Diversity of carboxylate binding in a new tetranuclear zinc cluster: correlation between spectroscopic investigations and carboxylate binding modes†
Ayan
Patra
a,
Tamal K.
Sen
b,
Rangeet
Bhattacharyya
c,
Swadhin K.
Mandal
b and
Manindranath
Bera
*a
aDepartment of Chemistry, University of Kalyani, Kalyani, Nadia, West Bengal 741235, India. E-mail: manindra05@yahoo.co.in; Fax: +91 33 25828282; Tel: +91 33 25828282 x306
bDepartment of Chemical Sciences, Indian Institute of Science Education & Research-Kolkata, Mohanpur, West Bengal 741252, India
cDepartment of Physical Sciences, Indian Institute of Science Education & Research-Kolkata, Mohanpur, West Bengal 741252, India
First published on 19th January 2012
Abstract
A new tetranuclear ZnII4 cluster has been prepared and characterized by exploiting the flexibility, chelating ability and bridging potential of a carboxylate rich μ-bis(tetradentate) ligand. The acetate and benzoate functionalities of the ligand display both monodentate terminal and syn–anti bidentate bridging binding modes in the cluster. The binding modes of carboxylate have been investigated by a combined approach of X-ray crystallography, FTIR, solution and solid state 13C NMR spectroscopic techniques.
Zinc compounds supported by carboxylate ligands are of great interest owing to their role in biochemical systems, catalysis, and materials chemistry. For instance, zinc carboxylates are attracting a great deal of attention because of their importance as highly active catalysts for the polymerization or copolymerization of a wide range of organic monomers.1 In this regard, zinc oxocarboxylates with the general formula Zn4O(O2CR)6 have also been known for decades,2 and interest in them was renewed primarily by the work of Yaghi and co-workers, who developed the synthesis of metal–organic frameworks (MOFs) with high porosities and tailor-made molecular cavities.3 Since then, MOFs based on zinc carboxylates have been studied for a variety of applications, including hydrogen storage, nonlinear optics, and catalysis.4
Zinc-containing enzymes range from those with simple one zinc binding sites with common coordination modes, such as in carbonic anhydrase and carboxypeptidase A, to metalloenzymes with more than one zinc ion and unusual protein coordination modes.5 So, the understanding of zinc coordination chemistry, in both biological systems and small molecules, is vital to the understanding of substrate binding and reaction mechanisms of zinc enzymes. The discovery of structural polyzinc nucleic acid polymerases and transcription factors, shown to bind as many as 11 zinc ions and in even more complicated coordination modes, has rekindled interest in the coordination chemistry of zinc.6 Most of the known polyzinc enzymes are coordinated to either His, Asp, or Glu amino acid residues and/or are bridged to each other through OH− and/or H2O or carboxylate groups in different binding modes. The bridging carboxylate is the most biologically pertinent in view of the occurrence of this bridge in enzymes such as phospholipase C and nuclease P1.7,8
The carboxylate rich ligand is of special interest because it can adopt a variety of coordination/binding modes9–11 resulting in diverse multidimensional architectures. However, most of the documented works are based upon rigid carboxylate ligands, such as benzenedicarboxylic acid based isomers, 1,3,5-benzenetricarboxylic acid and 1,2,4,5-benzenetetracarboxylic acid which possess di- or multi-carboxyl groups and have been widely used for the preparation of various MOFs.12–14 Polydentate ligands with flexible carboxyl groups are also under intensive study, because their flexibility and conformational freedom may provide more possibilities for the construction of frameworks with new topologies.9,15–18 Investigation of their coordination properties can lead to a deeper understanding of the supramolecular assembly processes.19 The carboxylate rich polydentate ligands have been used for some time as a way to assemble ZnII ions into aggregates with relevance in the area of bioinorganic chemistry.16,20 The focus here is on a μ-bis(tetradentate) ligand, N,N′-bis[2-carboxybenzomethyl]-N,N′-bis[carboxymethyl]-1,3-diaminopropan-2-ol (H5L), incorporating two acetate and two benzoate functionalities (Fig. 1).9 The ligand H5L has recently been shown to bind zinc(II) ions and cobalt(II) ions to yield mono- and hexanuclear zinc(II) complexes and tetra- and hexanuclear cobalt(II) complexes showing the bridging potential of carboxylates.9,21,22 Very recently, the unusual amide and carboxylate binding modes of a similar amide and carboxylate rich polydentate ligand have been explored in a self-assembled heptanuclear zinc cluster.15 We report here synthesis, characterization and spectroscopic investigations correlating the diverse binding modes of carboxylates of a new tetranuclear zinc-carboxylate cluster.
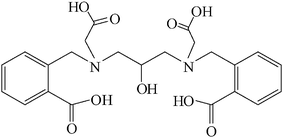 | ||
| Fig. 1 Chemical structure of the ligand H5L. | ||
The reaction of H5L with ZnCl2 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio in the presence of KOH at pH∼9 in methanol at room temperature afforded a white powder, that was easily crystallized into a colorless tetranuclear complex K2[Zn4L2(μ-H2O)]·3CH3OH·3H2O (1). The molecular structure of the complex 1 has been authenticated using analytical techniques such as elemental analysis, solution electrical conductivity, FTIR, 1H and 13C NMR spectroscopy and single crystal X-ray crystallography. The molar conductivity value of complex 1 in MeOH is 175 Ω−1 cm2 mol−1 at room temperature, indicating a behaviour attributable to 2
2 molar ratio in the presence of KOH at pH∼9 in methanol at room temperature afforded a white powder, that was easily crystallized into a colorless tetranuclear complex K2[Zn4L2(μ-H2O)]·3CH3OH·3H2O (1). The molecular structure of the complex 1 has been authenticated using analytical techniques such as elemental analysis, solution electrical conductivity, FTIR, 1H and 13C NMR spectroscopy and single crystal X-ray crystallography. The molar conductivity value of complex 1 in MeOH is 175 Ω−1 cm2 mol−1 at room temperature, indicating a behaviour attributable to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 electrolytes.
1 electrolytes.
Single crystal X-ray structure analysis23 reveals that cluster 1 consists of four zinc ions, two L5− ligands, one H2O molecule, and two K+ ions as counter cations. A total of three methanol and three water molecules cocrystallized with the complex. A structural view of the dianion tetranuclear zinc cluster 1 is depicted in Fig. 2. The dianionic species consists of four zinc ions, two polydentate ligands L5− and a H2O molecule.
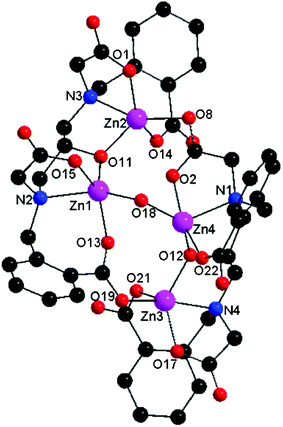 | ||
| Fig. 2 Molecular structure of the complex 1. | ||
The tetranuclear cluster anion is formed through the self-assembly of two dinuclear [Zn2L]− units which are in turn exclusively bridged by an acetate arm in syn–anti η1:η1:μ2 mode and one benzoate arm in syn–anti η1:η1:μ2 mode supported by a bridging μ-OH2 molecule. The metal ions within each [Zn2L]− unit are bridged and chelated by one L5− ligand. The ligand L5− within each dinuclear unit bridges the metal ions through one deprotonated alkoxo moiety while further coordinating the zinc ions through aliphatic and aromatic carboxylates, amine groups and a water molecule. It is most interesting that the two dinuclear [Zn2L]− units display two different structural arrangements with respect to the binding and bridging modes of carboxylates. The dinuclear unit (Fig. 3) in which two aliphatic carboxylate arms and two aromatic carboxylate arms of the ligand are cis to each other has Zn1 and Zn2 atoms in a slightly distorted square pyramidal geometry having the trigonality factors (τ)24 of 0.058 and 0.216, respectively. A bridging alkoxo oxygen, a monodentate aliphatic carboxylate oxygen, a tertiary amine nitrogen, a bridging aromatic carboxylate oxygen of one L5− ligand, and a bridging aqua molecule make up the coordination environment around the Zn1 center. The Zn2 center is surrounded by a bridging alkoxo oxygen, a monodentate aliphatic carboxylate oxygen, a tertiary amine nitrogen, and a monodentate aromatic carboxylate oxygen of one L5− ligand, and one bridging aliphatic carboxylate oxygen of the neighboring L5− ligand. The other dinuclear unit (Fig. 4) having two aliphatic carboxylate arms and two aromatic carboxylate arms of the ligand trans to each other, consists of Zn3 and Zn4 atoms. The coordination geometry around the Zn3 center is best described by a highly distorted trigonal bipyramidal geometry (τ = 0.705), surrounded by one bridging alkoxo oxygen, a monodentate aliphatic carboxylate oxygen, a tertiary amine nitrogen, a monodentate aromatic carboxylate oxygen of one L5− ligand, and a bridging aromatic carboxylate oxygen of the neighboring L5− ligand. The Zn4 center adopts a highly distorted trigonal bipyramidal geometry (τ = 0.523) consisting of one bridging alkoxo oxygen, a monodentate aromatic carboxylate oxygen, a tertiary amine nitrogen, a bridging aliphatic carboxylate oxygen of the L5− ligand, and a bridging aqua molecule. In particular, the flexible backbone of the ligand allows for various zinc–oxygen–zinc angles and various carboxylate bridges to coordinate between the dinuclear units, highlighting the versatility of the ligand.9,15,21,23
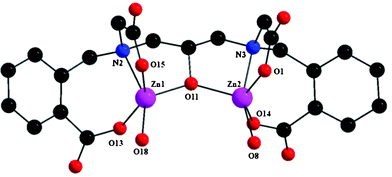 | ||
| Fig. 3 Molecular structure of the dinuclear unit of complex 1 having two aliphatic carboxylate arms and two aromatic carboxylate arms of the ligand cis to each other. | ||
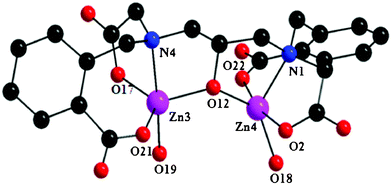 | ||
| Fig. 4 Molecular structure of the dinuclear unit of complex 1 having two aliphatic carboxylate arms and two aromatic carboxylate arms of the ligand trans to each other. | ||
A core structure of the complex 1 is shown in Fig. 5. The Zn–Obridging alkoxo bond distances are within the range of previously reported alkoxo bridged dinuclear and hexanuclear zinc systems at ∼1.976(6) Å.9,25 The Zn–Obridging alkoxo distances indicate that Zn–O alkoxo bridges in each dinuclear unit are close to symmetric [Zn1–O11, 1.983(6) Å; Zn2–O11, 1.998(6) Å; Zn3–O12, 1.975(6) Å; Zn4–O12, 1.951(6) Å]. The average Zn–Zn separation in an intra-dinuclear unit is 3.617 Å16,25a which is slightly longer than what is usually found in similar phenolate bridged dinuclear zinc complexes (3.35–3.40 Å).26 The average Zn–Zn separation where the two zinc centers are interligand to each other through a syn–anti carboxylate bridge is 4.885 Å. The separation between Zn1 and Zn4 atoms bridged by an aqua ligand is 3.306 Å. The interligand Zn–Zn separation is larger compared to intraligand Zn–Zn separation due to the syn–anti carboxylate bridges which is not surprising when considering the flexible carboxylate backbones of the ligand. The longer zinc-carboxylate arms with the shorter zinc-alkoxo arms provide the framework to support a wide bridging angle on average, Zn–Oalkoxo–Zn of 132.42°. To the best of our knowledge, complex 1 is the first crystallographically characterized solid-state structure of a tetranuclear zinc cluster where a symmetrical carboxylate rich polydentate ligand exhibits the unsymmetrical and versatile carboxylate binding modes towards the zinc(II) ions.
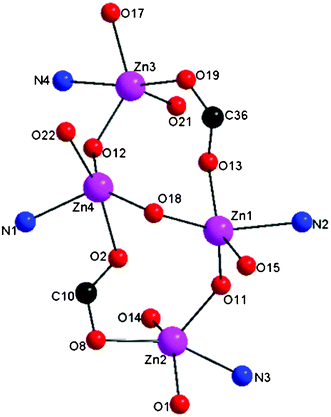 | ||
| Fig. 5 Core structure of the complex 1. | ||
In complex 1, the different modes of the carboxylate binding, namely syn–anti bidentate bridging and monodentate terminal coordination of both acetate and benzoate groups have been established from the FTIR spectra (Fig. S1†). Deacon and Phillips have carefully examined the FTIR spectra of many metal-carboxylate complexes with known X-ray crystal structures and drawn useful conclusions for the correlations between carboxylate stretching frequencies and their geometries.27 In the FTIR spectra of complex 1, two strong bands at 1594 and 1466 cm−1 are due to the asymmetric and symmetric stretching vibrations of a bridging acetate functionality of the ligand, respectively. The difference of ∼128 cm−1 between the asymmetric and symmetric stretching vibrations is attributed to the syn–anti bidentate bridging (η1:η1:μ2) of the acetate group. The other two strong bands at 1568 and 1451 cm−1 correspond to the asymmetric and symmetric stretching vibrations of a bridging benzoate functionality of the ligand, respectively. The difference of ∼117 cm−1 between the asymmetric and symmetric stretching vibrations is indicated by the syn–anti bidentate bridging (η1:η1:μ2) of the benzoate group. Significantly low values of separation for syn–anti bidentate bridging have been observed in the literature.11,28,29 In addition, the asymmetric and symmetric carboxylate stretches are observed at 1607 and 1388 cm−1 respectively, (Δ = 219 cm−1) which suggest the monodentate terminal coordination of the acetate and benzoate groups.29,30 Generally, the order of Δ (Δ = νas(COO−) − νs(COO−)) for divalent metal carboxylates is followed by Δ (bridging) < Δ (monodentate).27,31 The lower Δ values (Δ = 117 cm−1 and 128 cm−1) compared to that of higher value (Δ = 219 cm−1) strongly suggest the presence of syn–anti bidentate bridging along with the monodentate terminal coordinations. The FTIR spectrum of complex 1 also exhibits a broad band at 3434 cm−1 assignable to the ν(O–H) vibration of the coordinated water molecule.
We have employed both solution and solid state 13C NMR spectroscopic techniques as potential tools for diagnosing the different binding modes of carboxylate since it markedly depends on the bonding electrons, bond orders and electron densities. The 13C NMR spectrum of complex 1 in D2O solution at room temperature is shown in Fig. 6a. In the spectrum, there are two resonance peaks at 177.54 and 177.93 ppm with an approximate intensity ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 in the downfield which can be assigned to the syn–anti bidentate bridging benzoate carbon and monodentate terminal benzoate carbon, respectively (Fig. 6b). This intensity ratio of the signals indicates the presence of one syn–anti bidentate bridging benzoate and three monodentate terminal benzoates. The solution 13C NMR spectrum of complex 1 also exhibits another two resonance peaks at 177.84 and 178.66 ppm with an approximate intensity ratio of 1
3 in the downfield which can be assigned to the syn–anti bidentate bridging benzoate carbon and monodentate terminal benzoate carbon, respectively (Fig. 6b). This intensity ratio of the signals indicates the presence of one syn–anti bidentate bridging benzoate and three monodentate terminal benzoates. The solution 13C NMR spectrum of complex 1 also exhibits another two resonance peaks at 177.84 and 178.66 ppm with an approximate intensity ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 in the downfield indicating the presence of one syn–anti bidentate bridging acetate and three monodentate terminal acetates, respectively (Fig. 6b). The solid state 13C NMR spectrum (Fig. S2†) of complex 1 exhibits two broad signals in the region of 170 ppm to 180 ppm. The higher intense broad signal at ∼180 ppm can be assigned to the three monodentate terminal benzoate and three monodentate terminal acetate carbons. The lower intense broad signal at ∼177 ppm corresponds to one syn–anti bidentate bridging benzoate and one syn–anti bidentate bridging acetate carbon. Therefore, the solution and solid state 13C NMR spectroscopic investigations confirm that the integrity of the complex 1 is retained both in solution and solid state which has been further supported by solid state single crystal X-ray structure analysis. The monodentate and bidentate bridging binding modes of acetate functionalities in the di- and tetranuclear zinc complexes have also been identified with the help of solid state 13C NMR spectroscopic studies which are reported in the literature.32,33
3 in the downfield indicating the presence of one syn–anti bidentate bridging acetate and three monodentate terminal acetates, respectively (Fig. 6b). The solid state 13C NMR spectrum (Fig. S2†) of complex 1 exhibits two broad signals in the region of 170 ppm to 180 ppm. The higher intense broad signal at ∼180 ppm can be assigned to the three monodentate terminal benzoate and three monodentate terminal acetate carbons. The lower intense broad signal at ∼177 ppm corresponds to one syn–anti bidentate bridging benzoate and one syn–anti bidentate bridging acetate carbon. Therefore, the solution and solid state 13C NMR spectroscopic investigations confirm that the integrity of the complex 1 is retained both in solution and solid state which has been further supported by solid state single crystal X-ray structure analysis. The monodentate and bidentate bridging binding modes of acetate functionalities in the di- and tetranuclear zinc complexes have also been identified with the help of solid state 13C NMR spectroscopic studies which are reported in the literature.32,33
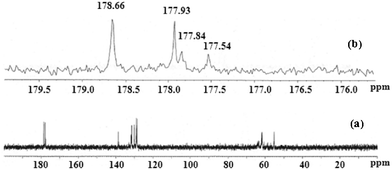 | ||
| Fig. 6 13C NMR spectra of (a) complex 1 in D2O solution at room temperature; and (b) complex 1 in the expanded region of 170 and 180 ppm. | ||
In conclusion, we have demonstrated that the ligand H5L effectively promotes the formation of a new tetranuclear zinc complex 1 in good yield under the specified experimental conditions. The tetranuclear zinc complex displays several versatile coordination/binding modes of the acetate and benzoate groups of the ligand which have been investigated by a combined approach of X-ray crystallography, FTIR and solution and solid state 13C NMR spectroscopic techniques. Despite being a symmetrical polydentate ligand having two acetate and two benzoate groups, the greater flexibility of the ligand H5L allows the dinuclear [Zn2L]− units to be self-assembled to a tetranuclear cluster 1 through syn–anti η1:η1:μ2 bridging modes of one acetate and one benzoate group. This versatile coordination ability of the ligand will positively contribute to the field of coordination and supramolecular chemistry.
Acknowledgements
The authors thank the Department of Science and Technology (DST) (Grant No.: SR/FT/CS-067/2009), New Delhi for funding and financial support. The instrumental facilities provided by the DST-FIST in the Department of Chemistry are greatly appreciated. A. P. also thanks the University of Kalyani for providing a university research fellowship.References
- (a) W. Kuran, Principles of Coordination Polymerisation, Wiley, Chichester, 2001, ch. 9 Search PubMed; (b) G. W. Coates and D. R. Moore, Angew. Chem., Int. Ed., 2004, 43, 6618 CrossRef CAS; (c) D. J. Darensbourg, Chem. Rev., 2007, 107, 2388 CrossRef CAS.
- (a) H. Koyama and Y. Saito, Bull. Chem. Soc. Jpn., 1954, 27, 112 CAS; (b) R. M. Gordon and H. B. Silver, Can. J. Chem., 1983, 61, 1218 CAS; (c) W. Clegg, D. R. Harbron, C. D. Homan, P. A. Hunt, I. R. Little and B. P. Straughan, Inorg. Chim. Acta, 1991, 186, 51 CrossRef CAS.
- O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705 CrossRef CAS.
- (a) J. L. C. Rowsell and O. M. Yaghi, Angew. Chem., Int. Ed., 2005, 44, 4670 CrossRef CAS; (b) H. Chun, D. N. Dybtsev, H. Kim and K. Kim, Chem.–Eur. J., 2005, 11, 3521 CrossRef CAS; (c) A. G. Wong-Foy, A. J. Matzger and O. M. Yaghi, J. Am. Chem. Soc., 2006, 128, 3494 CrossRef CAS; (d) T. Ohshima, T. Iwasaki and K. Mashima, Chem. Commun., 2006, 2711 RSC.
- (a) N. Strater, W. N. Lipscomb, T. Klabunde and B. Krebs, Angew. Chem., Int. Ed. Engl., 1996, 35, 2024 CrossRef; (b) H. Steinhagen and G. Helmchem, Angew. Chem., Int. Ed. Engl., 1996, 35, 2339 CrossRef CAS.
- (a) G. Parkin, Chem. Rev., 2004, 104, 699 CrossRef CAS; (b) J. Miller, A. D. McLachlan and A. Klug, EMBO J., 1985, 4, 1609 CAS.
- D. Lee, P. L. Hung, B. Spingler and S. J. Lippard, Inorg. Chem., 2002, 41, 521 CrossRef CAS.
- (a) T. Tanase, J. W. Yun and S. J. Lippard, Inorg. Chem., 1995, 34, 4220–4229 CrossRef CAS; (b) T. Tanase, J. W. Yun and S. J. Lippard, Inorg. Chem., 1996, 35, 3585 CrossRef CAS; (c) T. Tanase, H. Inukai, T. Onaka, M. Kato, S. Yano and S. J. Lippard, Inorg. Chem., 2001, 40, 3943 CrossRef CAS.
- A. B. S. Curtiss, M. Bera, G. T. Musie and D. R. Powell, Dalton Trans., 2008, 2717 RSC.
- R. L. Rardin, W. B. Tolman and S. J. Lippard, New J. Chem., 1991, 15, 417 CAS.
- V. Zelenak, Z. Vargova and K. Gyoryova, Spectrochim. Acta, Part A, 2007, 66, 262 Search PubMed.
- T. L. Hu, R. Q. Zou, J. R. Li and X. H. Bu, Dalton Trans., 2008, 1302 RSC.
- (a) Y. Qi, Y. Che, F. Luo, S. R. Batten, Y. Liu and J. Zheng, Cryst. Growth Des., 2008, 8, 1654 CrossRef CAS; (b) M. Du, X. J. Jiang and X. J. Zhao, Inorg. Chem., 2007, 46, 3984 CrossRef CAS; (c) Z. Shi, G. H. Li, L. Wang, L. Gao, X. B. Chen, J. Hua and S. H. Feng, Cryst. Growth Des., 2004, 4, 25 CrossRef CAS; (d) F. Luo, Y. X. Che and J. M. Zheng, Cryst. Growth Des., 2008, 8, 176 CrossRef CAS; (e) Z. Li, G. Zhu, X. Guo, X. Zhao, Z. Jin and S. Qin, Inorg. Chem., 2007, 46, 5174 CrossRef CAS; (f) C. Livage, N. Guillou, J. Marrot and G. Ferey, Chem. Mater., 2001, 13, 4387 CrossRef CAS.
- (a) A. Majumder, V. Gramlich, G. M. Rosair, S. R. Batten, J. D. Masuda, M. S. ElFallah, J. Ribas, J. P. Sutter, C. Desplanches and S. Mitra, Cryst. Growth Des., 2006, 6, 2355 CrossRef CAS; (b) J. D. Lin, J. W. Cheng and S. W. Du, Cryst. Growth Des., 2008, 8, 3345 CrossRef CAS; (c) R. Cao, D. F. Sun, Y. C. Liang, M. C. Hong, K. Tatsumi and Q. Shi, Inorg. Chem., 2002, 41, 2087 CrossRef CAS; (d) O. Fabelo, L. Canadillas-Delgado, F. S. Delgado, P. Lorenzo-Luis, M. M. Laz, M. Julve and C. Ruiz-Pérez, Cryst. Growth Des., 2005, 5, 1163 CrossRef CAS.
- M. Bera, G. T. Musie and D. R. Powell, Inorg. Chem., 2009, 48, 4625 Search PubMed.
- M. Jarenmark, S. Kappen, M. Haukka and E. Nordlander, Dalton Trans., 2008, 993 RSC.
- H. Carlsson, M. Haukka, A. Bousseksou, J. M. Latour and E. Nordlander, Inorg. Chem., 2004, 43, 8252 CrossRef CAS.
- K. Kanamori, N. Matsui, K. Takagi, Y. Miyashita and K. Okamoto, Bull. Chem. Soc. Jpn., 2006, 79, 1881 Search PubMed.
- (a) S. N. Wang, J. F. Bai, Y. Z. Li, Y. Pan, M. Scheer and X. Z. You, CrystEngComm, 2007, 9, 1084 RSC; (b) Y. H. He, Y. L. Feng, Y. Z. Lan and Y. H. Wen, Cryst. Growth Des., 2008, 8, 3586 CrossRef CAS; (c) S. Q. Zang, Y. Su, Y. Z. Li, H. Z. Zhu and Q. J. Meng, Inorg. Chem., 2006, 45, 2972 CrossRef CAS; (d) X. L. Wang, C. Qin, E. B. Wang and Z. M. Su, Chem.–Eur. J., 2006, 12, 2680 CrossRef CAS; (e) Y. B. Dong, Y. Y. Jiang, J. Li, J. P. Ma, F. L. Liu, B. Tang, R. Q. Huang and S. R. Batten, J. Am. Chem. Soc., 2007, 129, 4520 CrossRef CAS.
- (a) K. Z. Shao, Y. H. Zhao, X. L. Wang, Y. Q. Lan, D. J. Wang, Z. M. Su and R. S. Wang, Inorg. Chem., 2009, 48, 10 Search PubMed; (b) A. Erxleben, Inorg. Chem., 2001, 40, 208 CrossRef CAS; (c) F. Gao, B. H. Meng and M. L. Zhu, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2004, 60, m1225 Search PubMed.
- M. Bera, A. B. S. Curtiss, G. T. Musie and D. R. Powell, Inorg. Chem. Commun., 2008, 11, 1033 Search PubMed.
- M. Bera, G. T. Musie and D. R. Powell, Inorg. Chem. Commun., 2010, 13, 1029 Search PubMed.
-
Crystallographic data of 1: C49H62N4O25K2Zn4, Mr = 1446.786, monoclinic, space groupP21/c, a = 12.4598(13) Å, b = 20.593(2) Å, c = 27.475(3) Å, α = 90°, β = 81.456(7)°, γ = 90°, V = 6971.5(13) Å3, Z = 4, μ = 1.536 mm−1, ρcalcd = 1.477 mg m−3, 15
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 189 total reflections collected, 9161 unique reflections, R = 0.0966 [I > 2σ(I)], Rw = 0.2582 (all data).
189 total reflections collected, 9161 unique reflections, R = 0.0966 [I > 2σ(I)], Rw = 0.2582 (all data). - A. Addison, T. N. Rao, J. Reedijk, J. van Rijn and G. C. Verschoor, J. Chem. Soc., Dalton Trans., 1984, 1349 RSC.
- (a) T. Koike, M. Inoue, E. Kimura and M. Shiro, J. Am. Chem. Soc., 1996, 118, 3091 CrossRef CAS; (b) S. J. Brudenell, L. Spiccia, D. C. R. Hockless and E. R. T. Tiekink, J. Chem. Soc., Dalton Trans., 1999, 1475 RSC; (c) H. Adams, D. Bradshaw and D. E. Fenton, J. Chem. Soc., Dalton Trans., 2001, 3407 RSC.
- H. Adams, D. Bradshaw and D. E. Fenton, Inorg. Chim. Acta, 2002, 332, 195 CrossRef CAS.
- G. B. Deacon and R. J. Philllips, Coord. Chem. Rev., 1980, 33, 227 CrossRef CAS.
- T. Ishioka, Y. Shibata, M. Takahaski, I. Kanesaka, Y. Kitagawa and K. T. Nakamura, Spectrochim. Acta, Part A, 1998, 54, 1827 Search PubMed.
- V. Zelenak, I. Cisarova and P. Lewellyn, Inorg. Chem. Commun., 2007, 10, 27 CrossRef CAS.
- V. Zelenak, M. Sabo, W. Massa and P. Llewellyn, Inorg. Chim. Acta, 2004, 357, 2049 CrossRef CAS.
- (a) K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, John Wiley & Sons, New York, 1997 Search PubMed; (b) B. S. Manhas and A. K. Trikha, J. Indian Chem. Soc., 1982, 59, 315 Search PubMed; (c) D. Martini, M. Pellei, C. Pettinari, B. W. Skelton and A. H. White, Inorg. Chim. Acta, 2002, 333, 72 CrossRef CAS.
- B. H. Ye, X. Y. Li, I. D. Williams and X. M. Chen, Inorg. Chem., 2002, 41, 6426 CrossRef CAS.
- A. Demsar, J. Kosmrlj and S. Petricek, J. Am. Chem. Soc., 2002, 124, 3951 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, table of bond lengths and bond angles, FTIR spectra, and X-ray crystallographic data (CIF). CCDC 839232. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ra01317g |
| This journal is © The Royal Society of Chemistry 2012 |
