, P. Pedroso, A. Hernandez, E. Padilla and V. Ausina, Eur. J. Clin. Microbiol. Infect. Dis., 1999, 18, 896–898 CrossRef CAS.
- L. Min, L. Zhao-Yue, L. Qiang, Y. Hang, M. Lan, L. Jing-Hong, B. Yu-Bai and L. Tie-Jin, Chin. J. Chem., 2005, 23, 875–880 CrossRef.
- S. Nilsson, C. Lager, T. Laurell and S. Birnbaum, Anal. Chem., 1995, 67, 3051–3056 CrossRef CAS.
- X. Li, L. Wang, C. Zhou, T. Guan, J. Li and Y. Zhang, Clin. Chim. Acta, 2007, 378, 168–174 CrossRef CAS.
- M. Nichkova, D. Dosev, S. Gee, B. Hammock and I. Kennedy, Anal. Chem., 2005, 77, 6864–6873 CrossRef CAS.
- M. Bruchez Jr, M. Moronne, P. Gin, S. Weiss and A. Alivisatos, Science, 1998, 281, 2013–2016 CrossRef CAS.
- B. A. Koeneman, Y. Zhang, K. Hristovski, P. Westerhoff, Y. Chen, J. C. Crittenden and D. G. Capco, Toxicol. in Vitro, 2009, 23, 955–962 CrossRef CAS.
- Q. Ma, X. Wang, Y. Li, Y. Shi and X. Su, Talanta, 2007, 72, 1446–1452 CrossRef CAS.
- A. Gokarna, L.-H. Jin, J. S. Hwang, Y.-H. Cho, Y. T. Lim, B. H. Chung, S. H. Youn, D. S. Choi and J. H. Lim, Proteomics, 2008, 8, 1809–1818 CrossRef CAS.
- J. Li, K. Zhao, X. Hong, H. Yuan, L. Ma, Y. Bai and T. Li, Colloids Surf., B, 2005, 40, 179–182 CrossRef CAS.
- J. Xiao, Y. Bai, Y. Wang, J. Chen and X. Wei, Spectrochim. Acta, Part A, 2010, 76, 93–97 CrossRef.
- J. Xiao, T. Chen, L. Chen, H. Cao, F. Yang and Y. Bai, J. Inorg. Biochem., 2010, 104, 1148–1155 CrossRef CAS.
- W. Chen and J. Zhang, J. Nanosci. Nanotechnol., 2006, 6, 1159–1166 CrossRef CAS.
- L. Wang, R. Yan, Z. Huo, J. Zeng, J. Bao, X. Wang, Q. Peng and Y. Li, Angew. Chem., Int. Ed., 2005, 44, 6054–6057 CrossRef CAS.
- Y. Zhang, N. Kohler and M. Zhang, Biomaterials, 2002, 23, 1553–1561 CrossRef CAS.
- Y. Lin, S. Taylor, H. Li, K. A. S. Fernando, L. Qu, W. Wang, L. Gu, B. Zhou and Y.-P. Sun, J. Mater. Chem., 2004, 14, 527–541 RSC.
- G. Frens, Nat. Phys. Sci., 1973, 241, 20–22 CAS.
- Y. Chan, J. Zimmer, M. Stroh, J. Steckel, R. Jain and M. Bawendi, Adv. Mater., 2004, 16, 2092–2097 CrossRef CAS.
- L. Jin, D. Yu, Y. Liu, X. Zhao and J. Zhou, Talanta, 2008, 76, 1053–1057 CrossRef CAS.
- J. Li, L. Wang, K. Zhao, D. Li, Y. Bai and T. Li, Colloids Surf., A, 2005, 257–258, 329–332 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, optimization of sensing conditions, supplementary figures and scheme. See DOI: 10.1039/c2ra00976e |
| This journal is © The Royal Society of Chemistry 2012 |
