Plum-like and octahedral Co3O4 single crystals on and around carbon nanotubes: large scale synthesis and formation mechanism†
Yuanhua
Xiao
a,
Shaojun
Liu
a,
Shaoming
Fang
a,
Dianzeng
Jia
b,
Haiqiao
Su
c,
Weilie
Zhou
c,
John B.
Wiley
*c and
Feng
Li
*ab
aCollege of Materials and Chemical Engineering, State Laboratory of Surface and Interface Science and Technology, Zhengzhou University of Light Industry, Zhengzhou 450002, P. R. China. E-mail: lifeng696@yahoo.com; fengli@zzuli.edu.cn; Fax: +86-371-6355-6510; Tel: +86-371-6355-6510
bInstitute of Applied Chemistry, Xinjiang University, Urumqi 830046, P. R. China. Fax: +86-991-8588883; Tel: +86-991-8583083
cAdvanced Materials Research Institute, University of New Orleans, LA 70148, USA. E-mail: jwiley@uno.edu
First published on 1st March 2012
Abstract
An in situ growth strategy has been developed for the large scale production of novel nanoarchitectures consisting of single crystalline Co3O4 particles strung together by multi-walled carbon nanotubes (MWCNTs). Both Co3O4 plum-like and octahedra crystals have been grown on and around MWCNTs to form necklace-like structures. Solvents used in the reaction can dramatically affect the size and morphology of Co3O4 single crystals. Plum-like Co3O4 crystals are readily converted to octahedra, after changing the volume ratio of ethylene glycol (EG) to water. The size of Co3O4 single crystals also decreases on lowering the volume ratio of EG to water due to the enhanced polarity of the solvents. The MWCNTs are routinely oriented through the (400) planes of Co3O4 octahedra. This preferred growth orientation can be attributed to a lattice match between the MWCNTs and single crystalline Co3O4 particles.
Introduction
First synthesized in 1995 by Ajayan et al.,1 the nanoarchitectures constructed with carbon nanotubes (CNTs) and inorganic particles have been intensively investigated for applications in biochemical sensors,2,3 new catalysts,4 adsorbents,5 photo-electronic devices,6,7 supercapacitors,8–10 and batteries.11,12 Generally, the strategies for building the CNTs–inorganic nanoarchitectures can be categorized as either ex situ assembly or in situ growth.13 Based on covalent, noncovalent, or electrostatic interactions, inorganic building blocks can be attached onto the surfaces of CNTs via organic linkers with the ex situ assembly method.14–17 In contrast, the in situ techniques involve direct growth of inorganic building blocks onto the surfaces of CNTs; this approach allows for stable and close CNT contacts without the need for organic linkers.18 Nanoarchitectures composed of CNTs and diverse inorganic building blocks, such as single crystalline nanorods,19 polycrystalline nanospheres,7,20–27 and nanotubes,28–35 have already been fabricated with in situ growth techniques. Polycrystalline nanospheres of PtRu, Pt, Cu2O, MnO2, ZnO, Fe3O4, Co3O4 and MnFe2O4, for example, have been beaded along CNTs based on the technique.7,20–27 While inorganic nanotubes, including highly crystalline nanotubes of layered materials, can also be prepared with CNT templates,28–35 it is rare to find single crystalline nanotubes produced with this method.31 Single crystal ZnO nanorods can grow vertically onto the surfaces of CNTs.19Cobalt oxide, Co3O4 is an antiferromagnetic, p-type semiconductor with the spinel structure, AB2O4.36 Because the nanosized materials related to Co3O4 can be used as building blocks to construct functional systems,37–39 and the size and shape of nanocrystals are both crucial in tuning the properties of materials,40 the nanoarchitectures consisting of CNTs and nanosized Co3O4 building blocks have been especially attractive in recent years. Both in situ and ex situ techniques have been successfully utilized to construct nanoarchitectures of CNTs and Co3O4 nanoparticles for optimizing the properties of materials and designing devices with enhanced performances.10,11,14,25,29,41–52 Using ex situ assemblies, for instance, Li et al. reported a general procedure to attach metal and metal oxide nanoparticles, including Co3O4 nanoparticles, onto the surfaces of MWCNTs.14 Co3O4 nanotubes consisting of nanoparticles were also synthesized with an in situ procedure by employing MWCNTs as templates.29 Polycrystalline Co3O4 spheres beaded along CNTs were directly produced in supercritical solutions at low temperature for integrated microdevices.25 Recently, the composites of Co3O4 nanoparticles and 1D porous carbon with highly enhanced electrochemical performances were synthesized based on in situ growth for energy storage applications.10 To the best of our knowledge, however, there have so far been no reports in the literature involving the growth of necklace-like structures fabricated with CNTs and single crystalline Co3O4.
Herein we report a large-scale fabrication of novel nanoarchitectures consisting of multi-walled carbon nanotubes (MWCNTs) and Co3O4 particles based on in situ growth. Single crystalline Co3O4 plum-like particles and octahedra strung by MWCNTs have been successfully fabricated for the first time. It was found that the volume ratio of mixed solvents used in the reactions greatly influences the morphology and size of single crystalline Co3O4 particles where the shape varies from plum-like particles to octahedra, after changing the volume ratio of EG to water. Further, large scale production of the various necklace type structures is also possible. The orientation of the growth appears to be influenced by the surface of the CNT, giving some insight into the growth mechanism of these structures.
Experimental
Materials
All chemicals were purchased from Shanghai Analytical Chemicals Company and used as received. Carbon nanotubes were produced by Pyrograf Products, Inc.Growth of single crystalline plum-like Co3O4 particles strung with MWCNTs
In a typical synthesis, pristine MWCNTs (0.02 g) were placed in distilled water (30 ml) with hexadecyltrimethylammonium bromide (CTAB, 0.01 g). The black suspension was then sonicated for 30 min to disperse the MWCNTs into the water. Afterward, an ethylene glycol (EG) solution (10 ml) of Co(NO3)2·6H2O (5 mmol) was added to the MWCNT suspension drop-wise over a 20 min period with constant stirring. The mixture was sealed in a Teflon-lined stainless steel autoclave (50 ml) and kept at 160 °C for 20 h. After cooling to ambient temperature, the black products were collected by centrifugation, washed with ethanol and distilled water 5 times each and dried in vacuum at 60 °C for 12 h. Yield based on thermal analysis of the composites and obtained Co3O4: >90%.Growth of single crystalline Co3O4 octahedra around MWCNTs
Methodologies similar to those used in the growth of single crystalline Co3O4 plum-like particles were used to grow single crystalline Co3O4 octahedra along the pristine MWCNTs. The octahedral morphologies were produced by changing the volume ratio of EG to water from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 1
3 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and keeping the other reaction parameters the same. After cooling to ambient temperatures, black powders were isolated by centrifugation and washed with ethanol and distilled water for 5 times each. Yield: 85%.
5 and keeping the other reaction parameters the same. After cooling to ambient temperatures, black powders were isolated by centrifugation and washed with ethanol and distilled water for 5 times each. Yield: 85%.
Characterization
The microstructures of as-prepared samples were characterized by field emission scanning electron microscopy (FESEM, JEOL JSM-7001F) and transmission electron microscopy (TEM, JEOL JEM-2100 working at 200 kV). The composition of the materials was examined via X-ray diffraction (XRD) using a Rigaku D/max-2550 V diffractometer employing Cu-Kα radiation (λ= 1.54056 Å; scanning rate: 0.04° s−1 in the range of 10°–80° two theta).Results and discussion
The in situ growth of single crystalline Co3O4 plum-like particles strung together with MWCNTs was conducted in mixed solvent of EG and water with a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Fig. 1a–b show typical FESEM images of the necklace-like nanostructures formed with MWCNTs and Co3O4 particles along the full length of nanotubes. On average, the plum-like particles are about 1.1 μm in diameter (Fig. 1f) and the MWCNTs are ca. 170 nm in diameter and tens μm in length. The diameter of MWCNTs does not affect the formation of single crystalline particles of Co3O4. Particles typically have a rounded cube-like shape (Fig. 1c) and a grooved junction (Fig. 1d) on the surface of each sphere where, as the crystal growth faces intersect, they extend around the MWCNTs. The appearance of this combination of features is very reminiscent of plums. Interestingly, while most particles grow around a single MWCNT, in some cases multiple nanotubes can be clasped by a single particle (Fig. 1e); the two MWCNTs have diameters of about 200 nm and 100 nm, respectively, and are separated by a gap of about 30 nm. Further, one can see that the particle grows around the larger MWCNT and forms a solid junction between the two MWCNTs (as highlighted with arrow in Fig. 1e). Based on this in situ growth of single crystalline Co3O4 particles, it might be possible to create solid junctions between MWCNTs for incorporation into microdevices.
3. Fig. 1a–b show typical FESEM images of the necklace-like nanostructures formed with MWCNTs and Co3O4 particles along the full length of nanotubes. On average, the plum-like particles are about 1.1 μm in diameter (Fig. 1f) and the MWCNTs are ca. 170 nm in diameter and tens μm in length. The diameter of MWCNTs does not affect the formation of single crystalline particles of Co3O4. Particles typically have a rounded cube-like shape (Fig. 1c) and a grooved junction (Fig. 1d) on the surface of each sphere where, as the crystal growth faces intersect, they extend around the MWCNTs. The appearance of this combination of features is very reminiscent of plums. Interestingly, while most particles grow around a single MWCNT, in some cases multiple nanotubes can be clasped by a single particle (Fig. 1e); the two MWCNTs have diameters of about 200 nm and 100 nm, respectively, and are separated by a gap of about 30 nm. Further, one can see that the particle grows around the larger MWCNT and forms a solid junction between the two MWCNTs (as highlighted with arrow in Fig. 1e). Based on this in situ growth of single crystalline Co3O4 particles, it might be possible to create solid junctions between MWCNTs for incorporation into microdevices.
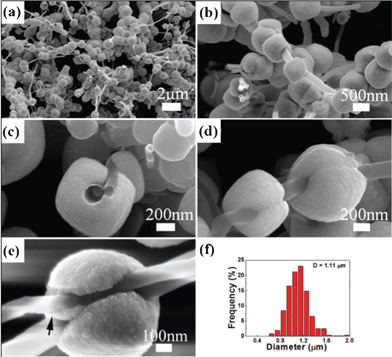 | ||
| Fig. 1 (a, b) Low and higher magnification FESEM image of nanoarchitectures constructed with MWCNTs and Co3O4 particles. (c) Top view of one cubic-like particle of Co3O4 with MWCNT drilling through it. (d) Side view of two cubic-like particles stringed with a MWCNT show a groove junction produced by the growth of particles at each of their surfaces. (e) High magnification FESEM image of one particle with opened groove holding two MWCNTs together. (f) Size distribution of plum-like particles. | ||
More structural information on the nanoarchitectures can be revealed by TEM observations as shown in Fig. 2. The two dimensional TEM image further highlights the rounded cubic-like shape of the particles. The MWCNTs pass through the particles along a direction just off the body diagonal of the crystal. The corresponding SAED pattern (Fig. 2b) of the one particle circled in white (Fig. 2a) consists of well ordered diffraction spots and can be indexed consistent with cubic Co3O4. We have carefully investigated the microstructure of the plum-like particles through taking SAED patterns of more than one hundred particles strung with MWCNTs and obtained similar, well ordered patterns. The results clearly indicate that all the Co3O4 particles strung with MWCNTs are single crystals. High resolution TEM (Fig. 2c), taken at the interface of Co3O4 particles and MWCNTs, exhibits lattices consistent with Co3O4 and MWCNTs, respectively. The lattice fringes with d-spacings of 0.34 nm correspond to the (002) plane of the MWCNT and those of 0.24 nm correspond to the (311) plane of Co3O4. The 0.28 nm lattice fringes, consistent with the (220) plane of cubic Co3O4, appear oriented relative to the MWCNT at an angle of about 60°. Overall, while there are steps and defects at the curved surface interface, HRTEM images of plum-like particles strung with MWCNTs exhibit single crystalline features expected for cubic Co3O4.
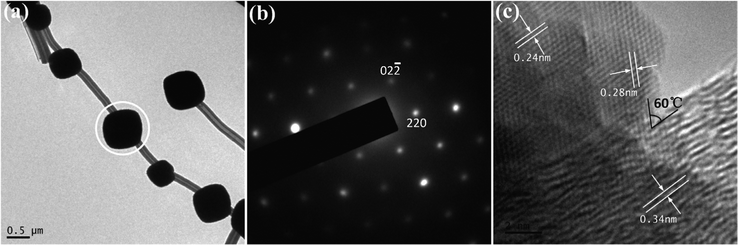 | ||
| Fig. 2 (a) Low magnification TEM image of nanoarchitectures constructed with MWCNTs and Co3O4 particles; (b) SAED pattern of one particle highlighted with white circle in (a); and (c) high resolution TEM image taken at the interface of the particle and MWCNT highlighted with white circle. | ||
The structure of nanoarchitectures consisting of MWCNTs and single crystalline Co3O4 plum-like particles was further studied by XRD as shown in Fig. 3a. The diffraction pattern shows that the composites are composed of cubic Co3O4 (JCPDS Card No. 42-1467) and MWCNTs (JCPDS Card No. 65-6212). The well-resolved diffraction peaks of cubic Co3O4 are consistent with the high crystallinity of the plum-like particles. Further, no impurities are detected with XRD.
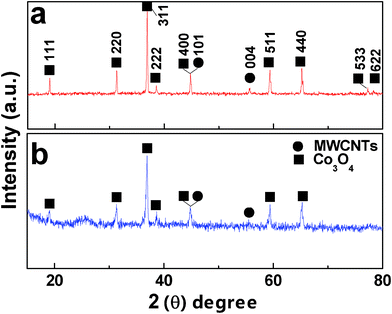 | ||
| Fig. 3 XRD patterns of nanoarchitectures constructed with Co3O4 plum-like particles (a) and octahedra (b) strung along MWCNTs. | ||
With the goal of better understanding particle formation in these systems, we carefully investigated the effect of the mixed solvent ratios on the growth of Co3O4 crystals. We found that on altering the volume ratio EG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water from 1
water from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 1
3 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, while maintaining the other reaction parameters, single crystalline Co3O4 octahedra were produced in large scale (Fig. 4a). FESEM images (Fig. 4b–d) reveal the production of Co3O4 octahedra with ca. 500 nm edges and long axes of ca. 800 nm. It is interesting that the MWCNTs are generally oriented through one of the two pyramidal halves of the Co3O4 octahedra and often run parallel to the direction of the octahedral diagonal through the (400) facet as shown in Fig. 4b. We can also find the MWCNT orientated along the [440] direction of the octahedron (center octahedron, Fig. 4c). In each octahedron, an indentation can be seen where the two fronts meet as the crystal growth encircles the MWCNT (Fig. 4d). Examination of a series of crystals shows that this junction can reach various stages of completion ranging from completely closed, to gaps at various stages of closure (Fig. 4). The growth of Co3O4 octahedra is very similar to that of plum-like particles, though the octahedra have much smoother surfaces and well-defined faces relative to those of the rounded rough surfaces of plum-like crystals.
5, while maintaining the other reaction parameters, single crystalline Co3O4 octahedra were produced in large scale (Fig. 4a). FESEM images (Fig. 4b–d) reveal the production of Co3O4 octahedra with ca. 500 nm edges and long axes of ca. 800 nm. It is interesting that the MWCNTs are generally oriented through one of the two pyramidal halves of the Co3O4 octahedra and often run parallel to the direction of the octahedral diagonal through the (400) facet as shown in Fig. 4b. We can also find the MWCNT orientated along the [440] direction of the octahedron (center octahedron, Fig. 4c). In each octahedron, an indentation can be seen where the two fronts meet as the crystal growth encircles the MWCNT (Fig. 4d). Examination of a series of crystals shows that this junction can reach various stages of completion ranging from completely closed, to gaps at various stages of closure (Fig. 4). The growth of Co3O4 octahedra is very similar to that of plum-like particles, though the octahedra have much smoother surfaces and well-defined faces relative to those of the rounded rough surfaces of plum-like crystals.
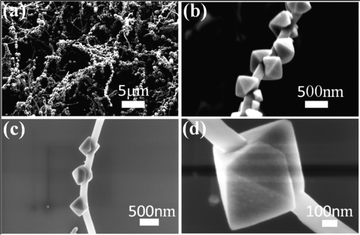 | ||
| Fig. 4 FESEM images of nanoarchitectures composed of MWCNTs and Co3O4 octahedra. (a) Low magnification image, (b) one MWCNT covered with Co3O4 octahedra, (c) three Co3O4 octahedra with opened gap toward MWCNT and (d) one Co3O4 octahedron with groove junction strung with MWCNT. | ||
The TEM image in Fig. 5a shows octahedral particles with edges of ca. 500 nm that have been strung together with MWCNTs. The relative orientation of the MWCNT is again close to the [400] direction of Co3O4. The results imply that the relative orientation of the Co3O4 octahedra is similar to that of plum-like particles. The SAED pattern (Fig. 5b) of one octahedron (Fig. 5a, white circle) shows a single-crystal spot pattern that can be indexed as cubic Co3O4.
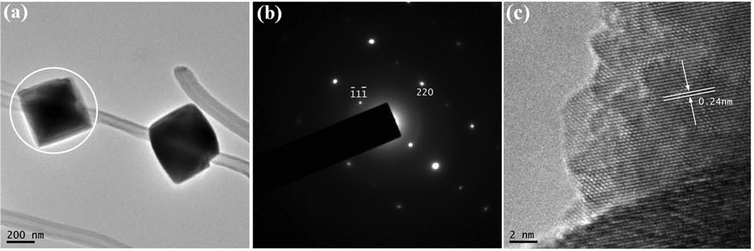 | ||
| Fig. 5 TEM image of nanoarchitectures consisting of Co3O4 octahedra and MWCNTs and (b) SAED pattern of one Co3O4 octahedron highlighted in (a) with white circle. (c) High resolution TEM image taken at the interface of the particle and MWCNT. | ||
To further investigate solvent effects on the growth of single crystalline Co3O4 particles on MWCNTs, a series of experiments were conducted in pure water and mixed solvents. Crystals were grown with volume ratios of EG to water of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1
4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 1
6, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 and 1
8 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, respectively. Fig. 6 shows the structure evolution of nanoarchitectures fabricated with increasing amounts of water. With high EG concentrations (Fig. 6a, EG
10, respectively. Fig. 6 shows the structure evolution of nanoarchitectures fabricated with increasing amounts of water. With high EG concentrations (Fig. 6a, EG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ≥ 1
H2O ≥ 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), it is rare to find nanoparticles on the surfaces of MWCNTs. Co3O4 particles with plum-like structures (Fig. 6b), however, can be grown on the surface of MWCNTs when the volume ratio is set at 1
1), it is rare to find nanoparticles on the surfaces of MWCNTs. Co3O4 particles with plum-like structures (Fig. 6b), however, can be grown on the surface of MWCNTs when the volume ratio is set at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Further lowering the volume ratio to 1
3. Further lowering the volume ratio to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, some octahedra can be found along with many plum-like particles (Fig. 6c). In the ratio of 1
4, some octahedra can be found along with many plum-like particles (Fig. 6c). In the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, octahedra dominate the mixture (Fig. 6d). On further lowering the volume ratio to 1
5, octahedra dominate the mixture (Fig. 6d). On further lowering the volume ratio to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 1
6, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 and 1
8 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, the size of the octahedra decreases as shown in Fig. 6e–g. If one uses only pure water as solvent in the reaction, it is rare to find any crystals in the product. The experimental results reveal that the growth rate of Co3O4 particles on the surfaces of MWCNTs can be slowed down by increasing the amount of water used in the reaction (Fig. S3†). Through simply adjusting the volume ratio of solvent used in the reaction and thus tuning the growth rate, we can manipulate both the shape and size of single crystalline Co3O4 particles strung with MWCNTs.
10, the size of the octahedra decreases as shown in Fig. 6e–g. If one uses only pure water as solvent in the reaction, it is rare to find any crystals in the product. The experimental results reveal that the growth rate of Co3O4 particles on the surfaces of MWCNTs can be slowed down by increasing the amount of water used in the reaction (Fig. S3†). Through simply adjusting the volume ratio of solvent used in the reaction and thus tuning the growth rate, we can manipulate both the shape and size of single crystalline Co3O4 particles strung with MWCNTs.
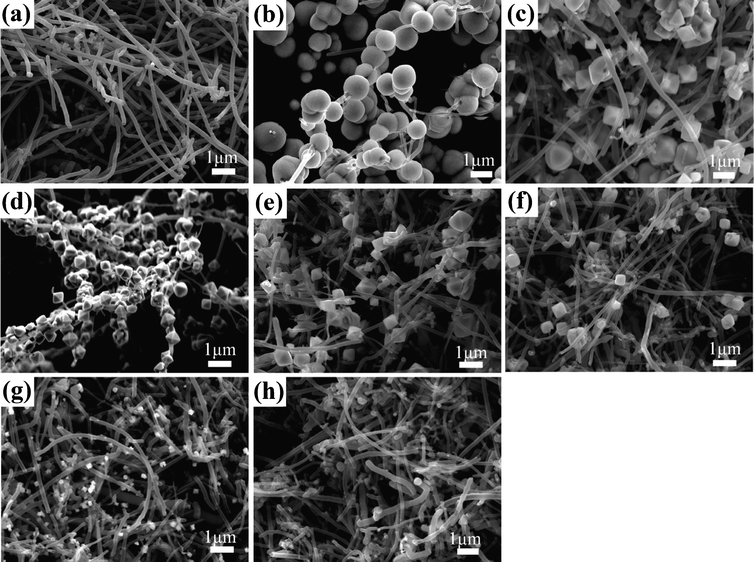 | ||
Fig. 6 FESEM images of the products synthesized in mixed EG and water solvents at volume ratio of (a) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (b)1 1, (b)1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, (c) 1 3, (c) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, (d) 1 4, (d) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, (e) 1 5, (e) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, (f) 1 6, (f) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, (g) 1 8, (g) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, and (h) pure water. 10, and (h) pure water. | ||
Since all of the reaction parameters were kept the same, except the solvent volume ratio, synthetic results indicate that the polarity of the mixed solvent has a direct effect on the growth of Co3O4 single crystals on MWCNTs. Dielectric constants (k) can often serve as a rough estimate of solvent polarity. Water, with a dielectric constant of about 80, exhibits quite a strong polarity. Solvents, such as EG, with a dielectric constant less than 15 are considered non-polar. Dielectric constants at 160 °C of mixed solvents of EG and water were calculated, based on published methods.53Fig. 7 shows the k curve of the mixed solvents and the size/shape change curve of the Co3O4 particles corresponding to the volume ratio of EG and water. The shape of the particles converts from plum-like to octahedral after increasing the dielectric constant to greater than 40. We can thus obtain the k threshold of 40 for controlling the shape of particles produced in the mixed solvent at 160 °C. The size of the particles declines on increasing the polarity of the solvent, and it changes dramatically after the polarity of the solvent increases to higher than 43 with the increased amount of water used in the reaction. Much smaller octahedral particles can be produced in solvent with a dielectric constant of greater than 43.
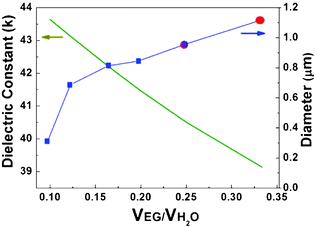 | ||
| Fig. 7 The evolution of dielectric constants (k, green line) and mean diameter (D, blue line) of Co3O4 particles corresponding to the changes in volume ratio of EG to water. The blue cubes indicate the octahedron shape of particles, red circles show the plum-like shape of the particles grown on and around the CNTs. | ||
In a control experiment, we examined the volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, but without MWCNTs in the reaction mixture and found that this produced no Co3O4 crystals at all. The results indicate that MWCNTs also play an important role in supplying nucleation sites for crystal growth. We also investigated the influence of CTAB on the growth of Co3O4 particles through conducting the reaction at a volume ratio of 1
3, but without MWCNTs in the reaction mixture and found that this produced no Co3O4 crystals at all. The results indicate that MWCNTs also play an important role in supplying nucleation sites for crystal growth. We also investigated the influence of CTAB on the growth of Co3O4 particles through conducting the reaction at a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 without adding CTAB into the reaction, and found there is no significant impact on the growth of particles (Fig. S2†).
3 without adding CTAB into the reaction, and found there is no significant impact on the growth of particles (Fig. S2†).
The size and shape of the final products in solid-state reactions generally depend on the rate of nucleation and growth as well as the surface modification.54 After the formation of nuclei on the surface of MWCNTs, the fast growth of Co3O4 particles takes place in the mixed solvents of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and thus produces plum-like particles strung along the surfaces of MWCNTs as shown in Scheme 1(1)–(3). It was found that the size of the crystals decreases with increasing the polarity of solvents by using more water as shown in Fig. 7. Single crystalline Co3O4 octahedra encased in 8 (111) facets can thus be produced in mixed solvents (1
3 and thus produces plum-like particles strung along the surfaces of MWCNTs as shown in Scheme 1(1)–(3). It was found that the size of the crystals decreases with increasing the polarity of solvents by using more water as shown in Fig. 7. Single crystalline Co3O4 octahedra encased in 8 (111) facets can thus be produced in mixed solvents (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) of higher polarity as shown in Scheme 1(4)–(5). Co3O4 nanocrystals with much smaller size can be deposited onto the surfaces of MWCNTs after decreasing the volume ratio of EG to water to 1
5) of higher polarity as shown in Scheme 1(4)–(5). Co3O4 nanocrystals with much smaller size can be deposited onto the surfaces of MWCNTs after decreasing the volume ratio of EG to water to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (Fig. 6g) for further increasing the polarity of the mixed solvents. Through simply tuning the polarity of mixed solvents used in the reaction, mixed solvents can affect both the size and morphology of nanocrystals stringed with MWCNTs.
10 (Fig. 6g) for further increasing the polarity of the mixed solvents. Through simply tuning the polarity of mixed solvents used in the reaction, mixed solvents can affect both the size and morphology of nanocrystals stringed with MWCNTs.
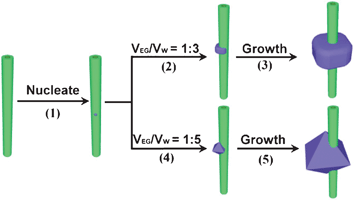 | ||
Scheme 1 The formation of single crystalline Co3O4 particles strung with MWCNTs. Co3O4 particles first nucleate on the surface of MWCNTs (1). They subsequently grow around the MWCNTs (2) in a mixed solvent of EG and water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), and finally form single crystalline plum-like particles around the MWCNTs (3). The growth of Co3O4 particles (4) in a mixed solvent of EG and water (1 3), and finally form single crystalline plum-like particles around the MWCNTs (3). The growth of Co3O4 particles (4) in a mixed solvent of EG and water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) yields octahedral single crystals around MWCNTs (5). 5) yields octahedral single crystals around MWCNTs (5). | ||
It is very interesting to find that the single crystalline Co3O4 particles can grow on and around MWCNTs. As the crystals grow, instead of only growing perpendicular to the MWCNT surface, they progressively grow so as to encircle the tube. This favorable growth around the nanotube indicates that surface interactions of the two components match very well. The XRD profile (Fig. 3) reveals that the (400) diffraction plane of Co3O4 overlaps with the (101) diffraction of MWCNTs. The SEM images as shown in Fig. 4 further verify that MWCNTs are perpendicular to the (400) plane and parallel to the diagonal of most Co3O4 octahedra. It is possible that the growth of Co3O4 single crystals on MWCNTs is favorable due to a good lattice matching between (400) and (101) facets, respectively.
Conclusions
Novel nanoarchitectures consisting of MWCNTs and Co3O4 particles with controlled structures have been produced in large scale. Single crystalline Co3O4 plum-like particles and octahedra have been grown on and around MWCNTs, to form necklace-like structures. It was found that the volume ratio of the mixed solvents used in the reaction dramatically affected the morphology and size of the single crystalline Co3O4 particles. Octahedral crystals are favored over plum-like ones when the volume ratio of EG to water is increased from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 1
3 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. This approach should be readily applied to a variety of other metal oxide materials. Nanoarchitectures consisting of MWCNTs and oxide single crystals, especially those with the spinel structure, could be fabricated with the in situ growth technique by carefully adjusting solvent polarity and considering inorganic materials with lattice parameters close to that of MWCNTs. It was reported that MWCNTs with ferromagnetic contacts exhibit interesting tunable transport properties.55 Compared to the antiferromagnetic properties of bulk Co3O4 materials, our primary investigation reveals that the single crystalline Co3O4 particles strung with MWCNTs show ferromagnetic properties. This might allow for creating active junctions with magnetic materials and CNTs, and thus for fabricating spin-electronic microdevices.
5. This approach should be readily applied to a variety of other metal oxide materials. Nanoarchitectures consisting of MWCNTs and oxide single crystals, especially those with the spinel structure, could be fabricated with the in situ growth technique by carefully adjusting solvent polarity and considering inorganic materials with lattice parameters close to that of MWCNTs. It was reported that MWCNTs with ferromagnetic contacts exhibit interesting tunable transport properties.55 Compared to the antiferromagnetic properties of bulk Co3O4 materials, our primary investigation reveals that the single crystalline Co3O4 particles strung with MWCNTs show ferromagnetic properties. This might allow for creating active junctions with magnetic materials and CNTs, and thus for fabricating spin-electronic microdevices.
Acknowledgements
We acknowledge the National Natural Science Foundation of P. R. China (NSFC. 21071130) and Outstanding Scholar Program of Henan Province (114200510012), P. R. China.References
- P. M. Ajayan, O. Stephan, P. Redlich and C. Colliex, Nature, 1995, 375, 564 CrossRef CAS.
- N. Du, H. Zhang, B. D. Chen, X. Y. Ma, Z. H. Liu, J. B. Wu and D. R. Yang, Adv. Mater., 2007, 19, 1641 CrossRef CAS.
- F. F. Zhang, X. L. Wang, C. X. Li, X. H. Li, Q. Wan, Y. Z. Xian, L. T. Jin and K. Yamamoto, Anal. Bioanal. Chem., 2005, 382, 1368 CrossRef CAS.
- W. Chen, X. L. Pan and X. H. Bao, J. Am. Chem. Soc., 2007, 129, 7421 CrossRef CAS.
- X. J. Peng, Z. K. Luan, Z. C. Di, Z. G. Zhang and C. L. Zhu, Carbon, 2005, 43, 880 CrossRef CAS.
- H. E. Unalan, P. Hiralal, D. Kuo, B. Parekh, G. Amaratunga and M. Chhowalla, J. Mater. Chem., 2008, 18, 5909 RSC.
- Y. W. Zhu, H. I. Elim, Y. L. Foo, T. Yu, Y. J. Liu, W. Ji, J. Y. Lee, Z. X. Shen, A. T. S. Wee, J. T. L. Thong and C. H. Sow, Adv. Mater., 2006, 18, 587 CrossRef CAS.
- H. Zhang, G. P. Cao, Z. Y. Wang, Y. S. Yang, Z. J. Shi and Z. N. Gu, Nano Lett., 2008, 8, 2664 CrossRef CAS.
- J. S. Ye, H. F. Cui, X. Liu, T. M. Lim, W. D. Zhang and F. S. Sheu, Small, 2005, 1, 560 CrossRef CAS.
- Y. Y. Liang, M. G. Schwab, L. J. Zhi, E. Mugnaioli, U. Kolb, X. L. Feng and K. Mullen, J. Am. Chem. Soc., 2010, 132, 15030 CrossRef CAS.
- W. L. Yao, J. L. Wang, J. Yang and G. D. Du, J. Power Sources, 2008, 176, 369 CrossRef CAS.
- F. Cheng, Z. Tao, J. Liang and J. Chen, Chem. Mater., 2008, 20, 667 CrossRef CAS.
- D. Eder, Chem. Rev., 2010, 110, 1348 CrossRef CAS.
- J. Li, S. B. Tang, L. Lu and H. C. Zeng, J. Am. Chem. Soc., 2007, 129, 9401 CrossRef CAS.
- M. A. Correa-Duarte, J. Perez-Juste, A. Sanchez-Iglesias, M. Giersig and L. M. Liz-Marzan, Angew. Chem., Int. Ed., 2005, 44, 4375 CrossRef CAS.
- J. Sun, L. Gao and M. Iwasa, Chem. Commun., 2004, 832 RSC.
- B. Liu and J. Y. Lee, J. Phys. Chem. B, 2005, 109, 23783 CrossRef CAS.
- X. H. Peng, J. Y. Chen, J. A. Misewich and S. S. Wong, Chem. Soc. Rev., 2009, 38, 1076 RSC.
- J. G. Ok, S. H. Tawfick, K. A. Juggernauth, K. Sun, Y. Y. Zhang and A. J. Hart, Adv. Funct. Mater., 2010, 20, 2470 CrossRef CAS.
- Q. Zhang, M. F. Zhu, Q. H. Zhang, Y. G. Li and H. Z. Wang, Mater. Chem. Phys., 2009, 116, 658 CrossRef CAS.
- D. J. Guo, L. Zhao, X. P. Qiu, L. Q. Chen and W. T. Zhu, J. Power Sources, 2008, 177, 334 CrossRef CAS.
- S. K. Kim, J. Zhang, M. Lee, H. Y. Choi and H. Lee, Curr. Appl. Phys., 2007, 7, 230 CrossRef.
- B. P. Jia, L. Gao and J. Sun, Carbon, 2007, 45, 1476 CrossRef CAS.
- Y. S. Luo, Q. F. Ren, J. L. Li, Z. J. Jia, Q. R. Dai, Y. Zhang and B. H. Yu, Nanotechnology, 2006, 17, 5836 CrossRef CAS.
- L. Fu, Z. M. Liu, Y. Q. Liu, B. X. Han, P. G. Hu, L. C. Cao and D. B. Zhu, Adv. Mater., 2005, 17, 217 CrossRef CAS.
- Z. L. Liu, B. Zhao, C. L. Guo, Y. J. Sun, Y. Shi, H. B. Yang and Z. A. Li, J. Colloid Interface Sci., 2010, 351, 233 CrossRef CAS.
- Y. Hou, Y. W. Cheng, T. Hobson and J. Liu, Nano Lett., 2010, 10, 2727 CrossRef CAS.
- Y. Qin, Y. Kim, L. B. Zhang, S. M. Lee, R. B. Yang, A. L. Pan, K. Mathwig, M. Alexe, U. Gosele and M. Knez, Small, 2010, 6, 910 CrossRef CAS.
- N. Du, H. Zhang, B. Chen, J. B. Wu, X. Y. Ma, Z. H. Liu, Y. Q. Zhang, D. Yang, X. H. Huang and J. P. Tu, Adv. Mater., 2007, 19, 4505 CrossRef CAS.
- H. Ogihara, M. Sadakane, Y. Nodasaka and W. Ueda, Chem. Mater., 2006, 18, 4981 CrossRef CAS.
- (a) M. Nath and C. Rao, Angew. Chem., Int. Ed., 2002, 41, 3451 CrossRef CAS; (b) M. Nath and C. Rao, J. Am. Chem. Soc., 2001, 123, 4841 CrossRef CAS; (c) C. Rao and M. Nath, Dalton Trans., 2003, 1 RSC; (d) M. Nath, A. Govindaraj and C. Rao, Adv. Mater., 2001, 13, 383 CrossRef.
- Z. Y. Sun, H. Q. Yuan, Z. M. Liu, B. X. Han and X. R. Zhang, Adv. Mater., 2005, 17, 2993 CrossRef CAS.
- L. W. Yin, Y. Bando, Y. C. Zhu, M. S. Li, C. C. Tang and D. Golberg, Adv. Mater., 2005, 17, 213 CrossRef CAS.
- Y. S. Min, E. J. Bae, K. S. Jeong, Y. J. Cho, J. H. Lee, W. B. Choi and G. S. Park, Adv. Mater., 2003, 15, 1019 CrossRef CAS.
- L. Fu, Z. M. Liu, Y. Q. Liu, B. X. Han, J. Q. Wang, P. G. Hu, L. C. Cao and D. B. Zhu, Adv. Mater., 2004, 16, 350 CrossRef CAS.
- J. H. Yang and T. Sasaki, Cryst. Growth Des., 2010, 10, 1233 CAS.
- X. W. Xie, Y. Li, Z. Q. Liu, M. Haruta and W. J. Shen, Nature, 2009, 458, 746 CrossRef CAS.
- B. Varghese, T. C. Hoong, Z. Yanwu, M. V. Reddy, B. V. R. Chowdari, A. T. S. Wee, T. B. C. Vincent, C. T. Lim and C. H. Sow, Adv. Funct. Mater., 2007, 17, 1932 CrossRef CAS.
- T. Zhu, J. S. Chen and X. W. Lou, J. Mater. Chem., 2010, 20, 7015 RSC.
- W. E. Buhro and V. L. Colvin, Nat. Mater., 2003, 2, 138 CrossRef CAS.
- S. Buratti, B. Brunetti and S. Mannino, Talanta, 2008, 76, 454 CrossRef CAS.
- W. Li, H. Jung, D. H. Nguyen, D. Kim, S. K. Hong and H. Kim, Sens. Actuators, B, 2010, 150, 160 CrossRef.
- G. X. Wang, X. P. Shen, J. N. Yao, D. Wexler and J. Ahn, Electrochem. Commun., 2009, 11, 546 CrossRef CAS.
- J. X. Liu, Z. S. Wang, W. Jiang, Y. Yang and F. S. Li, Rare Metal Mater. Eng., 2007, 36, 649 CAS.
- F. Lupo, R. Kamalakaran and A. Gulino, J. Phys. Chem. C, 2009, 113, 15533 CAS.
- Y. Wang, H. J. Zhang, L. Lu, L. P. Stubbs, C. C. Wong and J. Y. Lin, ACS Nano, 2010, 4, 4753 CrossRef CAS.
- L. J. Zhi, Y. S. Hu, B. El Hamaoui, X. Wang, I. Lieberwirth, U. Kolb, J. Maier and K. Mullen, Adv. Mater., 2008, 20, 1727 CrossRef CAS.
- J. Cheng, G. P. Cao and Y. S. Yang, Chem. J. Chin. Univ. Chin., 2007, 28, 2138 CAS.
- Y. Shan and L. Gao, Chem. Lett., 2004, 33, 1560 CrossRef CAS.
- W. L. Yao, J. Yang, J. L. Wang and L. A. Tao, Electrochim. Acta, 2008, 53, 7326 CrossRef CAS.
- Y. Shan and L. Gao, Mater. Chem. Phys., 2007, 103, 206 CrossRef CAS.
- S. K. Gill, A. M. Shobe and L. J. Hope-Weeks, Scanning, 2009, 31, 132 CrossRef CAS.
- A. Jouyban and S. Soltanpour, J. Chem. Eng. Data, 2010, 55, 2951 CrossRef CAS.
- F. Li, Y. Ding, P. X. Gao, X. Q. Xin and Z. L. Wang, Angew. Chem., Int. Ed., 2004, 43, 5238 CrossRef CAS.
- K. Tsukagoshi, B. W. Alphenaar and H. Ago, Nature, 1999, 401, 572 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: XRD pattern of MWCNTs, FESEM image of Co3O4 particles grown without adding CTAB, the size distribution profiles of Co3O4 particles. See DOI: 10.1039/c2ra00880g |
| This journal is © The Royal Society of Chemistry 2012 |
