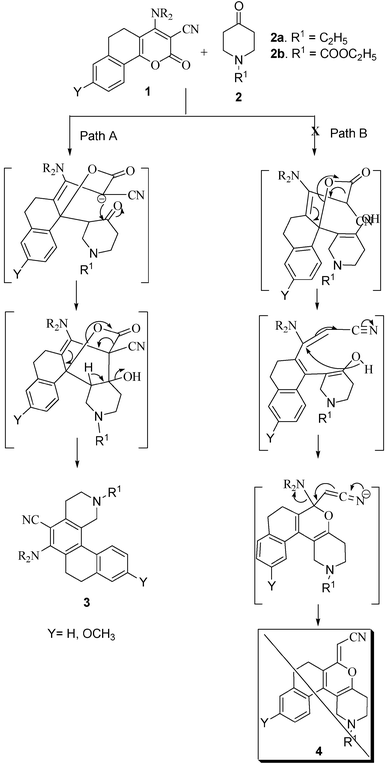
Naphtho[2,1-h]isoquinolines: a new class of partially reduced polycyclic aromatic nucleus†
Ramendra
Pratap
*a,
Resmi
Raghunandan
b,
P. R.
Maulik
b and
Vishnu
Ji Ram
*c
aDepartment of Chemistry, University of Delhi, North Campus, Delhi, 110007, India. E-mail: ramendrapratap@gmail.com
bMolecular & Structural Biology Division, Central Drug Research Institute, Lucknow, 226001, India
cDepartment of Chemistry, Lucknow University, Lucknow, 226007, India. E-mail: vjiram@yahoo.com
First published on 4th January 2012
Abstract
An efficient de novo synthesis of partially reduced naphtho[2,1-h]isoquinolines has been developed through base catalyzed ring transformation of 2-oxo-4-sec-amino-5,6-dihydro-2H-benzo[h]chromene-3-carbonitriles by a carbanion, generated in situ from 1-substituted-4-piperidones in DMF and powdered KOH, in excellent yields. The effect of nitrogen insertion in the D ring of partially reduced benzo[c]phenanthrene on conformational changes has also been studied by X-ray diffraction analysis.
The ubiquitous presence of isoquinoline ring systems in various natural products of therapeutic importance has made them a unique structural motif.1 Annoretine2Ia and litabamine3Ib are the two phenanthrene alkaloids isolated from Annona montana and Litsea cubeba respectively (Fig. 1), in which the ‘f’ site of the isoquinoline ring is fused with the 1,2-positions of naphthalene. The former Ia exhibits cytotoxic activity4 against different human cell cultures, whilst the latter Ib is active as a platelet antiaggregant5 and against acetyl choline esterase.6 These alkaloids belong to the family of the naphtho[2,1-f]isoquinoline ring system, having significant pharmacological properties. The therapeutic importance of this class of compounds inspired us to explore the chemistry of a synthetically viable new class of fused ring system, naphtho[2,1-h]isoquinoline, not reported so far.
![Structures of annoretine2 (Ia) and litabamine3 (Ib), designed naphthalene fused isoquinoline (II), benzo[c]phenanthrene (III) and dihydrobenzo[f]isoquinoline (IV).](/image/article/2012/RA/c2ra00767c/c2ra00767c-f1.gif) | ||
| Fig. 1 Structures of annoretine2 (Ia) and litabamine3 (Ib), designed naphthalene fused isoquinoline (II), benzo[c]phenanthrene (III) and dihydrobenzo[f]isoquinoline (IV). | ||
Polycyclic azaheteroarenes have been recognized as environmental contaminants, produced by incomplete combustion of fossil fuels, organic matter, coal tar distillation and tobacco smoke.7 Most of polycyclic aromatic hydrocarbons (PAHs) are potent carcinogens because of their activation and transformation by enzymes cytochrome P450 and epoxide hydrolase to diol epoxides that covalently bind with the amino function of purine bases of cellular DNA and mutate their replication, resulting in tumorigenesis.8 The naphtho[2,1-h]isoquinolineII ring system is very closely structurally analogous to partially reduced benzo[c]phenanthreneIII. The carcinogenic properties of PAHs are reduced or destroyed by inducing non-planarity through partial reduction of the molecule. The naphtha[2,1-h]isoquinoline, a partially reduced azapolycyclic system may not have carcinogenic properties due to the insertion of a nitrogen atom in the ring which may modify the electronic distribution of the tetracyclic ring system as well as induce non-planarity. The π bond reduction of the aromatic ring distorts the molecular planarity and reduces the binding affinity of metabolites for cellular DNA, resulting in a reduction in carcinogenecity.9 In addition, naphtho[2,1-h]isoquinoline possesses a fjord region which after activation may act as a potent DNA alkylating agent.10 These facts clearly imply that the antineoplastic action can be modified through structural and electronic modification.
In general the toxicity of polycyclic azaarenes depends upon the number of fused aryl rings as well as the hydrophobicity of the molecule.11
A computerized literature survey revealed that neither the chemistry nor the pharmacology of the naphtho[2,1-h]isoquinolineII ring system have been explored so far due to their unavailability. We therefore report a simple, efficient and economical synthesis of this class of compounds for the first time.
2-Oxobenzo[h]chromene 1 was selected as a precursor and prepared in two steps. The first step was the synthesis of the 2-oxo-4-methylsulfanyl-5,6-dihydro-2H-benzo[h]chromene-3-carbonitriles by stirring mixtures of methyl 2-cyano-3,3-dimethylthioacrylate and 1-tetralones, using powdered KOH as a base in DMSO at room temperature.12 This on amination with a sec-amine in ethanol at reflux temperature provided 2-oxo-4-sec-amino-5,6-dihydro-2H-benzo[h]chromene-3-carbonitriles 1 in good yields (Scheme 1).13
![Synthesis of 2-oxo-4-sec-amino-5,6-dihydro-2H-benzo[h]chromene-3-carbonitriles (1).](/image/article/2012/RA/c2ra00767c/c2ra00767c-s1.gif) | ||
| Scheme 1 Synthesis of 2-oxo-4-sec-amino-5,6-dihydro-2H-benzo[h]chromene-3-carbonitriles (1). | ||
During our previous research, the synthesis of benzo[f]isoquinolines14 was based on the base catalyzed ring transformation of 5,6-dihydro-4-sec-amino-2-oxobenzo[h]chromene-3-carbonitriles 1, by cyanoacetamide (Scheme 2). Our attempts to reproduce a similar ring transformation from 2-oxo-4-methylsulfanyl-5,6-dihydro-2H-benzo[h]chromene-3-carbonitriles by cyanoacetamide failed, possibly due to a preference for substitution reaction at C-4 over nucleophilic attack at C-10b for the ring transformation reaction. To avoid any complication due to side reactions, we preferred to use 5,6-dihydro-4-sec-amino-2-oxobenzo[h]chromene-3-carbonitriles 1 as a precursor.
![Synthesis of dihydrobenzo[f]isoquinolines (IV).](/image/article/2012/RA/c2ra00767c/c2ra00767c-s2.gif) | ||
| Scheme 2 Synthesis of dihydrobenzo[f]isoquinolines (IV). | ||
Following the synthesis of dihydrobenzo[f]isoquinolines through base catalyzed ring transformation of suitably functionalized 2-oxobenzo[h]chromenes 1 by cyanoacetamide,12 we planned a different strategy for the construction of naphtho[2,1-h] isoquinolines 3 using 1 as a precursor. The presence of a reduced aromatic ring in the precursor 1 has the advantage of obtaining partially reduced naphtho[2,1-h]isoquinolines, otherwise catalytic or chemical reduction at the final stage may lead to a complex mixture of reduced products.
The molecular make up of 5,6-dihydro-4-sec-amino-2-oxobenzo[h]chromene-3-carbonitriles 1 reveals that it possesses three electrophilic centres C-2, C-4 and C-10b, of which the latter is highly vulnerable to nucleophilic attack due to extended conjugation and the presence of an electron withdrawing substituent at position C-3 of the chromene ring 4. The carbanion used as a nucleophile in the present study for the ring transformation was generated in situ from N-substituted 4-piperidone by KOH in DMF.
Thus, an equimolar mixture of 1, N-substituted 4-piperidone 2 and powdered KOH in DMF was stirred for 2–3 h. During this period all the starting material was consumed and a new spot appeared on the TLC. The reaction mixture was poured onto crushed ice with vigorous stirring and thereafter neutralized with 10% aqueous HCl. The resulting precipitate was filtered, washed with water and purified using neutral alumina column chromatography and characterized as 2-substituted-6-sec-amino-1,2,3,4,7,8-hexahydro-naphtho[2,1-h]-isoquinolin-5-carbonitrile 3. There was also a possibility of the formation of another product, (5-oxa-2-substituted-1,2,3,4,7,8-hexahydronaphtho[2,1-h]isoquinolin-6-ylidene)acetonitrile 4, but practically the product isolated and characterized was 3. In the formation of both the products 3 and 4, the initial step is attack of the carbanion from 2 at C-10b with formation of the Michael adduct and thereafter, it may follow different courses of reactions. If the Michael adduct formed in situ undergoes cyclization involving C-3 of 1 and the carbonyl function of 4-piperidone with concomitant loss of carbon dioxide and water, product 3 is feasible. However, the formation of product 4 is based on the enolate addition to enamine followed by loss of secondary amine, but practically we did not succeed in isolating this compound even in trace amounts (Table 1).
|
|
||||
|---|---|---|---|---|
| 3 | Y | R1 | NR2 | Yield (%) |
| a | H | C2H5 | Piperidin-1-yl | 91 |
| b | H | C2H5 | 4-Methylpiperidin-1-yl | 92 |
| c | H | C2H5 | 4-Benzylpiperazin-1-yl | 87 |
| d | OCH3 | C2H5 | Piperidin-1-yl | 81 |
| e | H | COOC2H5 | Piperidin-1-yl | 88 |
| f | H | COOC2H5 | 4-Methylpiperidin-1-yl | 79 |
| g | H | COOC2H5 | 4-Benzylpiperidin-1-yl | 91 |
| h | H | COOC2H5 | 4-Benzylpiperazine-1-yl | 93 |
| i | OCH3 | COOC2H5 | Piperidin-1-yl | 76 |
| j | OCH3 | COOC2H5 | 4-Methylpiperidin-1-yl | 75 |
To study the conformational arrangements of the rings of naphthoisoquinoline, diffraction quality crystals of 2-ethyl-6-(4-methylpiperidin-1-yl)-1,2,3,4,7,8-hexahydronaphtho-[2,1-h]-isoquinoline-5-carbonitrile 3b were obtained by slow evaporation at room temperature. The conformation of this compound with arbitrary numbering is shown in the ORTEP diagram in Fig. 2, which indicates a non-planar conformation.
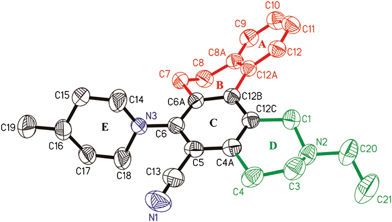 | ||
| Fig. 2 Displacement ellipsoid plot (30% probability) showing the molecular structure of 3b with the atomic labelling. | ||
The least squares plane calculation from the X-ray crystallographic data15 of 3b indicates that rings A and C are nearly planar. The average mean plane angle for the twist between the terminal rings A and C is 34.06°. The rings B and D adopt a half chair conformation. The ring E adopts an envelope conformation. The distance between the non-bonded carbon atoms is 3 Å, which is shorter than any van der Waals radii of carbon atoms by 0.4 Å in an over-crowded molecule, responsible for inducing distortion in the molecule. This strain is relieved either through stretching, bending of the chemical bonds and buckling of aromatic rings.
The torsion angles (C12–C12a–C12b–C12c and C1–C12c–C12b–C12a) of the compound 3b are −35.79 and −3.05. The torsion angles for benzo[c]phenanthrene and 1,4-dimethyl benzo[c]phenanthrene are 17.31 & 19.02 and −29.29 & −18.44° respectively. Some of the inner C–C bond lengths have been lengthened while the outer are closer to the normal single and double bond lengths. Thus, it is the central arene that accommodates most of the torsional strain.
Crystal packing of 3b revealed that there are four molecules in the unit cell (Fig. 3) in the form of enantiomeric pairs in which one pair has the tendency to acquire clockwise and the other anticlockwise helical conformations as evident from Fig. 4. The helix with the clockwise orientation is designated as P, while the helix with the anticlockwise orientation is designated as M.
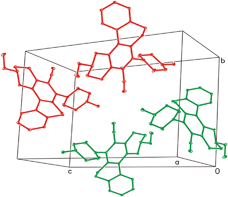 | ||
| Fig. 3 3b showing the arrangement of molecules in the unit cell. | ||
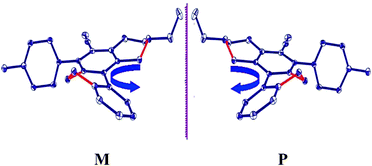 | ||
| Fig. 4 3b showing the enantiomeric pairs of the two compounds. | ||
All the synthesized compounds were characterized by spectroscopic analyses and synthetic protocols and analytical data for representative compounds are described in the experimental section.16
In summary, our synthetic protocol opens a new avenue for the construction of naphtho[2,1-h]isoquinolines, a new class of ring system, through base catalyzed ring transformation of suitably functionalized benzo[h]chromenes by carbanions, generated in situ from N-substituted 4-piperidones in DMF/KOH, in excellent yields. The reaction is very efficient and practical for the synthesis of a new class of aza polycyclic heteroaromatics.
Acknowledgements
The authors are thankful to CSIR, New Delhi and UGC, New Delhi, for financial support. RP is thankful to the University of Delhi, India, for providing a R and D grant.References
- K. Bentley, Nat. Prod. Rep., 2000, 17, 247 RSC.
- Y.-C. Wu, G.-Y. Chang, C.-Y. Duh and S. K. Wang, Phytochemistry, 1993, 33, 497 CrossRef CAS.
- Y.-C. Wu, J. Y. Liou, S.-S. Lee and S. J. Lu, Tetrahedron Lett., 1991, 32, 4169 CrossRef CAS.
- (a) W. M. Whaley and M. Meadow, J. Org. Chem., 1954, 19, 661 Search PubMed; (b) G. Galiazzo, P. Bortolus and F. Masetti, J. Chem. Soc., Perkin Trans. 2, 1975, 1712 RSC; (c) F. Masetti, G. Bartocci and U. Mazzucato, Gazz. Chim. Ital., 1982, 112, 255 CAS; (d) M. J. Tanga, R. G. Almquist and T. J. Smith, J. Heterocycl. Chem., 1985, 22, 1597 Search PubMed.
- Y.-C. Wu, J. Y. Liou, C.-Y. Duh, S.-S. Lee and S. T. Lu, J. Pharm. Pharmacol., 1997, 49, 706 Search PubMed.
- C. -M. Chiou, J.-J. Kang and S.-S. Lee, J. Nat. Prod., 1998, 61, 46 CrossRef.
- (a) R. G. Harvey, Polycyclic Aromatic Hydrocarbons: Chemistry and Carcinogenesis, Cambridge University, Cambridge, 1991 Search PubMed; (b) R. G. Harvey, in The Handbook of Environmental Chemistry, Part I: PAHs and Related Compounds, ed. O. Hutzinger and A. Neilson, Springer,Heidelberg, 1997, vol. 3, pp. 1–54, ch. 1 Search PubMed.
- (a) R. G. Harvey, Polycyclic Aromatic Hydrocarbons: Chemistry and Carcinogenesis, Wiley-VCH, New York, 1997 Search PubMed; (b) S. Kumar, J. Org. Chem., 1997, 62, 8535 CrossRef CAS.
- D. D. Phillips and A. W. Johnson, J. Am. Chem. Soc., 1955, 77, 5977 Search PubMed.
- E. R. Butch, H. Yagi, D. M. Jerina, S. Amin and W. M. Baird, Polycyclic Aromat. Compd., 1994, 6, 63 Search PubMed.
- E. A. J. Bleeker, S. Wiegman, P. de Voogt, H. A. Leslie, E. de Haas and W. Admiraal, Rev. Environ. Contam. Toxicol., 2002, 173, 39 Search PubMed.
- (a) R. Pratap and V. J. Ram, Tetrahedron Lett., 2007, 48, 5039–5042 Search PubMed; (b) R. Pratap and V. J. Ram, Tetrahedron Lett., 2007, 48, 2755 Search PubMed.
- R. Pratap and V. J. Ram, J. Org. Chem., 2007, 72, 7402 CrossRef CAS.
- R. Pratap, R. Raghunandan, P. R. Maulik and V. J. Ram, Tetrahedron Lett., 2007, 48, 7982 Search PubMed.
- The crystal data of 6b: C26H31N3, M = 385.54, monoclinic, P2(1)/c, a = 10.952(2) Å, b = 11.7610(1) Å, c = 17.935(4) Å, β = 105.090(1)°, V = 2230.5(7) Å3, Z = 4, Dc = 1.148 g cm−3, μ(Mo-Kα) = 0.068 mm−1, F(000) = 832, rectangular block, yellow, size = 0.325 × 0.275 × 0.175 mm, 5060 reflections measured (Rint = 0.0214), 3918 unique, wR2 = 0.2516 for all data, conventional R = 0.0700 [(Δ/σ)max = 000)] on F-values of 1558 reflections with I > 2σ(I), S = 1.016 for all data and 265 parameters. Unit cell determination and intensity data collection (2θ = 50°) were performed on a Bruker P4 diffractometer at 293(2) K. Structure solution by direct methods and refinements by full-matrix least-squares methods on F2. Programs: XSCANS [Siemens Analytical X-ray Instrument Inc., Madison, Wisconsin, USA, 1996], SHELXTL-NT [Bruker AXS Inc., Madison, Wisconsin, USA, 1997]. CCDC no. 890314.
- General procedure for the synthesis of 6-sec-amino1,2,3,4,7,8-hexahydronaphtho[2,1-h]isoquinoline-5-carbonitriles (6): A mixture of 2-oxo-4-sec-amino-5,6-dihydro-2H-benzo[h]chromene-3-carbonitrile (0.5 mmol), N-substituted piperidone (0.6 mmol) and KOH (0.7 mmol) in DMF (5 mL) was stirred for 2–3 h. Completion of reaction was monitored by TLC. Thereafter, the reaction mixture was poured onto crushed ice with vigorous stirring followed by neutralization with 10% HCl. The precipitate obtained was filtered, washed with water, dried and purified using a neutral alumina column with 1% ethyl acetate in hexane as eluent. (6a) White powder; yield: 91%; mp: 150–152 °C; IR (KBr): 2213 (CN) cm−1; 1H NMR (300 MHz, CDCl3): δ 1.11 (t, J = 7.17 Hz, 3H, CH3), 1.63–1.71 (m, 6H, CH2), 2.57 (q, J = 7.14 Hz, 2H, CH2), 2.62–2.65 (m, 2H, CH2), 2.73–2.77 (m, 2H, CH2), 2.82 (t, J = 6.33 Hz, 2H, CH2), 3.15 (t, J = 6.33 Hz, 6H, CH2), 3.75 (s, 2H, CH2), 7.25–7.32 (m, 3H, ArH), 7.42–7.46 (m, 1H, ArH); 13C NMR (75 MHz, CDCl3): δ 11.2, 22.9, 23.6, 25.6, 27.7, 28.2, 48.9, 50.8, 51.0, 55.9, 106.2, 116.9, 124.5, 126.2, 126.7, 126.9, 127.2, 132.2, 134.2, 136.6, 138.2, 139.2, 150.8; MSm/z 372 (M+ + 1); HRMS: (EI, 70 eV) calcd for C25H29N3 371.23615 (M+) found for m/z 371.23622.
Footnote |
| † CCDC reference number 890314. For crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ra00767c |
| This journal is © The Royal Society of Chemistry 2012 |