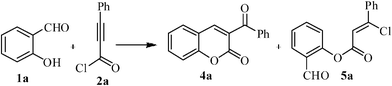
An unusual one-pot synthesis of 3-benzoylcoumarins and coumarin-3-carbaldehydes from 2-hydroxybenzaldehydes under esterification conditions
K. C.
Majumdar
*ab,
Srikanta
Samanta
a,
Inul
Ansary
a and
B.
Roy
a
aDepartment of Chemistry, University of Kalyani, Kalyani, 741235, W.B, India. E-mail: kcm_ku@yahoo.co.in; Fax: +913325828282; Tel: +913325827521
bDepartment of Chemical Sciences, Tezpur University, Napaam, Tezpur, 784028, Assam, India
First published on 19th January 2012
Abstract
Synthesis of 3-benzoylcoumarins and coumarin-3-carbaldehydes has been achieved in moderate to good yields (31–83%) by the reaction of easily available 2-hydroxybenzaldehydes and phenylpropiolyl chloride/propiolyl chloride under esterification condition.
Introduction
Coumarin (2H-Chromen-2-one) derivatives continue to be investigated over the years because of their tremendous biological importance.1Coumarin and its derivatives are associated with various biological activities viz. antiviral,2 antibacterial,3 antimicrobial,4anticoagulant,5 antiinflammatory,6 anticancer,7anticonvulsant,8antioxidant,9 antifungal,10anti-HIV,11anti-carcinogenic material,12 as well as inhibition of platelet aggregation,13 and inhibition of steroid 5a-reductase.14 Besides, they are attracting considerable attention of chemists due to their several applications e.g. optical brighterners,15 photosensitizers,16 and fluorescent & laser dyes17 as well as additives18 in food, perfumes, cosmetics and pharmaceuticals. These compounds are also utilized in drug and pesticidal preparations.19Coumarins have been synthesized by several routes e.g. Pechmann,20 Perkin,21 Knoevenagel,22 Reformatsky,23 Wittig,24vinyltriphenylphosphonium salt mediated aromatic electrophilic substitution,25 ultrasonic-assisted organic reactions,26palladium-catalyzed reactions of phenols with alkynoates27 and propiolic acids28 respectively, and Yb(OTf)3-catalyzed reactions of 5-alkylidene Meldrum's acids29 with phenolsetc. The main method for the synthesis of coumarins is the Pechmann reaction of substituted phenols with β-keto esters in the presence of protonic acid catalysts such as conc. H2SO4,20a,bTFA,30 lewis acid catalystsviz.AlCl3,31ZnCl2,32ZnCl2/Al2O3,33ZrCl4,34LiBr,1CuPy2Cl235etc., and in the presence of dehydrating agents P2O536 or montmorillonite clay.37 However, some of the catalysts used so far are harsh or hazardous, have to be used in considerable excess20,36 or lead to the formation of side products.38
Recently, Rao et al.39 have reported the synthesis of 3-aroylcoumarins by the condensation reaction of α-aroylketene dithioacetals and 2-hydroxyarylaldehydes. On the other hand, Tang et al.40 have also reported the synthesis of 3-benzoylcoumarin derivatives from the reaction of 2-hydroxybenzaldehydes with ethyl benzoylacetate in the presence of piperidine or pyrrolidine base. Herein, we wish to report a more convenient and efficient approach to the synthesis of 3-benzoylcoumarins and coumarin-3-carbaldehydes by the reaction of phenylpropiolyl chloride/propiolyl chloride, derived from commercially available phenylpropiolic acid/propiolic acid, with 2-hydroxybenzaldehydes under the esterification condition in moderate to good yields.
Results & discussion
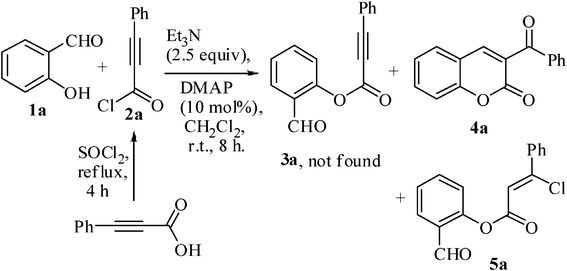
The starting precursors, substituted 2-hydroxybenzaldehydes 1b–j and 2-hydroxy-1-naphthaldehyde 6, were prepared according to our earlier published procedure.41 We chose 2-hydroxybenzaldehyde 1a as the model precursor for our investigation. When the substrate 1a was subjected to an esterification reaction with freshly prepared phenylpropiolyl chloride 2a (1.2 equiv) in the presence of Et3N (2.5 equiv) and 4-dimethylamino pyridine (DMAP, 10 mol%) in CH2Cl2 as solvent at room temperature for 8 h (Scheme 1), it was observed that a mixture of compounds 4a and 5a were formed in a ratio of 4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5a = 85
5a = 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 and in an overall 62% isolated yield (Table 1, entry 1). No trace of ester 3a was observed.
15 and in an overall 62% isolated yield (Table 1, entry 1). No trace of ester 3a was observed.
 | ||
| Scheme 1 Esterification of salicylaldehyde 1a. | ||
| Entry | Solvent | Base (equiv) | Additive | Isolated yield (%) | Ratio 4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5a 5a |
|---|---|---|---|---|---|
| a 10 mol% of additive was used for each case. b optimized reaction conditions. c dry CH2Cl2 and dry Et3N were used, DMAP = 4-Dimethylaminopyridine, DIPEA = N,N-Diisopropylethylamine, DBU = 1,8-Diazabicyclo[5.4.0]undec-7-ene, DABCO = 1,4-Diazabicyclo[2.2.2]octane and TBAHS = Tetrabutylammonium hydrogen sulphate. | |||||
| 1 | CH2Cl2 | Et3N (2.5) | DMAP a | 62 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
| 2 | CH2Cl2 | Et3N (3.5) | DMAP | 65 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05 05 |
| 3b | CH2Cl2 | Et3N (5.0) | DMAP | 71 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 00 00
|
| 4 | CH2Cl2 | Et3N (7.0) | DMAP | 70 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 00 |
| 5c | CH2Cl2 | Et3N (5.0) | DMAP | 70 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 00 |
| 6 | CHCl3 | Et3N (5.0) | DMAP | 58 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 00 |
| 7 | THF | Et3N (5.0) | DMAP | 43 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 00 |
| 8 | CH2Cl2 | DIPEA (5.0) | DMAP | 63 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 00 |
| 9 | CH2Cl2 | Pyridine (5.0) | DMAP | 60 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 00 |
| 10 | CH2Cl2 | DBU | DMAP | 5 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 00 |
| 11 | CH2Cl2 | DABCO | DMAP | trace | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 00 |
| 12 | CH2Cl2 | K2CO3 (5.0) | TBAHS | 25 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 00 |
| 13 | CH2Cl2 | — | DMAP | 92 | 00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
| 14 | CH2Cl2 | Et3N (5.0) | — | 55 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 00 |
Compound 4a was fully characterized from its spectral data which was consistent with literature values.39,40 Compound 5a was characterized as an uncyclized hydrochlorinated ester. This observation turned our attention to concentrate on the optimal reaction conditions for the formation of 3-benzoylcoumarin 4a to improve its yield. Therefore, our studies were directed towards performing a series of experiments with the substrate 1a where sequential changes in the conditions were made.
By increasing the amount of base up to 5.0 equiv, the yield of the product vinyl chloride derivative 5a was decreased and the desired cyclized product 3-benzoylcoumarin 4a was increased (Table 1, entries 2–4). The use of dry CH2Cl2 and dry Et3N gave the same result as that of ordinary CH2Cl2 and Et3N (Table 1, entry 5). Changing either solvent or base did not provide a better result (Table 1, entries 6–11). Under the phase transfer reaction the yield of the cyclized product 4a was found to be unsatisfactory (Table 1, entry 12). The cyclized product 4a was not obtained in the absence of base, but an overall yield of 92% of the vinyl chloride 5a was isolated (Table 1, entry 13). In the absence of DMAP the yield of the cyclized product decreased (Table 1, entry 14). Based on the above, the combination of 5.0 equiv of Et3N, 10 mol% of DMAP and the use of CH2Cl2 as the solvent at room temperature provided the best result (Table 1, entry 3).
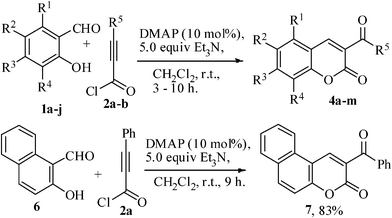
To test the generality of the reaction, the other substrates 1b–j and 6 were treated under optimized reaction condition and the 3-benzoylcoumarins and coumarin-3-carbaldehydes 4b–m and 7 were obtained in 31–83% yields (Table 2). From Table 2 it can be clear that the electron-donating groups like Me, Et, OMe, and OEt present in the substrates gave higher yields while electron-withdrawing groups, for example, Cl and Br gave relatively poor yields of the products.
| Entry | R1 | R2 | R3 | R4 | R5 | Product | Time (h) | Yield (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | H | H | H | H | Ph | 4a | 8 | 71 |
| 2 | H | Me | H | H | Ph | 4b | 8 | 75 |
| 3 | H | Et | H | H | Ph | 4c | 8 | 73 |
| 4 | H | OMe | H | H | Ph | 4d | 8 | 79 |
| 5 | H | OEt | H | H | Ph | 4e | 8 | 76 |
| 6 | H | Cl | H | H | Ph | 4f | 10 | 57 |
| 7 | H | Br | H | H | Ph | 4g | 9 | 60 |
| 8 | Me | H | H | H | Ph | 4h | 9 | 68 |
| 9 | Me | H | Me | H | Ph | 4i | 8 | 80 |
| 10 | Me | H | H | Me | Ph | 4j | 8 | 81 |
| 11 | H | H | H | H | H | 4k | 7 | 31 |
| 12 | H | Me | H | H | H | 4l | 5 | 37 |
| 13 | H | OMe | H | H | H | 4m | 3 | 53 |
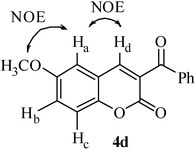
The spectral data and melting points for some of the known compounds (4a, 4b, 4d, 4f and 7) were consistent with the literature values.39 The structure of the product 3-benzoylcoumarin was also confirmed by NOESY (1H–1H correlation) experiment (Fig. 1).
 | ||
| Fig. 1 | ||
The NOESY spectrum of the compound 4d shows two important NOE interactions, one between C4–H (Hd, δH = 8.04, s) and C5–H (Ha, δH = 7.01, d, J = 2.7 Hz) and the other between C5–H (Ha, δH = 7.01, d, J = 2.7 Hz) and C6–OCH3 (δH = 3.87, s). These two NOE interactions support the formation of the suggested coumarin frame work.
The formation of products 4a–m and 7 may be explained by considering a Baylis–Hillman-type of reaction from the in situ formed ester 3 and the base (e.g.Et3N) present in the medium (Scheme 2). Thus, the base Et3N attacks at the polarized carbon atom of conjugated alkyne to generate the species 8, which on intramolecular coupling produces species 9. Finally, the species 9 eliminates the base to afford the products 4a–m, 7via the formation of intermediate 10. The compound 5a was formed by HCl addition to 3avia the formation of intermediate 11a when the reaction was carried out using a lower amount of Et3N as a base.
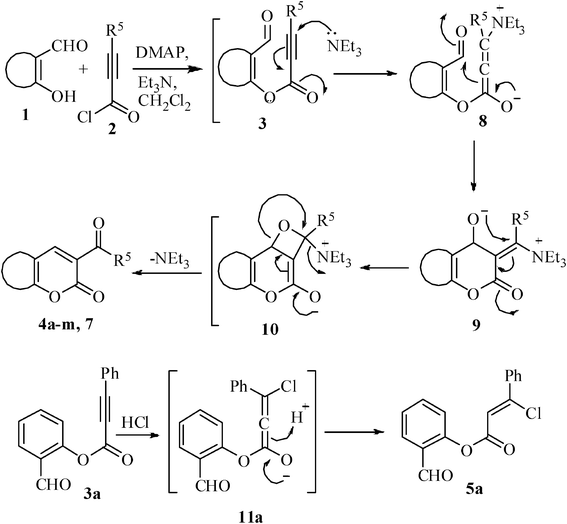 | ||
| Scheme 2 Probable mechanism for the formation of 3-benzoylcoumarins and coumarin-3-carbaldehydes. | ||
It is interesting to note that when the substrate 2-(3-phenylprop-2-ynyloxy)benzaldehyde 12 was mixed with 5.0 equiv of Et3N.HCl in CH2Cl2 at room temperature for 8 h, it did not afford (2H-Chromen-3-yl)methanone 13 (Scheme 3), and the starting material 12 was recovered unchanged. The reaction was also attempted with Et3N and DMAP in CH2Cl2 at room temperature for 10 h. However, the reaction did not occur.
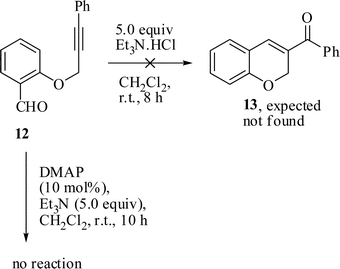 | ||
| Scheme 3 Reaction of 12 under esterification condition. | ||
The failure of the above reaction might be due to the absence of the ester C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond and the alkyne part remains unpolarized which makes an attack by the base difficult. Hence, the substrate 12 was recovered unchanged.
O bond and the alkyne part remains unpolarized which makes an attack by the base difficult. Hence, the substrate 12 was recovered unchanged.
2-Hydroxyacetophenone 14 was also tested with phenylpropiolyl chloride 2a under optimized reaction condition, and a mixture of ester 15 (20%) and its corresponding hydrochlorinated product 16 (60%) was obtained. But under phase transfer conditions, the ester derivative 15 (90%) was formed exclusively (Scheme 4). The isolation of the ester derivative 15 also supports the suggested mechanism that the cyclization goes through initial formation of the intermediate 3 (Scheme 2). Under phase transfer condition, the desired cyclized product 17 was not formed. This is perhaps due to the fact that K2CO3 is not a suitable base for the Baylis–Hillman reaction. The failure to obtain the cyclized product 17 under anhydrous conditions may be due to steric and/or electronic effects of the –COCH3group compared to that of the –CHO group. When excess base was present in the medium, the effective availability of the chloride anion was too low to form the addition product. In the absence of any base42 or in the presence of a lower amount of base, the available chloride anion concentration was sufficient to produce the addition product 5a or 16.
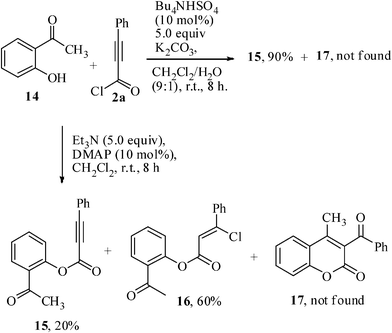 | ||
| Scheme 4 Esterification reaction of 2-hydroxy acetophenone 14. | ||
Conclusion
In conclusion, we have synthesized a library of 3-benzoylcoumarins and coumarin-3-carbaldehydes of potential interest via a Baylis–Hillman-type of reaction under very mild esterification conditions. This reaction is a promising one, because the 3-benzoylcoumarin moiety has a highly biologically active core and it has been used for the synthesis of biologically active analogues such as pyrano-chromene, benzo-coumarin, chromeno-pyridine and chromeno-pyrazole.3bExperimental
Melting points were determined in open capillaries and are uncorrected. IR spectra were run for KBr discs (and neat in case of liquid samples) on a Perkin-Elmer L 120-000A IR spectrometer (υmax in cm−1) and 1H NMR spectra were determined for solutions in CDCl3 with TMS as internal standard on a Bruker DPX-300 and DPX-400. 13C NMR spectra were determined for solutions in CDCl3 on a Bruker DPX-400. Mass spectra and HRMS were recorded on a Qtof Micro instrument. CHN was recorded on a Perkin-Elmer 2400 series II CHN-analyzer. Silica gel (60–120 mesh) and (230–400 mesh) were used for chromatographic separation. Silica gel-G [E-Merck (India)] was used for TLC. Petroleum–ether refers to the fraction between 60 and 80 °C.General procedure for the synthesis of 3-substituted-coumarin derivatives (4a–m and 7)
A mixture of compound 1 or 6 (300 mg, 1 equiv), triethyl amine (5 equiv) and a catalytic amount of DMAP (10 mol%) was stirred in dichloromethane (50 mL) at room temperature. A solution of phenylpropiolyl chloride 2a or simple propiolyl chloride 2b [generated separately from phenylpropiolic acid or propiolic acid (1.2 equiv)/SOCl2/reflux/4 h/followed by excess SOCl2 removal] in dichloromethane (30 mL) was added drop wise to the reaction mixture and stirred at room temperature for 3–10 h. The reaction mixture was then poured into 25 mL of water and then extracted with CH2Cl2 (3 × 15 mL), washed with water (2 × 10 mL), brine (10 mL) and dried over anhydrous Na2SO4. The solvent was removed to give a crude mass which was chromatographed over silica gel (230–400 mesh) using ethyl acetate–petroleum ether as an eluent to furnish the desired product 4 or 7 as a sole product.3-Benzoyl-2H-chromen-2-one (4a)
Using the general procedure, starting from 300 mg (2.45 mmol) of salicylaldehyde 1a, 1.7 mL (12.25 mmol) of triethyl amine, DMAP (30 mg, 10 mol%) and phenylpropiolyl chloride 2a [generated separately from phenylpropiolic acid (430 mg, 2.94 mmol)/SOCl2/reflux/4 h followed by excess SOCl2 removal], 435 mg of the title compound 4a was isolated as a white solid. Reaction time: 8 h. Eluent: EA/PE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 v/v). Yield: 71%. Mp: 132 °C (lit.39 134–136 °C). IR (KBr): 1656, 1714, 3062 cm−1. 1H NMR (CDCl3, 400 MHz): δH = 7.36 (dd ≈ t, 1H, J = 7.6 Hz, ArH), 7.42 (d, 1H, J = 8.4 Hz, ArH), 7.49 (dd ≈ t, 2H, J = 7.6 Hz, ArH), 7.60–7.68 (m, 3H, ArH), 7.89 (d, 2H, J = 8.0 Hz, ArH), 8.09 (s, 1H, C4–H of coumarin). 13C NMR (CDCl3, 100 Hz): δC = 116.9, 118.2, 125.0, 127.0, 128.6, 129.2, 129.6, 133.7, 133.8, 136.2, 145.5, 154.8, 158.4, 191.7. HRMS (ESI): calcd for C16H10O3Na [M+Na]+ 273.0528, found 273.0506.
19 v/v). Yield: 71%. Mp: 132 °C (lit.39 134–136 °C). IR (KBr): 1656, 1714, 3062 cm−1. 1H NMR (CDCl3, 400 MHz): δH = 7.36 (dd ≈ t, 1H, J = 7.6 Hz, ArH), 7.42 (d, 1H, J = 8.4 Hz, ArH), 7.49 (dd ≈ t, 2H, J = 7.6 Hz, ArH), 7.60–7.68 (m, 3H, ArH), 7.89 (d, 2H, J = 8.0 Hz, ArH), 8.09 (s, 1H, C4–H of coumarin). 13C NMR (CDCl3, 100 Hz): δC = 116.9, 118.2, 125.0, 127.0, 128.6, 129.2, 129.6, 133.7, 133.8, 136.2, 145.5, 154.8, 158.4, 191.7. HRMS (ESI): calcd for C16H10O3Na [M+Na]+ 273.0528, found 273.0506.
3-Benzoyl-6-methyl-2H-chromen-2-one (4b)
Using the general procedure, starting from 300 mg (2.20 mmol) of 2-hydroxy-5-methylbenzaldehyde 1b, 1.5 mL (11.00 mmol) of triethyl amine, DMAP (27 mg, 10 mol%) and phenylpropiolyl chloride 2a [phenylpropiolic acid (386 mg, 2.64 mmol)/SOCl2/reflux/4 h], 436 mg of the title compound 4b was isolated as a white solid. Reaction time: 8 h. Eluent: EA/PE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 v/v). Yield: 75%. Mp: 170 °C (lit.39 174 °C). IR (KBr): 1653, 1724, 3065 cm−1. 1H NMR (CDCl3, 400 MHz): δH = 2.44 (s, 3H, CH3), 7.31 (d, 1H, J = 8.8 Hz, ArH), 7.38 (s, 1H, ArH), 7.45–7.52 (m, 3H, ArH), 7.62 (dd ≈ t, 1H, J = 7.6 Hz, ArH), 7.88 (d, 2H, J = 8.0 Hz, ArH), 8.04 (s, 1H, C4–H of coumarin). 13C NMR (CDCl3, 100 MHz): δC = 20.8, 116.6, 117.9, 126.8, 128.6, 128.9, 129.6, 133.8, 134.8, 136.3, 145.5, 152.9, 158.7, 191.8. HRMS (ESI): calcd for C17H12O3Na [M+Na]+ 287.0684, found 287.0661.
19 v/v). Yield: 75%. Mp: 170 °C (lit.39 174 °C). IR (KBr): 1653, 1724, 3065 cm−1. 1H NMR (CDCl3, 400 MHz): δH = 2.44 (s, 3H, CH3), 7.31 (d, 1H, J = 8.8 Hz, ArH), 7.38 (s, 1H, ArH), 7.45–7.52 (m, 3H, ArH), 7.62 (dd ≈ t, 1H, J = 7.6 Hz, ArH), 7.88 (d, 2H, J = 8.0 Hz, ArH), 8.04 (s, 1H, C4–H of coumarin). 13C NMR (CDCl3, 100 MHz): δC = 20.8, 116.6, 117.9, 126.8, 128.6, 128.9, 129.6, 133.8, 134.8, 136.3, 145.5, 152.9, 158.7, 191.8. HRMS (ESI): calcd for C17H12O3Na [M+Na]+ 287.0684, found 287.0661.
3-Benzoyl-6-ethyl-2H-chromen-2-one (4c)
Using the general procedure, starting from 300 mg (2.00 mmol) of 5-ethyl-2-hydroxybenzaldehyde 1c, 1.40 mL (10.00 mmol) of triethyl amine, DMAP (25 mg, 10 mol%) and phenylpropiolyl chloride 2a [phenylpropiolic acid (350 mg, 2.40 mmol)/SOCl2/reflux/4 h], 406 mg of the title compound 4c was isolated as a white solid. Reaction time: 8 h. Eluent: EA/PE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 v/v). Yield: 73%. Mp: 156 °C. IR (KBr): 1655, 1719, 2962 cm−1. 1H NMR (CDCl3, 400 MHz): δH = 1.28 (t, 3H, J = 7.6 Hz, CH2CH3), 2.73 (q, 2H, J = 7.6 Hz, CH2CH3), 7.33 (d, 1H, J = 8.8 Hz, ArH), 7.40 (s, 1H, ArH), 7.48 (dd ≈ t, 3H, J = 8.0 Hz, ArH), 7.61 (dd ≈ t, 1H, J = 7.6 Hz, ArH), 7.88 (d, 2H, J = 7.6 Hz, ArH), 8.06 (s, 1H, C4–H of coumarin). 13C NMR (CDCl3, 100 MHz): δC = 15.5, 28.1, 116.7, 118.0, 126.7, 127.7, 128.6, 129.6, 133.8, 136.3, 141.2, 145.7, 153.1, 158.7, 191.9. MS (ESI): m/z = 301 [M+Na]+. Anal. Calcd. for C18H14O3: C, 77.68; H, 5.07%; Found: C, 77.71; H, 4.97%.
19 v/v). Yield: 73%. Mp: 156 °C. IR (KBr): 1655, 1719, 2962 cm−1. 1H NMR (CDCl3, 400 MHz): δH = 1.28 (t, 3H, J = 7.6 Hz, CH2CH3), 2.73 (q, 2H, J = 7.6 Hz, CH2CH3), 7.33 (d, 1H, J = 8.8 Hz, ArH), 7.40 (s, 1H, ArH), 7.48 (dd ≈ t, 3H, J = 8.0 Hz, ArH), 7.61 (dd ≈ t, 1H, J = 7.6 Hz, ArH), 7.88 (d, 2H, J = 7.6 Hz, ArH), 8.06 (s, 1H, C4–H of coumarin). 13C NMR (CDCl3, 100 MHz): δC = 15.5, 28.1, 116.7, 118.0, 126.7, 127.7, 128.6, 129.6, 133.8, 136.3, 141.2, 145.7, 153.1, 158.7, 191.9. MS (ESI): m/z = 301 [M+Na]+. Anal. Calcd. for C18H14O3: C, 77.68; H, 5.07%; Found: C, 77.71; H, 4.97%.
3-Benzoyl-6-methoxy-2H-chromen-2-one (4d)
Using the general procedure, starting from 300 mg (1.97 mmol) of 2-hydroxy-5-methoxybenzaldehyde 1d, 1.4 mL (9.85 mmol) of triethyl amine, DMAP (24 mg, 10 mol%) and phenylpropiolyl chloride 2a [phenylpropiolic acid (346 mg, 2.37 mmol)/SOCl2/reflux/4 h], 436 mg of the title compound 4d was isolated as a yellow solid. Reaction time: 8 h. Eluent: EA/PE (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 93 v/v). Yield: 79%. Mp: 158 °C (lit.39 160–162 °C). IR (KBr): 1655, 1711, 3046 cm−1. 1H NMR (CDCl3, 300 MHz): δH = 3.87 (s, 3H, OCH3), 7.01 (d, 1H, J = 2.7 Hz, ArH), 7.23 (dd, 1H, J = 9.0, 2.7 Hz, ArH), 7.34 (d, 1H, J = 9.0 Hz, ArH), 7.49 (dd ≈ t, 2H, J = 7.8 Hz, ArH), 7.62 (dd ≈ t, 1H, J = 7.5 Hz, ArH), 7.89 (d, 2H, J = 7.5 Hz, ArH), 8.04 (s, 1H, C4–H of coumarin). 13C NMR (CDCl3, 100 MHz): δC = 55.9, 110.6, 118.0, 118.5, 121.8, 127.2, 128.6, 129.6, 133.8, 136.2, 145.3, 149.3, 156.4, 158.6, 191.8. HRMS (ESI): calcd for C17H12O4Na [M+Na]+ 303.0633, found 303.0595.
93 v/v). Yield: 79%. Mp: 158 °C (lit.39 160–162 °C). IR (KBr): 1655, 1711, 3046 cm−1. 1H NMR (CDCl3, 300 MHz): δH = 3.87 (s, 3H, OCH3), 7.01 (d, 1H, J = 2.7 Hz, ArH), 7.23 (dd, 1H, J = 9.0, 2.7 Hz, ArH), 7.34 (d, 1H, J = 9.0 Hz, ArH), 7.49 (dd ≈ t, 2H, J = 7.8 Hz, ArH), 7.62 (dd ≈ t, 1H, J = 7.5 Hz, ArH), 7.89 (d, 2H, J = 7.5 Hz, ArH), 8.04 (s, 1H, C4–H of coumarin). 13C NMR (CDCl3, 100 MHz): δC = 55.9, 110.6, 118.0, 118.5, 121.8, 127.2, 128.6, 129.6, 133.8, 136.2, 145.3, 149.3, 156.4, 158.6, 191.8. HRMS (ESI): calcd for C17H12O4Na [M+Na]+ 303.0633, found 303.0595.
3-Benzoyl-6-methoxy-2H-chromen-2-one (4e)
Using the general procedure, starting from 300 mg (1.80 mmol) of 2-hydroxy-5-ethoxybenzaldehyde 1e, 1.3 mL (9.00 mmol) of triethyl amine, DMAP (22 mg, 10 mol%) and phenylpropiolyl chloride 2a [phenylpropiolic acid (315 mg, 2.16 mmol)/SOCl2/reflux/4 h], 402 mg of the title compound 4e was isolated as a white solid. Reaction time: 8 h. Eluent: EA/PE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 v/v). Yield: 76%. Mp: 162–163 °C. IR (KBr): 1657, 1712, 2976 cm−1. 1H NMR (CDCl3, 400 MHz): δH = 1.45 (t, 3H, J = 6.8 Hz, CH2CH3), 4.07 (q, 2H, J = 6.8 Hz, CH2CH3), 6.99 (d, 1H, J = 2.4 Hz, ArH), 7.22 (dd, 1H, J = 9.2, 2.4 Hz, ArH), 7.33 (d, 1H, J = 9.2 Hz, ArH), 7.48 (dd ≈ t, 2H, J = 8.0 Hz, ArH), 7.62 (dd ≈ t, 1H, J = 7.6 Hz, ArH), 7.88 (d, 2H, J = 7.6 Hz, ArH), 8.02 (s, 1H, C4–H of coumarin). 13C NMR (CDCl3, 100 MHz): δC = 14.7, 64.3, 111.3, 117.9, 118.5, 122.2, 127.2, 128.6, 129.6, 133.8, 136.3, 145.3, 149.2, 155.8, 158.8, 191.9. MS (ESI): m/z = 317 [M+Na]+. Anal. Calcd. for C18H14O4: C, 73.46; H, 4.79%; Found: C, 73.48; H, 4.74%.
9 v/v). Yield: 76%. Mp: 162–163 °C. IR (KBr): 1657, 1712, 2976 cm−1. 1H NMR (CDCl3, 400 MHz): δH = 1.45 (t, 3H, J = 6.8 Hz, CH2CH3), 4.07 (q, 2H, J = 6.8 Hz, CH2CH3), 6.99 (d, 1H, J = 2.4 Hz, ArH), 7.22 (dd, 1H, J = 9.2, 2.4 Hz, ArH), 7.33 (d, 1H, J = 9.2 Hz, ArH), 7.48 (dd ≈ t, 2H, J = 8.0 Hz, ArH), 7.62 (dd ≈ t, 1H, J = 7.6 Hz, ArH), 7.88 (d, 2H, J = 7.6 Hz, ArH), 8.02 (s, 1H, C4–H of coumarin). 13C NMR (CDCl3, 100 MHz): δC = 14.7, 64.3, 111.3, 117.9, 118.5, 122.2, 127.2, 128.6, 129.6, 133.8, 136.3, 145.3, 149.2, 155.8, 158.8, 191.9. MS (ESI): m/z = 317 [M+Na]+. Anal. Calcd. for C18H14O4: C, 73.46; H, 4.79%; Found: C, 73.48; H, 4.74%.
3-Benzoyl-6-chloro-2H-chromen-2-one (4f)
Using the general procedure, starting from 300 mg (1.92 mmol) of 5-chloro-2-hydroxybenzaldehyde 1f, 1.3 mL (9.60 mmol) of triethyl, DMAP (23 mg, 10 mol%) and phenylpropiolyl chloride 2a [phenylpropiolic acid (336 mg, 2.30 mmol)/SOCl2/reflux/4 h], 310 mg of the title compound 4f was isolated as a white solid. Reaction time: 10 h. Eluent: EA/PE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 v/v). Yield: 57%. Mp: 160 °C (lit.39 162–164 °C). IR (KBr): 1655, 1719, 3071 cm−1. 1H NMR (CDCl3, 400 MHz): δH = 7.36 (d, 1H, J = 9.2 Hz, ArH), 7.49 (dd ≈ t, 2H, J = 7.6 Hz, ArH), 7.58–7.65 (m, 3H, ArH), 7.87 (d, 2H, J = 8.0 Hz, ArH), 7.98 (s, 1H, C4–H of coumarin). 13C NMR (CDCl3, 100 MHz): δC = 118.7, 119.1, 127.0, 128.2, 128.7, 129.6, 130.3, 133.5, 134.1, 135.9, 143.8, 153.1, 157.8, 191.2. HRMS (ESI): calcd for C16H9ClO3Na [M+Na]+ 307.0138, found 307.0174.
19 v/v). Yield: 57%. Mp: 160 °C (lit.39 162–164 °C). IR (KBr): 1655, 1719, 3071 cm−1. 1H NMR (CDCl3, 400 MHz): δH = 7.36 (d, 1H, J = 9.2 Hz, ArH), 7.49 (dd ≈ t, 2H, J = 7.6 Hz, ArH), 7.58–7.65 (m, 3H, ArH), 7.87 (d, 2H, J = 8.0 Hz, ArH), 7.98 (s, 1H, C4–H of coumarin). 13C NMR (CDCl3, 100 MHz): δC = 118.7, 119.1, 127.0, 128.2, 128.7, 129.6, 130.3, 133.5, 134.1, 135.9, 143.8, 153.1, 157.8, 191.2. HRMS (ESI): calcd for C16H9ClO3Na [M+Na]+ 307.0138, found 307.0174.
3-Benzoyl-6-bromo-2H-chromen-2-one (4g)
Using the general procedure, starting from 300 mg (1.49 mmol) of 5-bromo-2-hydroxybenzaldehyde 1g, 1.0 mL (7.45 mmol) of triethyl amine, DMAP (18 mg, 10 mol%) and phenylpropiolyl chloride 2a [phenylpropiolic acid (261 mg, 1.79 mmol)/SOCl2/reflux/4 h], 295 mg of the title compound 4g was isolated as a white solid. Reaction time: 9 h. Eluent: EA/PE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 v/v). Yield: 60%. Mp: 175 °C. IR (KBr): 1656, 1717, 3070 cm−1. 1H NMR (CDCl3, 400 MHz): δH = 7.29–7.32 (m, 1H, ArH), 7.47–7.51 (m, 2H, ArH), 7.61–7.66 (m, 1H, ArH), 7.72–7.75 (m, 2H, ArH), 7.87 (dd, 2H, J = 8.8, 1.2 Hz, ArH), 7.98 (s, 1H, C4–H of coumarin). 13C NMR (CDCl3, 100 MHz): δC = 117.5, 118.7, 119.6, 128.1, 128.7, 129.6, 131.3, 134.1, 135.9, 136.3, 143.8, 153.5, 157.7, 191.1. MS (ESI): m/z = 351 [M+Na]+, 353 [M+Na+2]+. Anal. Calcd. for C16H9BrO3: C, 58.38; H, 2.76%; Found: C, 58.26; H, 2.67%.
19 v/v). Yield: 60%. Mp: 175 °C. IR (KBr): 1656, 1717, 3070 cm−1. 1H NMR (CDCl3, 400 MHz): δH = 7.29–7.32 (m, 1H, ArH), 7.47–7.51 (m, 2H, ArH), 7.61–7.66 (m, 1H, ArH), 7.72–7.75 (m, 2H, ArH), 7.87 (dd, 2H, J = 8.8, 1.2 Hz, ArH), 7.98 (s, 1H, C4–H of coumarin). 13C NMR (CDCl3, 100 MHz): δC = 117.5, 118.7, 119.6, 128.1, 128.7, 129.6, 131.3, 134.1, 135.9, 136.3, 143.8, 153.5, 157.7, 191.1. MS (ESI): m/z = 351 [M+Na]+, 353 [M+Na+2]+. Anal. Calcd. for C16H9BrO3: C, 58.38; H, 2.76%; Found: C, 58.26; H, 2.67%.
3-Benzoyl-5-methyl-2H-chromen-2-one (4h)
Using the general procedure, starting from 300 mg (2.20 mmol) of 2-hydroxy-6-methylbenzaldehyde 1h, 1.5 mL (11.00 mmol) of triethyl amine, DMAP (27 mg, 10 mol%) and phenylpropiolyl chloride 2a [phenylpropiolic acid (385 mg, 2.64 mmol)/SOCl2/reflux/4 h], 395 mg of the title compound 4h was isolated as a white solid. Reaction time: 9 h. Eluent: EA/PE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 v/v). Yield: 68%. Mp: 136 °C. IR (KBr): 1661, 1716, 3061 cm−1. 1H NMR (CDCl3, 400 MHz): δH = 2.57 (s, 3H, CH3), 7.17 (d, 1H, J = 7.2 Hz, ArH), 7.23 (d, 1H, J = 9.6 Hz, ArH), 7.47–7.54 (m, 3H, ArH), 7.62 (dd ≈ t, 1H, J = 7.6 Hz, ArH), 7.87–7.90 (m, 2H, ArH), 8.32 (s, 1H, C4–H of coumarin). 13C NMR (CDCl3, 100 MHz): δC = 18.4, 114.9, 126.2, 126.3, 128.6, 129.6, 133.5, 133.7, 133.8, 137.9, 142.7, 145.8, 155.4, 158.5, 192.1. MS (ESI): m/z = 287 [M+Na]+. Anal. Calcd. for C17H12O3: C, 77.26; H, 4.58%; Found: C, 77.17; H, 4.63%.
19 v/v). Yield: 68%. Mp: 136 °C. IR (KBr): 1661, 1716, 3061 cm−1. 1H NMR (CDCl3, 400 MHz): δH = 2.57 (s, 3H, CH3), 7.17 (d, 1H, J = 7.2 Hz, ArH), 7.23 (d, 1H, J = 9.6 Hz, ArH), 7.47–7.54 (m, 3H, ArH), 7.62 (dd ≈ t, 1H, J = 7.6 Hz, ArH), 7.87–7.90 (m, 2H, ArH), 8.32 (s, 1H, C4–H of coumarin). 13C NMR (CDCl3, 100 MHz): δC = 18.4, 114.9, 126.2, 126.3, 128.6, 129.6, 133.5, 133.7, 133.8, 137.9, 142.7, 145.8, 155.4, 158.5, 192.1. MS (ESI): m/z = 287 [M+Na]+. Anal. Calcd. for C17H12O3: C, 77.26; H, 4.58%; Found: C, 77.17; H, 4.63%.
3-Benzoyl-5,7-dimethyl-2H-chromen-2-one (4i)
Using the general procedure, starting from 300 mg (2.00 mmol) of 2-hydroxy-4,6-dimethylbenzaldehyde 1i, 1.4 mL (10 mmol) of triethyl amine, DMAP (25 mg, 10 mol%) and phenylpropiolyl chloride 2a [phenylpropiolic acid (350 mg, 2.40 mmol)/SOCl2/reflux/4 h], 445 mg of the title compound 4i was isolated as a white solid. Reaction time: 8 h. Eluent: EA/PE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 v/v). Yield: 80%. Mp: 196 °C. IR (KBr): 1663, 1714, 3067 cm−1. 1H NMR (CDCl3, 400 MHz): δH = 2.45 (s, 3H, CH3), 2.52 (s, 3H, CH3), 7.00 (s, 1H, ArH), 7.05 (s, 1H, ArH), 7.48 (dd ≈ t, 2H, J = 7.6 Hz, ArH), 7.61 (dd ≈ t, 1H, J = 7.6 Hz, ArH), 7.88 (d, 2H, J = 8.0 Hz, ArH), 8.30 (s, 1H, C4–H of coumarin). 13C NMR (CDCl3, 100 MHz): δC = 18.3, 22.0, 114.9, 115.0, 124.8, 127.6, 128.5, 129.5, 133.6, 136.6, 137.5, 143.1, 145.3, 155.7, 158.8, 192.3. HRMS (ESI): calcd for C18H14O3Na [M+Na]+ 301.0841, found 301.0823.
19 v/v). Yield: 80%. Mp: 196 °C. IR (KBr): 1663, 1714, 3067 cm−1. 1H NMR (CDCl3, 400 MHz): δH = 2.45 (s, 3H, CH3), 2.52 (s, 3H, CH3), 7.00 (s, 1H, ArH), 7.05 (s, 1H, ArH), 7.48 (dd ≈ t, 2H, J = 7.6 Hz, ArH), 7.61 (dd ≈ t, 1H, J = 7.6 Hz, ArH), 7.88 (d, 2H, J = 8.0 Hz, ArH), 8.30 (s, 1H, C4–H of coumarin). 13C NMR (CDCl3, 100 MHz): δC = 18.3, 22.0, 114.9, 115.0, 124.8, 127.6, 128.5, 129.5, 133.6, 136.6, 137.5, 143.1, 145.3, 155.7, 158.8, 192.3. HRMS (ESI): calcd for C18H14O3Na [M+Na]+ 301.0841, found 301.0823.
3-Benzoyl-5,8-dimethyl-2H-chromen-2-one (4j)
Using the general procedure, starting from 300 mg (2.00 mmol) of 2-hydroxy-3,6-dimethylbenzaldehyde 1j, 1.4 mL (10.00 mmol) of triethyl amine, DMAP (25 mg, 10 mol%), and phenylpropiolyl chloride 2a [phenylpropiolic acid (350 mg, 2.40 mmol)/SOCl2/reflux/4 h], 450 mg of the title compound 4j was isolated as a white solid. Reaction time: 8 h. Eluent: EA/PE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 v/v). Yield: 81%. Mp: 164 °C. IR (KBr): 1656, 1711, 3062 cm−1. 1H NMR (CDCl3, 400 MHz): δH = 2.45 (s, 3H, CH3), 2.53 (s, 3H, CH3), 7.07 (d, 1H, J = 7.6 Hz, ArH), 7.37 (d, 1H, J = 7.6 Hz, ArH), 7.48 (dd ≈ t, 2H, J = 7.6 Hz, ArH), 7.61 (dd ≈ t, 1H, J = 7.6 Hz, ArH), 7.90 (d, 2H, J = 7.2 Hz, ArH), 8.31 (s, 1H, C4–H of coumarin). 13C NMR (CDCl3, 100 MHz): δC = 15.3, 18.2, 116.9, 124.1, 125.7, 125.8, 128.5, 129.6, 133.7, 134.8, 135.2, 136.4, 143.2, 153.7, 158.6, 192.3. HRMS (ESI): calcd for C18H14O3Na [M+Na]+ 301.0841, found 301.0868.
19 v/v). Yield: 81%. Mp: 164 °C. IR (KBr): 1656, 1711, 3062 cm−1. 1H NMR (CDCl3, 400 MHz): δH = 2.45 (s, 3H, CH3), 2.53 (s, 3H, CH3), 7.07 (d, 1H, J = 7.6 Hz, ArH), 7.37 (d, 1H, J = 7.6 Hz, ArH), 7.48 (dd ≈ t, 2H, J = 7.6 Hz, ArH), 7.61 (dd ≈ t, 1H, J = 7.6 Hz, ArH), 7.90 (d, 2H, J = 7.2 Hz, ArH), 8.31 (s, 1H, C4–H of coumarin). 13C NMR (CDCl3, 100 MHz): δC = 15.3, 18.2, 116.9, 124.1, 125.7, 125.8, 128.5, 129.6, 133.7, 134.8, 135.2, 136.4, 143.2, 153.7, 158.6, 192.3. HRMS (ESI): calcd for C18H14O3Na [M+Na]+ 301.0841, found 301.0868.
2-Oxo-2H-chromene-3-carbaldehyde (4k)
Using the general procedure, starting from 300 mg (2.45 mmol) of 2-hydroxybenzaldehyde 1a, 1.7 mL (12.25 mmol) of triethyl amine, DMAP (30 mg, 10 mol%) and propiolyl chloride 2b [propiolic acid (206 mg, 2.94 mmol)/SOCl2/reflux/4 h], 132 mg of the title compound 4k was isolated as a white solid. Reaction time: 7 h. Eluent: EA/PE (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 93 v/v). Yield: 31%. Mp: 136 °C. IR (KBr): 1693, 1736, 2863, 3038 cm−1; 1H NMR (CDCl3, 400 MHz): δH = 7.37–7.42 (m, 2H, ArH), 7.69–7.72 (m, 2H, ArH), 8.44 (s, 1H, C4–H of coumarin), 10.26 (s, 1H, CHO). 13C NMR (CDCl3, 100 Hz): δC = 117.2, 118.2, 121.7, 125.3, 130.8, 135.1, 145.7, 155.5, 160.1, 187.8. MS (ESI): m/z = 197 [M+Na]+. Anal. Calcd. for C10H6O3: C, 68.97; H, 3.47%; Found: C, 68.89; H, 3.41%.
93 v/v). Yield: 31%. Mp: 136 °C. IR (KBr): 1693, 1736, 2863, 3038 cm−1; 1H NMR (CDCl3, 400 MHz): δH = 7.37–7.42 (m, 2H, ArH), 7.69–7.72 (m, 2H, ArH), 8.44 (s, 1H, C4–H of coumarin), 10.26 (s, 1H, CHO). 13C NMR (CDCl3, 100 Hz): δC = 117.2, 118.2, 121.7, 125.3, 130.8, 135.1, 145.7, 155.5, 160.1, 187.8. MS (ESI): m/z = 197 [M+Na]+. Anal. Calcd. for C10H6O3: C, 68.97; H, 3.47%; Found: C, 68.89; H, 3.41%.
6-Methyl-2-oxo-2H-chromene-3-carbaldehyde (4l)
Using the general procedure, starting from 300 mg (2.20 mmol) of 2-hydroxy-5-methylbenzaldehyde 1b, 1.5 mL (11.00 mmol) of triethyl amine, DMAP (27 mg, 10 mol%) and propiolyl chloride 2b [propiolic acid (185 mg, 2.64 mmol)/SOCl2/reflux/4 h], 153 mg of the title compound 4l was isolated as a white solid. Reaction time: 5 h. Eluent: EA/PE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 v/v). Yield: 37%. Mp: 112 °C. IR (KBr): 1690, 1730, 2882, 3037 cm−1; 1H NMR (CDCl3, 400 MHz): δH = 2.44 (s, 3H, CH3), 7.30 (d, 1H, J = 8.8 Hz, ArH), 7.47 (s, 1H, ArH), 7.50 (dd, 1H, J = 8.8, 2.0 Hz, ArH), 8.38 (s, 1H, C4–H of coumarin), 10.25 (s, 1H, CHO). 13C NMR (CDCl3, 100 Hz): δC = 20.7, 116.9, 117.9, 121.6, 130.4, 135.3, 136.3, 145.7, 153.7, 160.4, 187.9. MS (ESI): m/z = 211 [M+Na]+. Anal. Calcd. for C11H8O3: C, 70.21; H, 4.29%; Found: C, 70.34; H, 4.26%.
9 v/v). Yield: 37%. Mp: 112 °C. IR (KBr): 1690, 1730, 2882, 3037 cm−1; 1H NMR (CDCl3, 400 MHz): δH = 2.44 (s, 3H, CH3), 7.30 (d, 1H, J = 8.8 Hz, ArH), 7.47 (s, 1H, ArH), 7.50 (dd, 1H, J = 8.8, 2.0 Hz, ArH), 8.38 (s, 1H, C4–H of coumarin), 10.25 (s, 1H, CHO). 13C NMR (CDCl3, 100 Hz): δC = 20.7, 116.9, 117.9, 121.6, 130.4, 135.3, 136.3, 145.7, 153.7, 160.4, 187.9. MS (ESI): m/z = 211 [M+Na]+. Anal. Calcd. for C11H8O3: C, 70.21; H, 4.29%; Found: C, 70.34; H, 4.26%.
6-Methoxy-2-oxo-2H-chromene-3-carbaldehyde (4m)
Using the general procedure, starting from 300 mg (1.97 mmol) of 2-hydroxy-5-methoxybenzaldehyde 1d, 1.4 mL (9.85 mmol) of triethyl amine, DMAP (24 mg, 10 mol%) and propiolyl chloride 2b [propiolic acid (165 mg, 2.36 mmol)/SOCl2/reflux/4 h], 213 mg of the title compound 4m was isolated as a white solid. Reaction time: 3 h. Eluent: EA/PE (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 v/v). Yield: 53%. Mp: 118 °C. IR (KBr): 1683, 1734, 2883, 3069 cm−1; 1H NMR (CDCl3, 400 MHz): δH = 3.85 (s, 3H, OCH3), 7.19 (d, 1H, J = 2.8 Hz, ArH), 7.23 (dd, 1H, J = 8.8, 2.8 Hz, ArH), 7.38 (d, 1H, J = 8.8 Hz, ArH), 8.42 (s, 1H, C4–H of coumarin), 10.32 (s, 1H, CHO). 13C NMR (CDCl3, 100 Hz): δC = 55.8, 112.1, 120.6, 122.3, 127.2, 128.6, 145.8, 151.2, 157.3, 161.8, 187.7. MS (ESI): m/z = 227 [M+Na]+. Anal. Calcd. for C11H8O4: C, 64.71; H, 3.95%; Found: C, 64.63; H, 4.01%.
17 v/v). Yield: 53%. Mp: 118 °C. IR (KBr): 1683, 1734, 2883, 3069 cm−1; 1H NMR (CDCl3, 400 MHz): δH = 3.85 (s, 3H, OCH3), 7.19 (d, 1H, J = 2.8 Hz, ArH), 7.23 (dd, 1H, J = 8.8, 2.8 Hz, ArH), 7.38 (d, 1H, J = 8.8 Hz, ArH), 8.42 (s, 1H, C4–H of coumarin), 10.32 (s, 1H, CHO). 13C NMR (CDCl3, 100 Hz): δC = 55.8, 112.1, 120.6, 122.3, 127.2, 128.6, 145.8, 151.2, 157.3, 161.8, 187.7. MS (ESI): m/z = 227 [M+Na]+. Anal. Calcd. for C11H8O4: C, 64.71; H, 3.95%; Found: C, 64.63; H, 4.01%.
2-Benzoyl-3H-benzo[f]chromen-3-one (7)
Using the general procedure, starting from 300 mg (1.74 mmol) of 2-hydroxy-1-naphthaldehyde 6, 1.2 mL (8.70 mmol) of triethyl amine, DMAP (21 mg, 10 mol%) and phenylpropiolyl chloride 2a [phenylpropiolic acid (305 mg, 2.09 mmol)/SOCl2/reflux/4 h], 434 mg of the title compound 7 was isolated as a yellow solid. Reaction time: 9 h. Eluent: EA/PE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 v/v). Yield: 83%. Mp: 214–215 °C (lit.39 216–217 °C). IR (KBr): 1652, 1724, 3057 cm−1. 1H NMR (CDCl3, 400 MHz): δH = 7.48–7.53 (m, 3H, ArH), 7.60–7.65 (m, 2H, ArH), 7.73 (dd ≈ t, 1H, J = 7.6 Hz, ArH), 7.94 (dd ≈ t, 3H, J = 8.0 Hz, ArH), 8.11 (d, 1H, J = 8.8 Hz, ArH), 8.26 (d, 1H, J = 8.4 Hz, ArH), 8.92 (s, 1H, C4–H of coumarin). 13C NMR (CDCl3, 100 MHz): δC = 112.7, 116.8, 121.5, 125.3, 126.6, 128.6, 129.0, 129.2, 129.4, 129.6, 130.3, 133.7, 135.4, 136.5, 141.9, 155.4, 158.6, 192.1. MS (ESI): m/z = 323 [M+Na]+. Anal. Calcd. for C20H12O3: C, 79.99; H, 4.03%; Found: C, 79.92; H, 4.09%.
19 v/v). Yield: 83%. Mp: 214–215 °C (lit.39 216–217 °C). IR (KBr): 1652, 1724, 3057 cm−1. 1H NMR (CDCl3, 400 MHz): δH = 7.48–7.53 (m, 3H, ArH), 7.60–7.65 (m, 2H, ArH), 7.73 (dd ≈ t, 1H, J = 7.6 Hz, ArH), 7.94 (dd ≈ t, 3H, J = 8.0 Hz, ArH), 8.11 (d, 1H, J = 8.8 Hz, ArH), 8.26 (d, 1H, J = 8.4 Hz, ArH), 8.92 (s, 1H, C4–H of coumarin). 13C NMR (CDCl3, 100 MHz): δC = 112.7, 116.8, 121.5, 125.3, 126.6, 128.6, 129.0, 129.2, 129.4, 129.6, 130.3, 133.7, 135.4, 136.5, 141.9, 155.4, 158.6, 192.1. MS (ESI): m/z = 323 [M+Na]+. Anal. Calcd. for C20H12O3: C, 79.99; H, 4.03%; Found: C, 79.92; H, 4.09%.
2-Formylphenyl 3-chloro-3-phenylacrylate (5a)
Yield: 9.3%. White solid. Mp: 108–109 °C. IR (KBr): 1688, 1748, 2897, 3058 cm−1. 1H NMR (CDCl3, 400 MHz): δH = 6.87 (s, 1H, =CH), 7.30 (d, 1H, J = 8.0 Hz, ArH), 7.40–7.53 (m, 4H, ArH), 7.67 (dd ≈ t, 1H, J = 7.6 Hz, ArH), 7.79 (d, 2H, J = 7.6 Hz, ArH), 7.94 (d, 1H, J = 7.6 Hz, ArH), 10.21 (s, 1H, CHO). MS (ESI): m/z = 309 [M+Na]+, 311 [M+Na+2]+. Anal. Calcd. for C16H11ClO3: C, 67.03; H, 3.87% Found: 67.19; H, 3.83%.2-Acetylphenyl 3-phenylpropiolate (15)
A mixture of phenylpropiolyl chloride 2a [generated from phenylpropiolic acid (385 mg, 2.64 mmol)/SOCl2/reflux/4h/followed by excess SOCl2 removal], 2-hydroxy acetophenone 14 (300 mg, 2.20 mmol) and Bu4NHSO4 (38 mg, 10 mol%) in CH2Cl2 (45 mL) was stirred at room temperature. Then an aqueous solution of K2CO3 (1.52 g, 11.00 mmol) in 5 mL H2O was added to the reaction mixture and was stirred at room temperature for 8 h. It was then extracted with CH2Cl2, (3 × 15 mL), washed with water (2 × 10 mL), brine (10 mL) and dried over anhydrous Na2SO4. The solvent was then removed under reduced pressure to give a crude mass which was chromatographed over silica gel [230–400 mesh] using ethyl acetate-petroleum ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 v/v) as eluent to furnish the compound 15 (524 mg, 90%) as a colourless gummy mass. IR (KBr): 1689, 1729, 2205, 2231 cm−1. 1H NMR (CDCl3, 400 MHz): δH = 2.62 (s, 3H, CH3), 7.23 (dd, 1H, J = 8.4, 0.8 Hz, ArH), 7.36–7.43 (m, 3H, ArH), 7.47–7.52 (m, 1H, ArH), 7.58 (td, 1H, J = 7.6, 1.2 Hz, ArH), 7.64 (dd, 2H, J = 8.0, 1.2 Hz, ArH), 7.86 (dd, 1H, J = 8.0, 1.2 Hz, ArH). 13C NMR (CDCl3, 100 MHz): δC = 29.7, 89.2, 119.1, 123.7, 126.7, 128.7, 130.4, 130.8, 131.2, 133.3, 133.6, 148.2, 152.1, 197.3. MS (ESI): m/z = 287 [M+Na]+. Anal. Calcd. for C17H12O3: C, 77.26; H, 4.58%; Found: C, 77.33; H, 4.51%.
49 v/v) as eluent to furnish the compound 15 (524 mg, 90%) as a colourless gummy mass. IR (KBr): 1689, 1729, 2205, 2231 cm−1. 1H NMR (CDCl3, 400 MHz): δH = 2.62 (s, 3H, CH3), 7.23 (dd, 1H, J = 8.4, 0.8 Hz, ArH), 7.36–7.43 (m, 3H, ArH), 7.47–7.52 (m, 1H, ArH), 7.58 (td, 1H, J = 7.6, 1.2 Hz, ArH), 7.64 (dd, 2H, J = 8.0, 1.2 Hz, ArH), 7.86 (dd, 1H, J = 8.0, 1.2 Hz, ArH). 13C NMR (CDCl3, 100 MHz): δC = 29.7, 89.2, 119.1, 123.7, 126.7, 128.7, 130.4, 130.8, 131.2, 133.3, 133.6, 148.2, 152.1, 197.3. MS (ESI): m/z = 287 [M+Na]+. Anal. Calcd. for C17H12O3: C, 77.26; H, 4.58%; Found: C, 77.33; H, 4.51%.
2-Acetylphenyl 3-chloro-3-phenylacrylate (16)
Yield: 60%. White solid. Mp: 73–74 °C. IR (KBr): 1679, 1742, 3079 cm−1. 1H NMR (CDCl3, 400 MHz): δH = 2.59 (s, 3H, CH3), 6.84 (s, 1H, =CH), 7.21 (d, 1H, J = 8.0 Hz, ArH), 7.34–7.38 (m, 1H, ArH), 7.43–7.50 (m, 3H, ArH), 7.57 (td, 1H, J = 8.0, 1.6 Hz, ArH), 7.77 (dd, 2H, J = 8.0, 1.6 Hz, ArH), 7.84 (dd, 1H, J = 8.0, 1.6 Hz, ArH). MS (ESI): m/z = 323 [M+Na]+, 325 [M+Na+2]+. Anal. Calcd. for. C17H13ClO3: C, 67.89; H, 4.36%; Found: C, 67.73; H, 4.28%.Acknowledgements
We thank the CSIR (New Delhi) and DST (New Delhi) for financial assistance. Two of us are grateful to the UGC (New Delhi, I.A.) and to CSIR (New Delhi, S.S.) for their research fellowships. We also thank the DST (New Delhi) for providing Bruker DPX-400 NMR and Perkin-Elmer L 120-000A IR spectrometers and also a Perkin-Elmer 2400 series II CHN-analyzer under the DST-FIST programme.References
- S. Kumar, A. Saini and J. S. Sandhu, ARKIVOC, 2007,(xv), 18–23 CAS.
- (a) D. E. Zembower, S. Liao, M. T. Flavin, Z. Q. Xu, T. L. Stup, R. W. Buckheit, A. Khilevich, A. A. Mar and A. K. Sheinkman, J. Med. Chem., 1997, 40, 1005–1017 CrossRef CAS; (b) N. A. Al–Masoudi, I. A. Al–Masoudi, I. A. I. Ali, Y. A. Al-Soud, B. Saeed and P. L. Colla, Acta Pharm., 2006, 56, 175–188 Search PubMed.
- (a) J. Nawrot-Modranka, E. Nawrot and K. Graczy, Eur. J. Med. Chem., 2006, 41, 1301–1309 CrossRef CAS; (b) A. M. M. EI-Saghier, M. B. Naili, B. Kh. Rammash, N. A. Saleh and K. M. Kreddan, ARKIVOC, 2007,(xvi), 83–91 Search PubMed.
- B. S. Creaven, D. A. Egan, K. Kavanagh, M. McCann, M. Mahon, A. Noble, B. Thati and M. Walsh, Polyhedron, 2005, 24, 949–957 CrossRef CAS.
- R. Ruszat, S. Wyler, T. Forster, O. Reich, G. S. Christian, C. G. Thomas, T. Sulser and A. Bachmann, Eur. Urol., 2006, 50, 675–683 CrossRef.
- (a) P. Ronad, S. Dharbamalla, R. Hunshal and V. Maddi, Arch. Pharm., 2008, 341, 696–700 CrossRef CAS; (b) C. M. Lin, S. T. Huang, F. W. Lee, H. Sawkuo and M. H. Lin, Bioorg. Med. Chem., 2006, 14, 4402–4409 CrossRef CAS.
- (a) C. J. Wang, Y. J. Hsieh, C. Y. Chu, Y. L. Lin and T. H. Tseng, Cancer Lett., 2002, 183, 163–168 CrossRef CAS; (b) F. H. Dexeus, C. J. Logothetis, A. Sella, K. Fitz, R. Amato, J. M. Reuben and N. Dozier, J. Clin. Oncol., 1990, 8, 325–329 CAS.
- M. A. Bhat, N. Siddiqui and S. A. Khan, Indian J. Pharm. Sci., 2006, 68, 120–124 CrossRef CAS.
- Y. K. Tyagi, A. Kumar, H. G. Raj, P. Vohra, G. Gupta, R. Kumari, P. Kumar and R. K. Gupta, Eur. J. Med. Chem., 2005, 40, 413–420 CrossRef CAS.
- (a) S. Sardari, Y. Mori, K. Horita, R. G. Micetich, S. Nishibe and M. Daneshtalab, Bioorg. Med. Chem., 1999, 7, 1933–1940 CrossRef CAS; (b) H. H. Sayed, A. H. Shamroukh and A. E. Rashad, Acta Pharm., 2006, 56, 231–244 CAS.
- Y. Chen, Q. Zhang, B. Zhang, P. Xia, Y. Xia, Z-Y. Yang, N. Kilgore, C. Wild, S. L. Morris-Natschke and K-H. Lee, Bioorg. Med. Chem., 2004, 12, 6383–6387 CrossRef CAS.
- C. M. Elinos-Baez, F. Leon and E. Santos, Cell Biol. Int., 2005, 29, 703–708 CrossRef CAS.
- G. Cravotto, G. M. Nano, G. Palmisano and S. Tagliapietra, Tetrahedron: Asymmetry, 2001, 12, 707–709 CrossRef CAS.
- G. J. Fan, W. Mar, M. K. Park, E. W. Choi, K. Kim and S. Kim, Bioorg. Med. Chem. Lett., 2001, 11, 2361–2363 CrossRef CAS.
- M. Zahradnik, The Production and Application of Fluorescent Brightening Agents; Wiley & Sons: Chichester, 1992 Search PubMed.
- J. D. Hepworth, C. D. Gabbutt, B. M. Heron, In Comprehensive Heterocyclic Chemistry II; Katritzky, A. R., Rees, C. W., Scriven, E. F. V., ed.; Pergamon Press: Oxford, 1996, 5, 301–350 Search PubMed.
- G. R. Green J. M. Evans A. K. Vong, In Comprehensive Heterocyclic Chemistry II; Katritzky, A. R., Rees, C. W., Scriven, E. F. V., ed.; Pergamon Press: Oxford, 1996, 5, 469–500 Search PubMed.
- R. O'Kennedy, R. D. Thornes, Coumarins: Biology, Applications and Mode of Action; Wiley & Sons: Chichester, 1997 Search PubMed.
- W. C. Meuly, Kirk-Othmer Encyclopedia of Chemical Technology, 3rd ed.; John Wiley & Sons: New York, 1979, 7, 196 Search PubMed.
- (a) H. von Pechmann and C. Duisberg, Ber. Dtsch. Chem. Ges., 1883, 16, 2119–2128 CrossRef; (b) H. von Pechmann and C. Duisberg, Ber. Dtsch. Chem. Ges., 1884, 17, 929–979 CrossRef; (c) E. John and S. Israelstam, J. Org. Chem., 1961, 26, 240–242 CrossRef CAS.
- J. R. Johnson, Org. React., 1942, 1, 210–265 Search PubMed.
- (a) G. Jones, Org. React., 1967, 15, 204–599 CAS; (b) R. Adams and T. E. Bockstahler, J. Am. Chem. Soc., 1952, 74, 5346–5348 CrossRef CAS; (c) S. B. Kadin, J. Org. Chem., 1966, 31, 620–622 CrossRef CAS.
- R. L. Shriner, Org. React., 1942, 1, 1–37 Search PubMed.
- (a) E. Galariniotou, V. Fragos, A. Makri, K. E. Litinas and D. N. Nicolaides, Tetrahedron, 2007, 63, 8298–8304 CrossRef CAS; (b) T. N. Glasnov and I. C. Ivanov, Synth. Commun., 2008, 38, 1579–1588 CrossRef CAS.
- (a) I. Yavari, R. Hekmat-Shoar and A. Zonouki, Tetrahedron Lett., 1998, 39, 2391–2392 CrossRef CAS; (b) K. C. Majumdar, I. Ansary, S. Samanta and B. Roy, Synlett, 2011, 694–698 CrossRef CAS.
- (a) H. Xu, W.-M. Liao and H.-F. Li, Ultrason. Sonochem., 2007, 14, 779–782 CrossRef CAS; (b) S. Puri, B. Kaur, A. Parmar and H. Kumar, Ultrason. Sonochem., 2009, 16, 705–707 CrossRef CAS.
- (a) B. M. Trost, F. D. Toste and K. Greenman, J. Am. Chem. Soc., 2003, 125, 4518–4526 CrossRef CAS; (b) B. M. Trost and F. D. Toste, J. Am. Chem. Soc., 1998, 120, 9074–9075 CrossRef CAS.
- (a) B. M. Trost and F. D. Toste, J. Am. Chem. Soc., 1996, 118, 6305–6306 CrossRef CAS; (b) M. Kotani, K. Yamamoto, J. Oyamada, Y. Fujiwara and T. Kitamura, Synthesis, 2004, 1466–1470 CAS.
- E. Fillion, A. M. Dumas, B. A. Kuropatwa, N. R. Malhotra and T. C. Sitler, J. Org. Chem., 2006, 71, 409–412 CrossRef CAS.
- L. L. Woods and J. Sapp, J. Org. Chem., 1962, 27, 3703–3705 CrossRef CAS.
- S. M. Sethna, N. M. Shah and R. C. Shah, J. Chem. Soc., 1938, 228–232 RSC.
- J. E. T. Corrie, J. Chem. Soc., Perkin Trans. 1, 1990, 7, 2151–2152 RSC.
- A. Shockravi, M. M. Heravi and H. Valizadeh, Phosphorus, Sulfur Silicon Relat. Elem., 2003, 178, 143–147 CrossRef CAS.
- G. Smitha and Ch. Sanjeeva Reddy, Synth. Commun., 2004, 34, 3997–4003 CrossRef CAS.
- B. Rajitha, V. Naveen Kumar, P. Someshwar, J. Venu Madhav, P. Narsimha Reddy and Y. Thirupathi Reddy, ARKIVOC, 2006,(xii), 23–27 CAS.
- A. Robertson, W. F. Sandrock and C. B. Henry, J. Chem. Soc., 1931, 2426–2432 RSC.
- T. S. Li, Z. H. Zhang, F. Yang and C. G. Fu, J. Chem. Res. (S), 1998, 1, 38–39 RSC.
- P. Livant and W. Xu, J. Org. Chem., 1998, 63, 636–641 CrossRef CAS (and references cited therein)..
- H. S. P. Rao and S. Sivakumar, J. Org. Chem., 2006, 71, 8715–8723 CrossRef CAS.
- X. Tang, A. J. Blake, W. Lewis and S. Woodward, Tetrahedron: Asymmetry, 2009, 20, 1881–1891 CrossRef CAS.
- K. C. Majumdar, I. Ansary, B. Sinha and B. Chattopadhyay, Synthesis, 2009, 3593–3602 CrossRef CAS.
- M. Hapke, K. Kral and A. Spannenberg, Synthesis, 2011, 0642–0652 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |