Enhanced electrochemical capacitance of nanoporous NiO based on an eggshell membrane
Wentao
Deng
a,
Yong
Liu
*b,
Ying
Zhang
b,
Fang
Lu
a,
Qiyuan
Chen
a and
Xiaobo
Ji
*a
aCollege of Chemistry and Chemical Engineering, Central South University, Changsha, 410083, P.R. China. E-mail: xji.csu.edu@gmail.com; Fax: +86 0731-88879616; Tel: +86 0731-88879616
bState Key Laboratory of Powder Metallurgy, Central South University, Changsha, 410083, P.R. China. E-mail: yongliu11@yahoo.com.cn; Fax: +86 0731-88710855; Tel: +86 0731-88836939
First published on 10th January 2012
Abstract
Porous NiO nanospheres are synthesised with the assistance of an eggshell membrane. The resulting NiO nanospheres are composed of well distributed nanoparticles of 5–10 nm in diameter with high surface areas and good electrolyte wetting, which result in a good capacitive performance of 550 F g−1 at a current density of 1 A g−1 in 2 M KOH aqueous solution.
The rapidly growing market in portable electronic devices and electric vehicles (EVs) coupled with ever worsening global warming issues calls for not only urgent development of clean alternative energies and emission control of global warming gases, but also more environmentally friendly, high-performance energy-storage systems. Electrochemical supercapacitors (ECs), also called supercapacitors or ultracapacitors, are electronic components that can be rapidly charged/discharged and have a longer cycle life than batteries.1 ECs used to store energy are generally based on carboneous materials, transition metal oxides and conducting polymers.2,3 Transition metal oxides used in supercapacitor, such as RuO2, Co3O4, MnO2 and NiO, have been developed admirably in recent years due to their high pseudocapacitance caused by a fast redox reaction. Impressively, their specific capacitance can be 10–100 times higher than those obtained from the conventional electrochemical double layer capacitors (EDLCs).4 In particular, nickel is a great candidate for capacitors due to its low cost and high abundance on earth,5,6 it can display a theoretical specific capacitance of 2573 F g−17 It has been used as active material of batteries for years.8 It's not surprising to see that nickel oxide is one of the most promising materials for ECs owing to its unique physical and electrochemical properties based on the Ni(OH)2/NiOOH transformation.9
The morphology of electrode materials can exert a wide influence over the speed of ion transfer and the ability related to electrolyte wetting. Fast ion transportation and excellent electrolyte infiltration can be obtained by porous structures, owning to their higher surface areas and less jammed ion paths.10 In this study, we report a strategy to fabricate porous NiO nanospheres by calcination of basic nickel carbonate that is prepared by using eggshell membrane as a template under mild conditions. The obtained NiO was applied as an active material in a supercapacitor and good capacitive properties were observed.
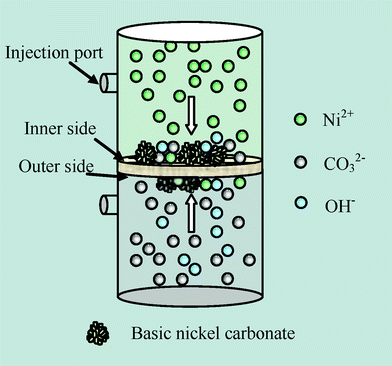
The eggshell membrane in this experiment was prepared by directly peeling the membrane from the shell of a hen egg and was washed in ultra pure water.11 In this work, the eggshell membrane was used as a removable template for the preparation of the precursor basic nickel carbonate. All the reagents were of analytical grade and used without further purification. The growth of the precursor progressed in a device which was placed vertically as shown in Scheme 1. Nickel chloride (1 M) and sodium carbonate (1 M, pH 11.53) aqueous solutions were separately placed in the two parts of the device divided by the eggshell membrane. After being left for 9 days at 40 °C, the precipitates that grew on the inner side were scraped from the membrane, washed with ultra pure water three times and dried in an oven for 24 h at 40 °C. To obtain NiO, the dry precipitates were calcined in a tube furnace by first heating at a speed of 5 °C min−1 and then keeping at 400 °C for 1 h.
 | ||
| Scheme 1 Schematic diagram of the crystallizer. | ||
Electrochemical characterizations were carried out at room temperature (Modulab, Solartron Analytical). The working electrode was fabricated by first mixing prepared NiO, acetylene black and polytetrafluoroethylene (PTFE) at a weight ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and then the prepared mixture was pressed on nickel foam at 10 MPa for 30 s. A three electrode system was used. A Hg/HgO electrode filled with 1 M NaOH was used as the reference electrode, and a platinum sheet as the counter electrode. The electrolyte was 2 M KOH aqueous solution.
5 and then the prepared mixture was pressed on nickel foam at 10 MPa for 30 s. A three electrode system was used. A Hg/HgO electrode filled with 1 M NaOH was used as the reference electrode, and a platinum sheet as the counter electrode. The electrolyte was 2 M KOH aqueous solution.
Fig. 1 is shown to reveal the morphology characters of NiO. A scanning electron microscopy (SEM; Nova NanoSEM 230) image of basic nickel carbonate formed on eggshell membrane is shown in Fig. 1(a). Basic nickel carbonate formed on the both sides of the membrane when the Ni2+ and CO32− diffused from one side to the other and bonded with each other through the membrane as the arrows shown in Scheme 1.12 It is easy to decompose the as-prepared basic nickel carbonate and get NiO through calcination. The corresponding reactions can be described as in eqn (1).13
 | (1) |
 | ||
| Fig. 1 Morphology characterization of porous NiO nanospheres. (a) SEM image. (b) XRD pattern. (c) TEM image and the image at the top right corner is electron diffraction pattern (ED). (d) High resolution transmission electron microscopy (HRTEM) image, the image at top right corner is the enlargement of the part in circle. | ||
The solutions were separated by the eggshell membrane, providing a link for ion-exchange. As can be clearly seen in Fig. 1(a), the precipitates aggregate to form spheres of about 1 μm in diameter on the fibers which are part of the eggshell membrane.11SEM image of NiO in Fig. 1(b) indicates that the obtained NiO nanospheres had rough surfaces with many protuberances and were less than 100 nm in diameter. It is supposed that NiO nanospheres were assembled from smaller nanoparticles. The structure was further elucidated by Transmission electron microscopy (TEM; JEM-2100F) (Fig. 1(d)). It is clear that the NiO nanospheres were composed of well distributed nanoparticles, which had a typical size ranging from 5 to 10 nm in diameter, giving higher surface areas and better electrolyte wetting. The electron diffraction (ED) pattern at the top right corner in Fig. 1(d) shows the crystallization of NiO. The crystallinity of NiO was determined by X-ray diffraction (XRD; D/ruax 2550PC) in Fig. 1(c). All of the diffraction peaks can be indexed to the NiO (PDF 47-1049). The high resolution transmission electron microscopy image in Fig. 1(e) shows that the lattice fringes interplanar spacing is 0.14 nm, consistent with the distance of the (220) planes of NiO.
Cyclic voltammetry (CV) measurements were shown in Fig. 2(a). A pair of cathodic and anodic peaks in the CV curves can be clearly seen, indicating the fast redox reaction of NiO in alkaline solution, which can be displayed as in eqn (2)14,15
| NiO + OH− ⇌ NiOOH + e− | (2) |
 | ||
| Fig. 2 Electrochemical properties of porous NiO nanospheres in 2 M KOH solution: (a) Cyclic votammograms of NiO in the potential range from 0 to 0.55 V. (b) Galvanostatic current charge/discharge curves of NiO for different current densities. (c) Charge/discharge measurements over 1000 cycles at a current density of 3 A g−1. The inset shows the Galvanostatic current charge/discharge curves of NiO at 3 A g−1. | ||
Fig. 2(b) shows the galvanostatic current charge/discharge curves (CD) of porous NiO nanospheres. The broad platforms of the curves suggested that NiO nanospheres had good capacitive properties. The average specific capacitances were calculated to be 550 F g−1, 452 F g−1, 300 F g−1 at discharge current densities of 1, 2, 10 A g−1, respectively. Fig. 2(c) indicates that the capacitance decreases about 55% after 1000 cycle measurements, which can be attributed to the nanostructure of the as-prepared NiO nanospheres which were constituted of tiny particles of about 5–10 nm that would easily agglomerate into large-size grains in the process of the fast redox reaction.3 The re-agglomeration of nano materials reduces the active material area, resulting in a reduction of specific capacitance.
In summary, we fabricated porous NiO nanospheres by an eggshell membrane assisted method. Through this method the precursor basic nickel carbonate formed on the eggshell membrane which was used as a template in the process. The obtained NiO consisted of nanoparticles with typical sizes of 5–10 nm in diameter and exhibited a good specific capacitance of 550 F g−1 at a current density of 1 A g−1. Its good capacitive properties make it a promising electrode material for supercapacitors.
Financial support from the National Natural Science Foundation of China (No. 21050110115), International Joint Project from The Royal Society (No. JP090644), Hunan Province Foundation of Natural Science (10JJ6026) and Central South University Annual Mittal-Founded Innovation Project (71143000032) are greatly appreciated.
References
- Electrochemical Supercapacitors: Scientific Fundermentals and Technological Applications, ed. B. E. Conway, Kluwer Academic/Plenum Publishers, New York, 1999 Search PubMed.
- B. E. Conway and W. G. Pell, J. Solid State Electrochem., 2003, 7, 637–644 CrossRef CAS.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS.
- B. E. Conway, J. Electrochem. Soc., 1991, 138, 1539–1548 CrossRef CAS.
- C. Bradu, L. Frunza, N. Minalche, S. Avramescu, M. Neata and I. Udrea, Appl. Catal., B, 2010, 96, 548–556 CrossRef CAS.
- B. Q. Xu, J. M. Wei, H. Y. Wang, K. Q. Sun and Q. M. Zhu, Catal. Today, 2001, 68, 217–225 CrossRef CAS.
- K.-W. Nam and K.-B. Kim, J. Electrochem. Soc., 2002, 149, A346 CrossRef CAS.
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS.
- K.-C. Liu and M. A. Anderson, J. Electrochem. Soc., 1996, 143, 124–130 CrossRef CAS.
- P. G. Bruce, B. Scrosati and J. M. Tarascon, Angew. Chem., Int. Ed., 2008, 47, 2930–2946 CrossRef CAS.
- M. Takiguchi, K. Igarashi, M. Azuma and H. Ooshima, Cryst. Growth Des., 2006, 6, 2754–2757 CAS.
-
N. Zubryckyj,
1972, US patent 3
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 640
640![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 706 Search PubMed.
706 Search PubMed. - Y. Wang and J. J. Ke, Mater. Res. Bull., 1996, 31, 55–61 CrossRef CAS.
- Q. Lu, M. W. Lattanzi, Y. P. Chen, X. M. Kou, W. F. Li and X. Fan, Angew. Chem., 2011, 123, 6979–6982 CrossRef.
- D.-D. Zhao, S.-J. Bao, W.-J. Zhou and H.-L. Li, Electrochem. Commun., 2007, 9, 869–874 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
