Polyaniline/multiwalled carbon nanotubes nanocomposite-an excellent reversible CO2 capture candidate
Ashish
Kumar Mishra
and
Sundara
Ramaprabhu
*
Alternative Energy and Nanotechnology Laboratory (AENL), Nano Functional Materials Technology Centre (NFMTC), Department of Physics, Indian Institute of Technology Madras, Chennai, 600036, India. E-mail: ramp@iitm.ac.in
First published on 10th January 2012
Abstract
Here we demonstrate the reversible CO2 capture capacity of polyaniline/multiwalled carbon nanotubes nanocomposite at high pressures for the first time to the best of our knowledge. High CO2 capture capacity was achieved for nanocomposite, which can be associated to the possible chemical interaction of CO2 molecules with nanocomposite. This study promotes the investigation of other polymer-carbon composites for CO2 capture.
It has been estimated that man-made emissions of CO2 into the atmosphere have resulted in an increase in CO2 concentration of at least 30% over pre-industrial age concentrations. CO2 emissions over the next 100 years could see an increase of 3 to 4 times from current levels, which may lead to climate change and/or global warming and therefore serious environmental impact. Accordingly, there has been a consequential focus on improving techniques for CO2 sequestration or capture. Today, different industries (like coal fired power plants, cement, automobiles and steel industries etc.) are required to remove or substantially reduce CO2 levels in exhaust streams before they are vented to the atmosphere. The most likely options for CO2 capture include geological sequestration, absorption, physical and chemical adsorption, gas-separation membranes and mineralization/biomineralization.1–4 The CO2 absorption process using aqueous amine solutions have been extensively used for the removal of CO2 from gas streams in many industries. This process is very expensive and energy intensive. This technology also suffers from inherent regeneration cost and inefficiency due to the possible corrosion in the presence of O2 and other impurities and the large amounts of energy required for regeneration. In addition, the amine solution loses viability through amine degradation and loss. Some amine based ionic liquids have attracted attention due to their good CO2 uptake capacity. But preparation of such ionic liquids is a tedious process.5–7 Additionally, most of these absorbents seriously suffer with adsorption capacity limited to room temperature.
Among the various technologies and processes that have been developed and are emerging for CO2 capture, storage and utilization (CSU) of CO2, solid CO2-adsorbents are widely applied. For any practical application of these solid CO2-adsorbents, along with sorption capacity, recyclability should also be considered.8 The current world research is focused on the investigation of materials which can capture a large amount of CO2 through physical or chemical adsorption. Recently, several solid sorbents have been utilized to remove carbon dioxide from enclosed environments such as submarine, aircraft, spacecraft or enclosed pressurized chambers.9,10 Leal et al., have demonstrated the reversible adsorption of CO2 on amine surface-bonded silica gel.11 The porous support provides the amine with structural integrity and a surface for gas/solid contact. The lack of stability in solid materials often limits them to a one-time use hence the ability to regenerate an adsorbent is also an important consideration. In terms of achieving high adsorption capacities, carbonaceous materials (activated carbon, carbon nanotubes, graphene) and zeolite-based molecular sieves have shown much promise. Activated carbon (AC) generally gives higher additional capacity at pressures greater than atmospheric compared to zeolites. CO2 adsorption capacities of activated carbons not only depends on their pore structure but also on the surface chemistry properties.12,13 Recently, enhanced CO2 adsorption capacity is reported in nitrogen treated AC.14 Studies of CO2 adsorption have been performed on single and multiwalled carbon nanotubes (CNTs).15,16 The amine-functionalized CNTs have received increasing attention for CO2 capture from flue gas due to its unique physicochemical properties as well as high thermal and chemical stability.17 These sorbents include single-walled carbon nanotubes15 and multiwalled carbon nanotubes18,19 functionalized with various amine solutions. In our earlier work, we have demonstrated the physicochemical adsorption behavior of magnetite decorated multiwalled carbon nanotubes (MWNTs) with good CO2 capture capacity and reusability.20Amine based solvents and amine based ionic liquids show high performance for CO2 capture due to the presence of amine groups.7 In this context, in the present work we have modified the surface of MWNTs with polyaniline (PANI) with simple chemical route and demonstrated the physicochemical adsorption behavior of PANI decorated MWNTs (PANI-f-MWNTs) nanocomposite for CO2 capture. PANI consists of a chain of amine groups, hence it is anticipated that this nanocomposite will have high CO2 capture capacity. To the best of our knowledge, this study is the first study on PANI-carbon nanotubes nanocomposite for CO2 capture.
MWNTs were synthesized by catalytic chemical vapor deposition method. In this method hydrogen decrepitated AB3 alloy was used as catalyst material. Pyrolysis of acetylene takes place at 700 °C under inert atmosphere, which results in the growth of MWNTs.21 These MWNTs were further purified by air oxidation followed by acid treatment to remove amorphous carbon and catalytic impurities. These purified MWNTs were further functionalized to make it hydrophilic by the stirring of MWNTs in concentrated nitric acid for 2 h. Functionalization provides anchoring sites for the uniform coating of PANI over MWNTs surface. Coating of PANI over functionalized MWNTs (f-MWNTs) was performed by poly-condensation of aniline with K2Cr2O7 in solution of 1 M HCl. The nanocomposite material (PANI-f-MWNTs) was then filtered and washed with a large amount of water and subsequently with ethanol to remove the residual oxidant. Finally, all composites were washed with acetone and dried at 60 °C.22
A morphological study of PANI-f-MWNTs nanocomposite was performed using an electron microscopy technique. Fig. 1 shows the SEM (a,c) and TEM (b,d) images of MWNTs and PANI-f-MWNTs nanocomposite. Images clearly show the uniform coating of PANI over the surface of f-MWNTs. Functionalization of MWNTs provide anchoring sites for polymer decoration and results in uniform decoration of PANI. Fig. 2 shows the Raman spectra of f-MWNTs and PANI-f-MWNTs nanocomposite. Fig. 2a shows the comparative intensity of D-band (1349 cm−1) with G-band (1578 cm−1) for f-MWNTs,23 which arises because of further acid treatment of purified MWNTs, which leads to the defects on the surface of MWNTs due to the attachment of functional groups. Fig. 2b exhibits the Raman spectra of PANI-f-MWNTs nanocomposite. Peaks at 1346 and 1578 cm−1 belong to MWNTs, while peaks at 1161.9, 1212.5 and 1485.8 cm−1 correspond to the characteristic peaks of PANI.24 Thus, Raman spectrum of PANI-f-MWNTs nanocomposite shows the peaks corresponding to different vibrations of polyaniline and MWNTs in nanocomposite. These vibrations were also identified in FTIR spectrum of PANI-f-MWNTs nanocomposite also (Fig. 3). FTIR spectrum of f-MWNTs (shown in Fig. 3a) confirms the defective sites at the surface of f-MWNTs and the presence of >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (1635 cm−1), >C
C (1635 cm−1), >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1022 cm−1),
O (1022 cm−1), ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 (2854, 2925 cm−1) and –OH (3437 cm−1) functional groups at the surface of MWNTs. These functional groups act as anchoring sites for the polymer. Fig. 3b shows the FTIR spectrum of PANI-f-MWNTs nanocomposite with low intensity of anti-symmetric
CH2 (2854, 2925 cm−1) and –OH (3437 cm−1) functional groups at the surface of MWNTs. These functional groups act as anchoring sites for the polymer. Fig. 3b shows the FTIR spectrum of PANI-f-MWNTs nanocomposite with low intensity of anti-symmetric ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 vibrations (around 2928 cm−1) of MWNTs along with hydroxyl group (–OH stretching, 3435 cm−1).23 In addition, peaks around 501, 803, 1289, 1488 and 1567 cm−1 were also observed for PANI-f-MWNTs nanocomposite corresponding to deformation of the benzenoid ring (B), C–C stretching of quinonoid, C–N stretching of secondary aromatic amines, benzenoid ring stretching and quinonoid (Q) ring stretching, respectively. The peak around 1243 cm−1 may correspond to the C–NH+ stretching (characteristic of polaron form of PANI emeraldine salt), while the peak around 1122 cm−1 may be assigned to the stretching vibration of –NH+
CH2 vibrations (around 2928 cm−1) of MWNTs along with hydroxyl group (–OH stretching, 3435 cm−1).23 In addition, peaks around 501, 803, 1289, 1488 and 1567 cm−1 were also observed for PANI-f-MWNTs nanocomposite corresponding to deformation of the benzenoid ring (B), C–C stretching of quinonoid, C–N stretching of secondary aromatic amines, benzenoid ring stretching and quinonoid (Q) ring stretching, respectively. The peak around 1243 cm−1 may correspond to the C–NH+ stretching (characteristic of polaron form of PANI emeraldine salt), while the peak around 1122 cm−1 may be assigned to the stretching vibration of –NH+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) (in the B–NH+
(in the B–NH+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Q segment) in the bipolaron form of PANI emeraldine salt.25,26 These peaks clearly suggest the formation of emeraldine salt type polyaniline in PANI-f-MWNTs nanocomposite.
Q segment) in the bipolaron form of PANI emeraldine salt.25,26 These peaks clearly suggest the formation of emeraldine salt type polyaniline in PANI-f-MWNTs nanocomposite.
 | ||
| Fig. 1 (a) SEM and (b) TEM images of MWNTs, (c) SEM and (d) TEM images of PANI-f-MWNTs nanocomposite. | ||
 | ||
| Fig. 2 Raman spectra of (a) f-MWNTs and (b) PANI-f-MWNTs nanocomposite. | ||
 | ||
| Fig. 3 FTIR spectra of (a) f-MWNTs and (b) PANI-f-MWNTs nanocomposite. | ||
Capture capacity of PANI-f-MWNTs nanocomposite for CO2 was studied using Sieverts apparatus and capture capacity was calculated using gas equation with van der Waals corrections. Schematic of the apparatus is given elsewhere.20 Capture of CO2 in PANI-f-MWNTs nanocomposite was studied at three different temperatures (25, 50 and 100 °C) and at high pressures (3–13 bar). Amount of CO2 adsorbed in mole was measured by following equations:
| Δnabsorbed = n − (n′ + n′′) | (1) |
| abn i 3 + aVini2 + (RT + Pib)Vi2ni − PiVi3 = 0 | (2) |
| abn′3 + aVin′2 + (RT + Peqb)Vi2n′ − PiVi3 = 0 | (3) |
| abn′′3 + aVcn′′2 + (RT + Peqb)Vc2n′′ − PeqVc3 = 0 | (4) |
Fig. 4 shows the adsorption isotherms of MWNTs and PANI-f-MWNTs nanocomposite in the pressure range 2–13 bar and at three different temperatures (25, 50 and 100 °C). Adsorption capacity of 11.7, 8.3 and 7 mmol g−1 was observed at 11 bar pressure and 25, 50 and 100 °C temperatures, respectively for MWNTs. However, adsorption capacities of 67, 46 and 27 mmole/g were observed for PANI-f-MWNTs nanocomposite at 11 bar pressure and 25, 50 and 100 °C temperatures, respectively. As it is known that, amine based solvents are known to be a good adsorbent of CO2 gas due to the interaction of CO2 with amine groups. Higher adsorption capacity of PANI-f-MWNTs nanocomposite compared to MWNTs may directly associate with chemical interaction of CO2 molecules with number of amine groups present in the polyaniline based nanocomposites along with possible physical adsorption of CO2 at high pressures. Isotherms suggest that capture capacity increases with an increase in pressure. This may be due to the possible multilayer adsorption of gas at nanocomposite surface and presence of more number of CO2 molecules to interact with amine groups of polymeric chain at high pressures.
 | ||
| Fig. 4 Pressure-composition adsorption isotherms of PANI-f-MWNTs nanocomposite. | ||
Fig. 5 shows the temperature variation of capture capacity of nanocomposite for CO2. It clearly suggests that the capture capacity of nanocomposite decreases with an increase in temperature. This may be due to the higher kinetic energy of CO2 molecules at higher temperatures, which results in greater desorption rate and reduces the possibility of molecular adsorption of CO2. In addition, the possibility of interaction of CO2 molecules with amine groups may be reduced due to high energy CO2 molecules. At lower temperatures, better interaction of CO2 molecules with amine groups may be responsible for high CO2 capture capacity.
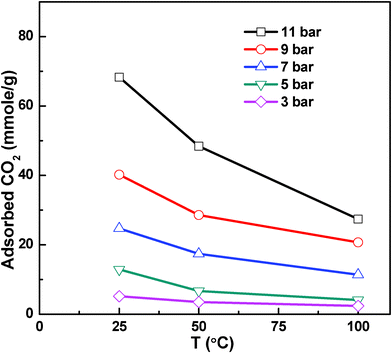 | ||
| Fig. 5 Temperature dependence of CO2 capture capacity of PANI-f-MWNTs nanocomposite. | ||
In order to confirm the mechanism for CO2 capture in PANI-f-MWNTs nanocomposite, FTIR study was performed. Fig. 6 shows the FTIR spectrum of CO2 adsorbed PANI-f-MWNTs nanocomposite. Along with peaks corresponding to the characteristics of PANI-f-MWNTs nanocomposite, additional peaks were observed at 1384 and 1749 cm−1. These peaks correspond to the chemical interaction of CO2 with amine groups of nanocomposite, leading to the formation of bidentate carbonates (C–O stretching vibrations) and carbamates (C–O asymmetric stretching vibrations), respectively.7,27 Additionally, a significant peak was observed at 2350 cm−1, which corresponds to the physically adsorbed CO2 at high pressures.20,28FTIR results clearly indicate the formation of a chemically bound carbonate and carbamates with additional physically absorbed CO2 at higher pressures. Thus the FTIR study suggests the physicochemical adsorption behavior of PANI-f-MWNTs nanocomposite for CO2.
 | ||
| Fig. 6 FTIR spectra of CO2 adsorbed PANI-f-MWNTs nanocomposite. | ||
Regeneration of nanocomposite was performed by degassing the sample after each cycle of CO2 capture at 140 °C under vacuum (∼10−8 bar) using a diffusion pump. This helps in removing captured CO2 from the nanocomposite. High performance of CO2 capture capacity was observed in the next cycle, suggesting the regeneration of nanocomposite. Upon application of vacuum at high temperature, desorption of physically adsorbed CO2 takes place.20 Additionally, upon the application of vacuum the carbonates and carbamate asymmetric stretchings disappear, and the original spectrum is restored. This regenerating property of nanocomposite for CO2 capture make it a suitable candidate for commercial use to reduce the CO2 from the industrial exhaust.
The present nanocomposite (PANI-f-MWNTs) shows higher CO2 capture capacity compared to other solid sorbents reported. Zhang et al. have reported around 20% enhancement in CO2 uptake by modifying the activated carbon with nitrogen at room temperature and shown around 16 mmole/g of CO2 capture in nitrogen doped activated carbon at 11 bar pressure.14 High pressure CO2 adsorption study on different metal organic frameworks by Millward and Yaghi exhibits CO2 capture capacity ranging from 2–10 mmole/g at nearly 11 bar pressure and room temperature.29 Cavenati et al. have reported around 5 mmole/g of CO2 adsorption in 13X zeolite at 16 bar pressure and room temperature.30CO2 adsorption capacity of 5 mmole/g at nearly 12 bar pressure and at room temperature is observed for MCM 41 silica.31 The present nanocomposite shows higher CO2 capture performance compared to our earlier study of MWNTs and Fe3O4-f-MWNTs. The CO2 capture capacity of 11.7 and 49 mmole/g was observed for MWNTs and Fe3O4-f-MWNTs, respectively, at 11 bar pressure and room temperature.20 An enhancement of 37% in capture capacity was observed for PANI-f-MWNTs compared to Fe3O4-f-MWNTs nanocomposite. High capture capacity of PANI-f-MWNTs may be attributed to the chemical interaction of CO2 molecules with a number of amine groups present in the polymeric chain of aniline.
Conclusion
In summary, we have demonstrated for the first time polyaniline coated multiwalled carbon nanotubes nanocomposite as an excellent CO2 capture candidate under elevated pressure and temperatures. This nanocomposite shows higher CO2 capture capacity compared to other reported solid sorbents (like zeolites, activated carbon, carbon nanotubes, magnetite nanocomposite) under the same conditions. A simple preparation technique of this nanocomposite provides an edge over ionic liquids for use at a large scale. Regeneration capability of this nanocomposite and considerable sustainability of capture capacity even at high temperature (∼100 °C), make it a suitable CO2 capture candidate for commercial application such as for the storage of discharged CO2 from thermal power plants, steel and cement industries. Additionally, desorbed CO2 during the recovery of nanocomposite can be used in food, oil and chemical industries. This study promotes the investigation of other polymer-carbon composites for CO2 capture.Acknowledgements
The authors acknowledge the support of IIT Madras and the Office of Alumni Affairs, IIT Madras. One of the authors (Ashish) is thankful to DST, India for providing financial support. Authors are also thankful to SAIF, IIT Madras for helping with FTIR analysis.References
- S. Shimada, H. Y. Li, Y. Oshima and K. Adachi, Environ. Geol., 2005, 49, 44 CrossRef CAS.
- W. N. Sams, G. Bromhal, S. Jikich, T. Ertekin and D. H. Smith, Energy Fuels, 2005, 19, 2287 CrossRef CAS.
- DOE Report DOE/ER-30194, A research needs assessment for the capture, utilization and disposal of carbon dioxide from fossil fuel-fired power plants, 1993.
- DOE Report DOE/SC/FE-1, Carbon sequestration research and development, 1999.
- R. E. Baltus, R. M. Counce, B. H. Culbertson, H. Luo, D. W. DePaoli, S. Dai and D. C. Duckworth, Sep. Sci. Technol., 2005, 40, 525 CrossRef CAS.
- J. Huang and T. Ruther, Aust. J. Chem., 2009, 62, 298 CrossRef CAS.
- B. Gurkan, B. F. Goodrich, E. M. Mindrup, L. E. Ficke, M. Massel, S. Seo, T. P. Senftle, H. Wu, M. F. Glaser, J. K. Shah, E. J. Maginn, J. F. Brennecke and W. F. Schneider, J. Phys. Chem. Lett., 2010, 1, 3494 CrossRef CAS.
- Q. Wang, J. Luo, Z. Zhong and A. Borgna, Energy Environ. Sci., 2011, 4, 42 CAS.
- H. A. Zinnen, A. R. Oroskar, C. H. Chang, U.S. Patent 4,810,266, 1989 Search PubMed.
- P. J. Birbara, T. A. Nalette, U.S. Patent 5,492,683, 1996 Search PubMed.
- O. Leal, C. Bolivar, C. Ovalles, J. J. Garcia and Y. Espidel, Inorg. Chim. Acta, 1995, 240, 183 CrossRef CAS.
- M. M. Maroto-Valer, Z. Tang and Y. Z. Zhang, Fuel Process. Technol., 2005, 86, 1487 CrossRef CAS.
- M. Fr`ere, G. de Weireld and R. Jadot, J. Porous Mater., 1998, 5, 275 CrossRef.
- Z. Zhang, M. Xu, H. Wang and Z. Li, Chem. Eng. J., 2010, 160, 571 CrossRef CAS.
- M. Cinke, J. Li, C. W. Bauschlicher Jr., A. Ricca and M. Meyyappan, Chem. Phys. Lett., 2003, 376, 761 CrossRef CAS.
- C. S. Hsu, C. Lu, F. Su, W. Zeng and W. Chen, Chem. Eng. Sci., 2010, 65, 1354 CrossRef.
- S. K. Smart, A. I. Cassady, G. Q. Lu and D. J. Martin, Carbon, 2006, 44, 1034 CrossRef CAS.
- F. Su, C. Lu, W. Chen, H. Bai and J. F. Hwang, Sci. Total Environ., 2009, 407, 3017 CrossRef CAS.
- C. Lu, H. Bai, B. Wu, F. Su and J. F. Hwang, Energy Fuels, 2008, 22, 3050 CrossRef CAS.
- A. K. Mishra and S. Ramaprabhu, Energy Environ. Sci., 2011, 4, 889 CAS.
- M. M. Shaijumon and S. Ramaprabhu, Chem. Phys. Lett., 2003, 374, 513 CrossRef CAS.
- A. L. M. Reddy, F. E. Amitha, I. Jafri and S. Ramaprabhu, Nanoscale Res. Lett., 2008, 3, 145 CrossRef.
- U. J. Kim, C. A. Furtado, X. Liu, G. Chen and P. C. Eklund, J. Am. Chem. Soc., 2005, 127, 15437 CrossRef CAS.
- Z. Wang, J. Yuan, M. Li, D. Han, Y. Zhang, Y. Shen, L. Niu and A. Ivaska, J. Electroanal. Chem., 2007, 599, 121 CrossRef CAS.
- M. I. Boyer, S. Quillard, E. Rebourt, G. Louarn, J. P. Buisson, A. Monkman and S. Lefrant, J. Phys. Chem. B, 1998, 102, 7382 CrossRef CAS.
- C. Marjanovic, G. Dragicevic, L. Milojevic, M. Mojovic, M. Mentus, S. Dojcinovic, B. Marjanovic and B. Stejskal, J. Phys. Chem. B, 2009, 113, 7116 CrossRef.
- R. Srivastava, D. Srinivas and P. Ratnasamy, J. Catal., 2005, 233, 1 CrossRef CAS.
- W. M. Hlaing Oo, M. D. McCluskey, A. D. Lalonde and M. G. Norton, Appl. Phys. Lett., 2005, 86, 073111 CrossRef.
- A. R. Millward and O. M. Yaghi, J. Am. Chem. Soc., 2005, 127, 17998 CrossRef CAS.
- S. Cavenati, C. A. Grande and A. E. Rodrigues, J. Chem. Eng. Data, 2004, 49, 1095 CrossRef CAS.
- Y. Belmabkhout, R. S. Guerrero and A. Sayari, Chem. Eng. Sci., 2009, 64, 3721 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
