Efficient, rapid synthesis of bis(indolyl)methane using ethyl ammonium nitrate as an ionic liquid
Shafeek A. R.
Mulla
*,
A.
Sudalai
,
Mohsinkhan Y.
Pathan
,
Shafi A.
Siddique
,
Suleman M.
Inamdar
,
Santosh S.
Chavan
and
R. Santosh
Reddy
Chemical Engineering & Process Development Division, National Chemical Laboratory, Dr Homi Bhabha Road, Pune-411008, Maharashtra, India. E-mail: sa.mulla@ncl.res.in; Fax: +91-20-25902676; Tel: +91-20-25902316
First published on 2nd March 2012
Abstract
A simple and rapid protocol has been developed for an efficient synthesis of bis(indolyl)methane in excellent yields using ethyl ammonium nitrate (EAN) as reusable ionic liquid at room temperature. The protocol involves an electrophilic substitution reaction of indoles with several aldehydes.
Introduction
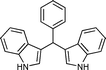
Several bis(indolyl)alkanes and their derivatives have been isolated from plant and marine sources.1 Among the various derivatives of indoles, bis(indolyl)methanes have wide medicinal applications such as to induce apoptosis in human cancer cells and normalize abnormal cell growth associated with cervical dysplasia,2 to promote beneficial estrogen metabolism in both women and men, to prevent breast cancer3 and also to increase the natural metabolism of the body's hormones.4 Due to the vast biological activity of bis(indolyl)methanes and their wide medicinal applications, various methods of their synthesis have been reported in the literature. However, almost all the methods have employed conventional Lewis acids as well as protic acids as catalysts to promote electrophilic substitution reaction of indoles with various aldehydes or carbonyl compounds.5 A variety of other catalysts such as H-Y zeolite,6 sulphamic acid,7 In(OTf)3,8 LiClO4,9 bis(cyclopentadienyl)ZrCl2,10 CuBr2,11 ZrCl4,12 Zn(HSO4)2,13 polyindole salt,14 CAN,15N-tert-butanesulfinyl aldimines,16 ion exchange resin,17 acetic acid,18 InCl3,19 InF3,20 Dy(OTf)3,21 Ln(OTf)3,22 FeCl3·6H2O,23 V(HSO4)3,24 SBA-15/SO3H,25 oxalic acid,26 TBBDA,27 silica bonded S-sulfonic acid,28 Bi(NO3)3,29 Cu(BF4)2.SiO2,30 vanadomolybdophosphoric acid,31 Ph–PMO–SO3H,32 glycerin and CeCl3,33 B(C6F5)3,34 H6P2W18O62,35 phosphated zirconia,36 Ph3CCl,37 have been reported for the synthesis of bis(indolyl)methanes. However, these methods suffer from drawbacks, such as toxic metal ions, expensive solvents, long reaction times, tedious work-up, low product yields, higher catalyst loading and formation of large amounts of wastes.38–40Recently, use of ionic liquids (IL) has attracted an increasing interest in the area of organic synthesis due to their unique properties such as their hydrophobicity and/or hydrophilicity as well as their good solvating capability, easy recoverability, reusability and non-flammability with almost no vapour pressure. Significant progress has been made in the application of ionic liquids in catalytic processes for the synthesis of heterocyclic compounds.41–44 Also the ionic liquid catalyzed synthesis of bis(indolyl)methane using [bmim]BF4,45 [bmim]PF6,45 [acmim]Cl, [hmim]HSO446 and TMGT,47 at room temperature have been reported so far in the literature. However, these methods suffer from certain disadvantages such as very long reaction times and lower product yields. Hence, there has been considerable interest in the development of an elegant, rapid and efficient method for the synthesis of bis(indolyl)methanes using ionic liquids as a recyclable, eco-friendly reaction medium. Ethyl ammonium nitrate (EAN) is an ionic liquid with acidic properties (pH = 5),48 cheap and easily recoverable, reusable at room temperature, when compared to other ionic liquids. Recently, EAN has been used in various organic reactions such as Kabachnik–Field reaction, Knoevenagel condensation, nitration of phenol and also in the synthesis of 2-amino-4,6-diphenylpyridine-3-carbonitrile and flavones.49 Herein, we report the use of ethyl ammonium nitrate [C2H5NH3]NO3 as a media as well as catalyst in the electrophilic substitution of indoles with a variety of aldehydes to provide bis(indolyl)methanes at ambient conditions (Scheme 1).
 | ||
| Scheme 1 ethyl ammonium nitrate (EAN) mediated synthesis of bis(indolyl)methane. | ||
Results and discussion
Using indole and benzaldehyde as test substrates, the reaction parameters were optimized to determine the optimal condition for the synthesis of bis(indolyl)methanes (Table 1). When Indole (2 mmol) was treated with benzaldehyde (1 mmol) for 5 h in ethyl ammonium nitrate (EAN) (0.2 mmol) as an ionic liquid without solvent at 25 °C, the corresponding bis(indolyl)methane (3a) was obtained in 30% yield. When we carried out the same reaction in absence of EAN no reaction took place even at a higher temperature (85 °C); however the yield and reaction time could be significantly improved to 60% yield in 1.5 h when 0.8 mmol of EAN was used. Interestingly, increasing the molar ratio of EAN (2 mmol) resulted in a dramatic improvement in the yield of 3a (95%) in 3 min. In general, a higher EAN concentration (2 mmol) gave better yields in a shorter time. These results clearly indicate that the EAN acts as a catalyst as well as a green reaction media. To gauge the scope of this methodology, a variety of substituted aldehydes (2a–q) were reacted with indole 1 in the presence of 2 mmol of EAN at 25 °C to produce the corresponding bis(indolyl)methanes (3a–q, Table 2). The nature of substitution on the aromatic ring showed some effect on product yields and reactions times. Aromatic aldehydes with electron-withdrawing groups at o- and p- positions, provided products in excellent yields with shorter reactions time (Table 2, entry d, g & i), the reaction is extremely fast often completing in < 3 min. However, in the case of 3-chlorobenzaldehyde, a low yield of the corresponding product was obtained in longer reaction time (Table 2, entry f). Substrates with electron-donating groups took a longer time with moderate yields (Table 2, entry b, c & e). In the case of 3,4,5-trimethylbenzaldehyde, the rate of reaction is found to be very slow, due to the presence of three electron donating methyl groups (Table 2, entry e). Also, a similar effect is observed in the case of 5-methoxy indole with 4-methyl and 4-methoxy benzaldehyde (Table 2, entry p and q). This clearly indicates that the position and nature of the substitutions on the aromatic ring play a key role in the rate of reaction. Heterocyclic aldehydes reacted smoothly with the indole to give the corresponding products in excellent yield (Table 2, entry h & j). Furthermore aliphatic aldehydes also react smoothly with the indole to give the corresponding products in good yields (Table 2, entry k and l).| Entry | ethyl ammonium nitrate (mmol) | Reaction time (min) | Yield (%)b |
|---|---|---|---|
| a Reaction conditions: indole (2 mmol), benzaldehyde (1 mmol), ethyl ammonium nitrate at 25 °C. b Isolated yields after chromatographic purification. | |||
| 1 | 0 | 300 | 0 |
| 2 | 0.2 | 300 | 30 |
| 3 | 0.4 | 300 | 45 |
| 4 | 0.6 | 210 | 50 |
| 5 | 0.8 | 90 | 60 |
| 6 | 1.0 | 10 | 90 |
| 7 | 2.0 | 03 | 95 |
To further elaborate the scope of this protocol with substituted indole, a reaction of 5-nitro indole and 5-methoxy indole was carried out with different aromatics as well as aliphatic aldehyde, which provided the corresponding bis(indolyl)methanes in good to moderate yields (Table 2, entries m, n, o, p and q).
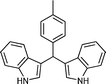
| Entry | Indole (1) | Aldehyde (2) | Product (3) | Time (min) | %Yield b[ref] |
|---|---|---|---|---|---|
| a Reaction conditions: indole (2 mmol), aldehyde (1 mmol), ethyl ammonium nitrate (2 mmol) at 25 °C. b Isolated yields after chromatographic purification. | |||||
| a |
|
|
|
03 | 9311 |
| b |
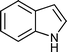
|
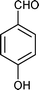
|
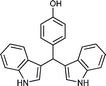
|
05 | 9211 |
| c |
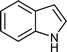
|

|
|
08 | 9111 |
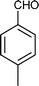
| d |
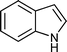
|
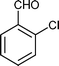
|
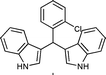
|
03 | 9211 |
| e |
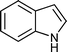
|
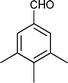
|
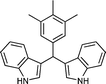
|
10 | 904 |
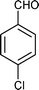
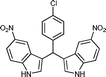
| f |
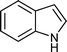
|
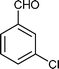
|
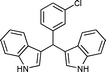
|
10 | 884 |
| g |
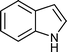
|
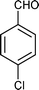
|
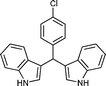
|
01 | 9511 |
| h |
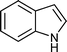
|
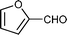
|
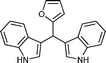
|
03 | 9211 |
| i |
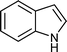
|
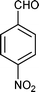
|
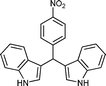
|
01 | 9511 |
| j |
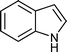
|
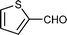
|
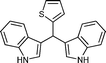
|
04 | 938 |
| k |
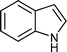
|
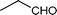
|
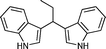
|
02 | 938 |
| l |
|
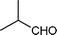
|
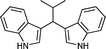
|
02 | 895 |
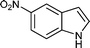
| m |
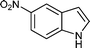
|
|
|
08 | 8714 |
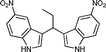
| n |
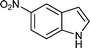
|
|
|
08 | 8612 |
| o |
|
|
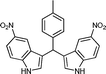
|
05 | 946 |
| p |
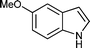
|
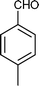
|
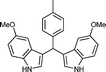
|
11 | 87 |
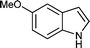
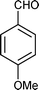
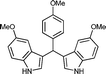
| q |
|
|
|
12 | 83 |
A probable mechanism is shown in Scheme 2 for the synthesis of bis(indolyl)methanes. The mechanism involves activation of the carbonyl group by EAN followed by nucleophilic addition of the indole to the aldehyde to afford the intermediate A. The subsequent dehydration of A gave B which on nucleophilic addition of another mole of indole gave C, followed by aromatization to give the product 3a–q (Scheme 2).
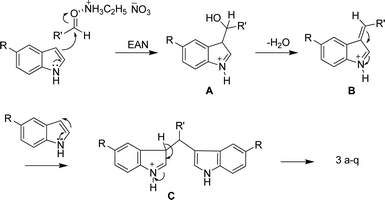 | ||
| Scheme 2 Plausible reaction mechanism for synthesis of bis(indolyl)methane over EAN. | ||
Recyclability of ethyl ammonium nitrate
The EAN was recovered from the aqueous layer by removal of water at 70 °C under reduced pressure and recycled several times with almost no loss of activity (Table 3, Run1–4).| No. of cycles | Fresh | Run 1 | Run 2 | Run 3 | Run 4 |
|---|---|---|---|---|---|
| a Reaction conditions: indole (2 mmol), benzaldehyde (1 mmol), ethyl ammonium nitrate (2 mmol) at 25 °C. b Isolated yields after chromatographic purification. | |||||
| Yield (%)b | 95 | 95 | 94 | 94 | 93 |
| EAN recovery (%) | >98 | >97 | >96 | >96 | >95 |
The efficacy of EAN for the synthesis of bis(indolyl)methanes as compared to other acid catalysts and ionic liquids (ILs) reported in the literature can be understood from the results shown in Table 4. The high rate can be rationalized due to high acidity associated with it (pH = 5) coupled with its ability to absorb water formed during the reaction in the synthesis of bis(indolyl)methanes.
| Sr. No. | Reaction conditions | Time | Catalyst | Yield (%)[Reff.] |
|---|---|---|---|---|
| 1 | EAN, rt | 3 min | EAN | 93 |
| 2 | Ionic liquid , 1 ml , rt | 15 min | TMGT | 9347 |
| 3 | Microwave oven, 450 W | 5 min | [bmim] [HSO4] | 9346 |
| 4 | Acetonitrile 3 ml, rt | 5.5 h | Acidic ionic liquid immobilized on silica (ILIS) | 9750 |
| 5 | Ionic liquid 1ml, rt | 1.5 h | FeCl3·6H2O | 9823 |
| 6 | [bmim]BF4 or [bmim]PF6 2 ml, rt | 4.5 h | 8745 | |
| 7 | Ionic liquid 1 ml, rt | 15 min | In(OTf)3 | 908 |
Conclusions
In conclusion, this communication describes how EAN mediated an efficient, rapid synthesis of bis(indolyl)methane from indoles and aldehydes in excellent yields at room temperature in shorter reaction times. Novel EAN acts as a catalyst and green media for the reaction, making this method cheaper, simple, convenient, and an environmentally friendly process for the synthesis of substituted bis(indolyl)methanes.Experimental
All chemicals and reagents required for the reactions were procured from Sigma-Aldrich with purity >98% and used without further purification. The products were characterized using 1H NMR, 13C NMR spectra. NMR spectra of the products were obtained using Bruker AC-200 and AC-400 MHz spectrometers with TMS as the internal standard. Column chromatography was performed on silica gel, Merck grade 60–120 mesh size. TLC was performed on 0.25 mm E. Merck pre-coated silica gel plates (60 F254). The compounds are already well known in the literature.6–10Typical procedure for the synthesis of EAN ionic liquid
The ethyl ammonium nitrate used was synthesized according to the procedures reported in the literature.49 The aqueous solution of ethylamine (70%, 100 ml) was taken in a 1 L round bottom flask, which was maintained below 10 °C using an ice–water bath. To this cold solution, nitric acid (30%, 330 ml) was added slowly drop-wise with vigorous stirring to attain a pH of the mixture of 7.3, the addition was stopped and the mixture was stirred further for 0.5 h. The water from the mixture was removed in a rotary evaporator in a boiling water bath at a pressure of 200 mmHg. The traces of water were removed at 100 °C and 1 mmHg pressure to afford the ethyl ammonium nitrate in quantitative yield (170 g). The ethyl ammonium nitrate obtained was used as the catalyst in this reaction.A typical experimental procedure for synthesis of bis(indolyl)methane
A mixture of indole (2 mmol), benzaldehyde (1 mmol) and EAN (2 mmol) was stirred at room temperature. The completion of the reaction was monitored by TLC. After the completion of the reaction, the reaction mixture was diluted by water and extracted with ethyl acetate. The organic layer was dried over anhydrous sodium sulphate and concentrated in vacuo to get the crude product. The crude product was purified by column chromatography. All the isolated reaction products were characterized and confirmed by NMR, IR and mass spectroscopy.Characterization of selected compounds
Acknowledgements
SMI, MYP and SSC thank CSIR New Delhi for SRF and JRF, respectively. The authors also thank Dr B. D. Kulkarni and Dr V. V. Ranade, Chair, CE-PD for their encouragement and support.References
- (a) R. J. Sundberg, The Chemistry of Indoles, Academic Press, New York, 1996 Search PubMed; (b) A. Casapullo, G. Bifulco, I. Bruno and R. Riccio, J. Nat. Prod., 2000, 63, 447 CrossRef CAS; (c) T. R. Garbe, M. Kobayashi, N. Shimizu, N. Takesue, M. Ozawa and H. Yukawa, J. Nat. Prod., 2000, 63, 596 CrossRef CAS; (d) B. Bao, Q. Sun, X. Yao, J. Hong, C. O. Lee, C. J. Sim, K. S. Im and J. H. Jung, J. Nat. Prod., 2005, 68, 711 CrossRef CAS.
- (a) M. C. Bell, Gynecol. Oncol., 2000, 78, 123 Search PubMed; (b) C. H. Thomas, US Pat., 4500689, 1985 Search PubMed.
- X. Ge, S. Yannai, G. Rennert, N. Gruener and F. A. Fares, Biochem. Biophys. Res. Commun., 1996, 228, 153 Search PubMed.
- J. J. Michnovicz and H. L. Bradlow, Cancer 199, 16, 58 Search PubMed.
- (a) P. T. Perumal and R. Nagarajan, Tetrahedron, 2002, 58, 1229 CrossRef CAS; (b) M. Chakrabarty, N. Ghosh, R. Basak and Y. Harigaya, Tetrahedron Lett., 2002, 43, 4075 CrossRef CAS; (c) D. Chen, L. Yu and P. G. Wang, Tetrahedron Lett., 1996, 37, 4467 CrossRef CAS; (d) J. S. Yadav, B. V. Reddy, C. V. Murthy, G. M. Kumar and C. Madan, Synthesis, 2001, 783 CrossRef CAS; (e) M. L. Deb and P. J. Bhuyan, Synlett, 2008, 325 CAS.
- A. Vijender Reddy, K. Ravinder, V. L. Niranjan Reddy, T. Venkateshwer, V. Ravikanth and Y. Venkateswarlu, Synth. Commun., 2003, 33, 3687 Search PubMed.
- P. R. Singh, D. U. Singh and S. D. Samant, Synth. Commun., 2005, 35, 2133 CrossRef.
- Shun- Jun Ji, Min-Feng Zhou, Da-Gong Gu, Shun-Yi Wang and Teck-Peng Loh, Synlett, 2003, 13, 2077 Search PubMed.
- J. S. Yadav, B. V. S. Reddy, V. S. R. Murthy and G. M. Kumar, Synthesis, 2001, 783 CrossRef CAS.
- M. Lakshmi Kantam, K. Aziz and P. R. Likhar, Catal. Lett., 2004, 98, 117 Search PubMed.
- L. Mo, Z. Ma and Z. Zhang, Synth. Commun., 2005, 35, 1997 CrossRef CAS.
- R. R. Nagawade and D. B. Shinde, Bull. Korean Chem. Soc., 2005, 26(12), 1962 Search PubMed.
- K. Niknama, M. A. Zolfigolb, T. Sadabadia and A. Nejati, Journal of the Iranian Chemical Society, 2006, 3, 318 Search PubMed.
- S. Palaniappan and A. John, J. Mol. Catal. A: Chem., 2005, 42, 168 Search PubMed.
- X. Zeng, S. Ji and S. Wang, Tetrahedron, 2005, 61, 10235 CrossRef CAS.
- B. Ke, Y. Qin, Q. He, Z. Huang and P. Wang, Tetrahedron Lett., 2005, 46, 1751 CrossRef CAS.
- F. Xin-Liang, G. Chuan-Jin and Z. Cheng-Xue, Synth. Commun., 2004, 34, 487 Search PubMed.
- A. Kamal and A. A. Qureshi, Tetrahedron, 1963, 19, 513 CrossRef CAS.
- G. Babu, N. Sridhar and P. T. Perumal, Synth. Commun., 2000, 30, 1609 CrossRef CAS.
- B. P. Bandgar and K. A. Shaikh, J. Chem. Res. Synop., 2004, 34 Search PubMed.
- X.-L. Mi, S.-Z. Luo, J.-Q. He and J.-P. Cheng, Tetrahedron Lett., 2004, 45, 4567 CrossRef CAS.
- D. Chen, L. Yu and P. G. Wang, Tetrahedron Lett., 1996, 37, 4467 CrossRef CAS.
- Shun-Jun Ji, Min-Feng Zhou, Da-Gong Gu, Zhao-Qin Jiang and Teck-Peng Loh, Eur. J. Org. Chem., 2004, 1584 CrossRef CAS.
- F. Shirin, A. Yahyazabeh, M. Abedini and D. Imami, Bull. Korean Chem. Soc., 2010, 31, 1715 Search PubMed.
- M. A. Naik, D. Sachdev and A. Dubey, Catal. Commun., 2010, 11, 1148 CrossRef CAS.
- R. G. Vaghei, H. Veisi, H. Keypour and A. A. Firouzabadi, Mol. Diversity, 2010, 14, 87 Search PubMed.
- M. A. Zolfigol, A. Khazaei and A. R. Moosavi, Org. Prep. Proced. Int., 2010, 42, 175 Search PubMed.
- K. Niknam, D. Saberi and M. Baghernejad, Phosphorus, Sulfur Silicon Relat. Elem., 2010, 185, 875 Search PubMed.
- L. Iglesias, C. Aguilar, D. Bandyopadhyay and B. K. Banik, Synth. Commun., 2010, 40, 3678 Search PubMed.
- G. Meshram and V. D. Patil, Synth. Commun., 2010, 40, 29 Search PubMed.
- E. Rafiee, Z. Zolfagharifar, M. Joshaghani and S. Eavani, Appl. Catal., A, 2009, 365, 287 Search PubMed.
- A. Karam, J. C. Alonso, T. I. Gerganova, P. Ferreira and N. Bion, Chem. Commun., 2009, 7000 RSC.
- C. C. Silveira, S. R. Mendes, F. M. Líbero, E. J. Lenardão and G. Perin, Tetrahedron Lett., 2009, 50, 6060 CrossRef CAS.
- S. Chandrasekhar, S. Khatun, G. Rajesh and C. R. Reddy, Tetrahedron Lett., 2009, 50, 6693 CrossRef CAS.
- M. M. Heravi, K. Bakhtiari, A. Fatehi and F. F. Bamoharram, Catal. Commun., 2008, 9, 289 Search PubMed.
- S. V. Nadkarni, M. B. Gawande, R.V. Jayaram and J. M. Nagarkar, Catal. Commun., 2008, 9, 1728 CrossRef CAS.
- A. Khalafi-Nezhad, A. Parhami, A. Zare, A. R. Zare, A. Hasaninejad and F. Panahia, Synthesis, 2008, 4, 617 Search PubMed.
- P. T. Anastas and J. C. Warner ed. “Green Chemistry Theory and Practice”, Oxford University Press, Oxford, 1998 Search PubMed.
- P. T. Anasta and T. C. Williamson, ed. Green Chemistry Frontier in Benign Chemical Synthesis and Processes, Oxford Univ. Press, Oxford, 1998 Search PubMed.
- C. Christ, Ed. “Production Integrated Environmental Protection and waste Managements in the Chemical Industry, Wiley–VCH, Weinheim, 1999 Search PubMed.
- G. W. Kabalka and A. R. Mereddy, Tetrahedron Lett., 2005, 46, 6315 CrossRef CAS.
- M. Y. Pathan, V. V. Paike, P. R. Pachmase, S. P. More, S. S. Ardhapure and R. P. Pawar, ARKIVOC (XV), 2006, 205 Search PubMed.
- S. R. Sarda, M. Y. Pathan, V. V. Paike, P. R. Pachmase, W. N. Jadhav and R. P. Pawar, ARKIVOC (XVi), 2006, 43 Search PubMed.
- (a) T. Walton, Chem. Rev., 1999, 99, 2071 CrossRef CAS; (b) P. Walden, Bull. Acad. Imper. Sci. (St. Petersburg), 1914, 1800 Search PubMed.
- J. S. Yadav, B. V. S. Reddy and S. Sunita, Adv. Synth. Catal., 2003, 345, 349 CrossRef CAS.
- S. A. Sadaphal, K. F. Shelke, S. S. Sonar and M. S. Shingare, Cent. Eur. J. Chem., 2008, 4, 622 Search PubMed.
- K. Rad-Moghadam and M. Sharifi-Kiasaraie, Tetrahedron, 2009, 65, 8816 CrossRef CAS.
- R. Kanzaki, K. Uchida, S. Hara and Y. Umebayashi, Chem. Lett., 2007, 36, 684 CrossRef CAS.
- (a) S. A. Dake, D. S. Raut, K. R. Kharat, R. S. Mhaske, S.U. Deshmukh and R. P. Pawar, Bioorg. Med. Chem. Lett., 2011, 21, 2527 Search PubMed; (b) R. V. Hangarge, D. V. Jarikote and M. S. Shingare, Green Chem., 2002, 4, 266 RSC; (c) S. R. Sarda, M. Y. Pathan, V. V. Paike, P. R. Pachmase, W. N. Jadhav and R. P. Pawar, ARKIVOC (XVi), 2006, 43 Search PubMed; (d) S. R. Sarda, J. D. Kale and S. K. Wasmatkar, Mol. Diversity, 2009, 13, 545 Search PubMed.
- H. Hagiwara, M. Sekifuji, T. Hoshi, K. Qiao and C. Yokoyama, Synlett, 2007, 8, 1320 Search PubMed.
| This journal is © The Royal Society of Chemistry 2012 |
