Hierarchical Co3O4 nanosheet@nanowire arrays with enhanced pseudocapacitive performance†
Qiu
Yang‡
,
Zhiyi
Lu‡
,
Zheng
Chang
,
Wei
Zhu
,
Jiaqiang
Sun
,
Junfeng
Liu
*,
Xiaoming
Sun
* and
Xue
Duan
State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, China. E-mail: ljf@mail.buct.edu.cn; sunxm@mail.buct.edu.cn; Fax: +86-10-64425385; Tel: +86-10-64448751
First published on 22nd December 2011
Abstract
Electrodes with hierarchical nanoarchitectures could offer many opportunities for improved performance in energy storage. Herein, we report the synthesis of a hierarchical Co3O4 nanosheet@nanowire arrays (NSWAs) by a facile hydrothermal and annealing treatment. The synthesis of Co3O4 NSWAs, composed of Co3O4 nanowires standing on nanosheet arrays, was achieved in two steps. The formation of nanosheet arrays on nickel foam and the growth of the nanowires around the sheets in the form of nanowires was achieved by studying the morphology evolution process upon reaction time. This novel structure of the material provides a high specific capacitance of 715 F g−1 and remarkable rate capability (at least 69% can be maintained when the current density increased 6 times). Furthermore, the excellent cycling performance (100% after 1000 cycles) is another essential factor in making the Co3O4 hierarchical NSWAs an advanced supercapacitor material.
Introduction
To meet the urgent needs for sustainable and renewable power sources in the modern electronics industry, many efforts have been made in developing advanced energy storage devices. Among the various power source devices, supercapacitors (SCs), also known as electrochemical capacitors, have garnered considerable attention over the past decade due to their high power density, fast charging/discharging rate and long cycle life compared to secondary batteries and conventional dielectric capacitors.1–5 Generally, SCs can be classified into two types depending on the different charge storage mechanisms, traditional electrical double layer capacitors (EDLCs) and pseudocapacitors. The most commonly used materials for EDLCs are carbonaceous materials including active carbon,6graphene7 and carbon nanotubes,8,9 but their low energy density is a major drawback which reaches at most 250 F g−1. In contrast, new pseudocapacitors based on metal oxides/hydroxides, such as NiO,10,11Ni(OH)2,12–14Co3O415,16 and MnO2,17–19 have a higher energy density which stores the charge in a faradic process. Amongst these, cobalt oxides have been demonstrated to be promising electrode materials for pseudocapacitors due to their low environmental footprint, low cost and extremely high theoretical specific capacitance (3560 F g−1).16,20 However, various cobalt oxides previously reported that commonly show capacitances in the range of 90–580 F g−1, suffer from poor rate capability and/or poor capacitance retention upon cycling.21–25 How to improve the capacitive characteristics is an intriguing and challenging goal.It is well accepted that the growth of hierarchical complex nanostructures with multi-dimensions can avoid many drawbacks in the field of energy storage (lithium-ion batteries26–28 and supercapacitors17,29–31). The electrochemical performance can be improved by the hierarchical nanostructural design, integrating the high conductivity of the inner core and large surface areas of the outer branches to permit homogeneous interface/chemical distributions at the nanoscale and fast ion and electron transfers. For example, an ultra-high specific capacity (800 F g−1), which is close to its theoretical value was achieved by coating MnO2 onto SnO2 nanowires grown on a stainless steel substrate.17 Furthermore, a hybrid Co3O4 core and MnO2 shell nanowire array exhibited high capacitance with good cycle performance and remarkable rate capability with respect to pure Co3O4 arrays as a supercapacitor.29 Very recently, a ZnO nanowires@ultrathin nickel hydroxidenitrate nanoflakes hybrid array has demonstrated high-rate energy storage in pseudocapacitor applications.31 In our previous work, we have also observed a NiO nanorod array and a Ni(OH)2 nanowall film with a remarkable pseudocapacitive performance.32,33 Herein, we present a novel three dimensional design for an electrode material, nanosheet@nanowire arrays (NSWAs), which consist of a nickel foam, a Co3O4 nanosheet array and a Co3O4 nanowire array from bottom to top (as shown in Fig. 1). Nickel foam was chosen as the substrate due to its high electronic conductivity and a desirable three dimensional (3D) structure.13,34Co3O4 nanosheets were vertically grown on nickel foam as secondary structures to form a 3D framework at the nanoscale. Finally, Co3O4 nanowires grow epitaxially along a nanosheet lead to a three-ordered structure with a large surface area and high porosity. Thus, by employing this hierarchical design of the electrode, high supercapacitive performance can be expected.
 | ||
| Fig. 1 Schematic image of a novel three-ordered nano-array design. | ||
The hierarchical Co3O4 NSWAs was achieved by a simple two-step synthesis including a facile urea precipitation method with a successive annealing treatment. The length of the Co3O4 nanowires can be simply tuned by adjusting the growth time and a possible growth mechanism is proposed. A specific capacitance of 715 F g−1 by chronopotentiometry at a current density of 5 mA cm−2 was achieved in 1 M KOH aqueous solution. The hierarchical electrode also exhibited a remarkable rate capability with the a specific capacitance of 491 F g−1 (69% capacitance retention) at a current density of 30 mA cm−2, as well as good long-term cycling stability (exactly 100% of its initial specific capacitance after 1000 cycles). Compared with pure Co3O4 nanosheet arrays (NSAs) and nanowire arrays (NWAs), hierarchical Co3O4 NSWAs show an improved rate capability over NSAs (58%) and NWAs (42%), while keeping the high specific capacitance competitive to NWAs by integrating the higher conductivity of the inner nanosheet core and large surface areas of the outer nanowires to permit homogeneous interface/chemical distributions at the nanoscale as well as the fast ion and electron transfer. These results suggest that the hierarchical design of the nanoarrays, which take advantage of the synergistic effect of the nanocomponents at different scales, can achieve a high specific capacitance, good rate characteristic and long-term life for supercapacitor.
Experimental
The preparation procedures of the Co3O4 NSWAs, NSAs and NWAs are described as follows. All of the chemicals were of analytical grade and used without further purification. In a typical procedure, Co(NO3)2·6H2O (0.58 g, 2 mmol), NH4F (0.30 g, 8 mmol) and urea (0.6 g, 10 mmol) were dissolved in 36 mL distilled water and stirred to form a clear solution. Nickel foam (approximately 3 cm × 2 cm) was carefully cleaned with concentrated HCl solution (37 wt%) in an ultrasound bath for 5 min in order to remove the surface NiO layer, and then deionized water and absolute ethanol were used for 5 min each to ensure the surface of the Ni foam was well cleaned. The aqueous solution and the Ni foam were transferred to a 40 mL Teflon-lined stainless-steel autoclave, which was sealed, maintained at 100 °C for 6 h (for synthesis of Co3O4 NSAs), 100 °C 9 h (for synthesis of Co3O4 NSWAs) and 120 °C for 9 h (for synthesis of Co3O4 NWAs), and then allowed to cool to room temperature within 15 min using cooling water. The thin film on the metal substrate was rinsed several times with distilled water and ethanol with the assistance of ultrasonication, and dried at 80 °C for 6 h. Finally the sample was put into a quartz tube and annealed at 523 K for 3 h.X-ray powder diffraction (XRD) patterns were recorded on a X-ray diffractometer (Rigaku D/max 2500) at a scan rate of 10° min−1 in the 2θ range from 15 to 90°. The size and morphology of the samples were characterized using a field-emission scanning electron microscope (SEM) (Zeiss SUPRA 55) operating at 20 kV. High-resolution transmission electron microscopy (HRTEM) measurements were carried out using a JEOL JEM 2100 system operating at 200 kV.
The electrochemical measurements were carried out at 298 K in a three-electrode glass cell connected to an electrochemical workstation. A thin film on the metal substrate (1 cm2) was used as the working electrode. A platinum electrode (1 cm2) and a saturated calomel electrode were used as the counter and reference electrodes, respectively. Freshly prepared 1 mol L−1 KOH aqueous solution was used as the electrolyte. The electrochemical performance of the samples was evaluated on a CHI 660D (Cheng Hua, Shang Hai) workstation for cyclic voltammetry (CV) and chronopotentiometry (CP) tests.
Results and discussion
Structure characterization, growth mechanism and morphology control
Fig. 2A, B and C show SEM images of the as-synthesized Co3O4 NSWAs. It demonstrates that the products exhibit urchin-like structures with multidirectional tertiary nanowires grown on the secondary nanosheets and forming a highly dense film on the major skeleton of a nickel foam. The nanosheets are vertically aligned on the substrate and the total size of the assembled structure is in the range of 6–8 μm. The average diameter and length of the nanowires are estimated to be about 50–100 nm and 0.5–1 μm, respectively. It was expected that this unique structure might have high surface area due to the hierarchical and free standing nanowires and high morphology stability due to the large size of nanosheets, and consequently, could provide high specific capacitance due to easy access of the active species in the redox process to the interface of the electrode. Fig. 2D shows a typical TEM image of an individual nanosheet branched with nanowires. These Co3O4 nanowires are single-crystals grown along the [220] direction as identified by high-resolution TEM (HRTEM) images and fast Fourier transform (FFT) patterns (Fig. 2E, corresponds to the selected region in Fig. 2D). The XRD pattern in Fig. 2F reveals the crystal structure and phase purity of the as-obtained Co3O4 NSWAs. All the diffraction peak positions and their relative intensities are in good accordance with the standard pattern of Co3O4 (JCPDF: 42–1467) while the peaks marked “#” stand for the substrate. Additionally, no other diffraction peak was detected, implying the absence of any impurities in the product. | ||
| Fig. 2 A, B, C) Typical SEM images of NSWAs in different magnifications; D, E) Typical TEM and HRTEM image of NSWAs (inset is FFT); F) XRD pattern of Co3O4 NSWAs. | ||
In order to have a closer inspection of the evolution processes of hierarchical NSWAs, samples at different reaction stages were collected. Fig. 3A–D are the SEM images of the products obtained at various growth times, indicating the morphological and structural transformation from nanosheet array to nanosheet@nanowire array. After the initial 6 h reaction, the nanosheet array appeared and aligned, grown with a high density on the Ni foam. However, after another 1 h (total reaction time of 7 h), nanoparticles of ∼10 nm diameter began to nucleate on the surfaces of the nanosheets. These small nanoparticles exhibited a thorn-like shape, indicating the tendency to anisotropic growth (see inset of Fig. 3B). When the reaction time was further prolonged to 8 h, the earlier formed thorn-like nanoparticles elongated, establishing the rudiments of nanowires while the length (less than 500 nm) is also shorter than the final sample. At last, by further increasing the reaction time to 9 h, perfect hierarchical nanostructures were formed with multidirectional nanowires. As revealed by XRD analysis, the samples are almost in accordance to β–Co(OH)2 (JCPDF: 30–0443, seen Figure S1, ESI†). Finally, the precursor topologically transforms into the Co3O4 NSWA by thermal annealing. In this procedure, we found that the lengths of the Co3O4 sub-nanowires can be tuned by simply adjusting the growth time, which increased with the reaction time (7–9 h). Fig. 3E shows the scheme of growth process of Co3O4 NSWAs.
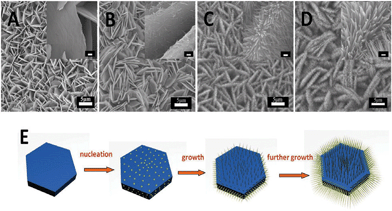 | ||
| Fig. 3 SEM images of the products at various reaction stages by setting the reaction time to A) 6 h, B) 7 h, C) 8 h, D) 9 h. The insets are the corresponding magnified SEM images with scale bars of 200 nm. E) Scheme of the possible formation process of the Co3O4 hierarchically structure. | ||
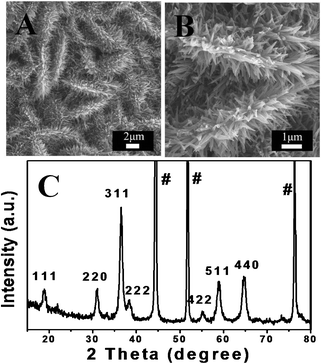
Other morphologies, like Co3O4 NSAs and NWAs were also hydrothermally synthesized by controlling the reaction time or temperature. In Fig. 4A, very dense NSAs, which are vertically aligned on a Ni foam substrate, are fabricated by reducing the reaction time to 6 h. The nanosheets are quite uniform with the average size of the edge and thickness about 6 μm and 100 nm, respectively. In another case, uniform Co3O4 NWAs (5–7 μm in length, 50 nm in diameter) can be achieved when the temperature is increased to 120 °C (Fig. 4B). The phases of the as-prepared samples were examined by the XRD, as shown in Fig. 4C. All of the diffraction peaks indicated that these two samples are the same as the Co3O4 NSWAs. Noteworthy, mass loading of active material per unit area and a tight binding between active material and substrate are two important factors for supercapacitor.33,35NH4F plays an essential role in the hydrothermal process for achieving these two goals. Without NH4F, the as-prepared film can be removed easily by several minutes of ultrasonication and less than 1.5 mg cm−2 of the active material can be retained on substrate. But for the Co3O4 NSWA sample, a high mass loading (7.6 mg cm−2) is achieved and the red color on the film cannot be dislodged after 2 h of ultrasonication, implying a tight binding to the nickel foam substrate. This phenomenon evidently reveals the fact that NH4F is largely associated with adhesion between the substrate and the nano-arrays which is in good accordance with previously reported literature.36
 | ||
| Fig. 4 A, B) SEM images of Co3O4 NSAs and NWAs; C) XRD pattern of NSAs and NWAs. | ||
Function as supercapacitor electrodes
The characteristics of high specific capacitances, high rate capability and high cycling stabilities are all critical to high performance electrochemical supercapacitors.9,12 We thus tested the capacitive performance of the hybrid array electrode in a three-electrode configuration with a Pt counter electrode and a SCE reference electrode in 1 M KOH aqueous electrolyte. Co3O4 NSAs and NWAs were also investigated for comparison. Fig. 5A shows the cyclic voltammograms (CVs) of a Co3O4 NSWA electrode at scan rates of 1–10 mV s−1. The shapes of the CV reveal that the capacitance characteristic is very distinct from that of the electric double-layer capacitance in which the shape is normally close to an ideal rectangular shape. One couple of redox peaks is clearly observed for Co3O4 NSWAs, which corresponds to the reversible reactions of Co3+/Co4+ associated with anions OH−.29 It should be noted that with the sweep rate increased, the shape of the CV changed, the anodic peak potential and cathodic peak potential shift in more anodic and cathodic directions, respectively, and the capacitance inevitably decreased. Fig. 5B shows the results obtained for the Co3O4 NSWAs electrode in the potential range of 0–0.44 V at various current rates (5, 10, 20 and 30 mA cm−2). The specific capacitance was calculated to be 5.44 F cm−2 (715 F g−1) at a low current density (5 mA cm−2), and 3.73 F cm−2 (491 F g−1) at a high current density (30 mA cm−2) and at least 69% can be maintained (Fig. 4D). As a long cycle life is very important in supercapacitors, cycle charge/discharge testing was employed to examine the service life of the Co3O4 NSWA electrode at the current density of 20 mA cm−2 (Fig. 5C). It can be seen that the specific capacitance increases slightly in the beginning 100 cycles and that there is little reduction over the following 1000 cycles. This demonstrates that the charge and discharge processes do not induce significant structural or micro-structural changes in the Co3O4 NSWA electrode, as expected for pseudo-capacitance reactions. Fig. 6 further confirms the high stability of the Co3O4 NSWA electrode. As seen in Fig. 6A and B, the morphology of NSWAs was preserved after over 1000 cycles while the phase was also unchanged in view of Fig. 6C. This result highlights the capability of the 3D pseudocapacitive material based hybrid array electrode to meet the requirements of a long cycle lifetime. These three important tips (high specific capacitance based on mass or volume, excellent rate capability and stable cycling performance) imply that the Co3O4 NSWA is an excellent electrode for supercapacitors.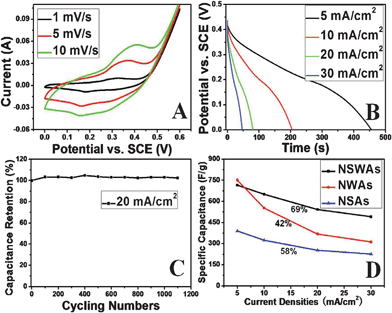 | ||
| Fig. 5 Electrochemical characterization of the Co3O4 NSWAs: (A) CV curves of the Co3O4 NSWAs at different scan rates; (B) galvanostatic discharge curves of the Co3O4 NSWAs at various discharge current densities; (C) average specific capacitance versus cycle number of the Co3O4 NSWAs at a galvanostatic charge and discharge current density of 20 mA cm−2; (D) specific capacitance versus current densities of NSWAs, NSAs and NWAs. | ||
 | ||
| Fig. 6 Typical SEM images (A, B) and XRD pattern (C) of Co3O4 NSWAs after 1000 cycles. | ||
Various morphologies of Co3O4 including nanosheets,25 nanopaticles,37 hollow spheres21 and nanotubes24 have been reported for supercapacitor applications. The capacitance of those previously reported is typically in the range 90–580 F g−1 and our sample is even higher. There some literature does exist with competitive or higher capacitance values, for instance, long Co3O4 Co3O4 nanowire arrays or flower-like structures grown on nickel foam showed a maximum specific capacitance as high as 746 F g−1 and 1952 F g−1, but in one case the cycling stability data (86% maintained after 500 cycles15) is not satisfied, implying it is an irreversible one, and in the other case only a small amount of active material was loaded on the substrate, leading to a low capacitance value based on volume as well as the poor cycling retention (only 78% can be preserved).20
Also in strong contrast to the Co3O4 NSWAs, Co3O4 NSAs and NWAs showed much lower specific capacitance or inferior rate capability as revealed by CV and galvanostatic measurement (Fig. 5D and Fig. S2 and S3, ESI†). The average specific capacitance of Co3O4 NSAs and NWAs is 390 and 751 F g−1at a current density of 5 mA cm−12. While the specific capacitance further decreased to 226 F g−1 (58%) and 315 F g−1 (42%) at a current density of 30 mA cm−2. We can easily conclude that the composite electrodes demonstrate higher capacitance or a better rate capability compared to these two individual components.
Clearly, the synergistic effect of the nanowire and nanosheet is significant to the capacitance performance in the view of the above phenomena. On one hand, the enhanced capacitance and rate capability of the Co3O4 NSWAs compared with the bare Co3O4 NSAs can be easily understood by introducing more active nanowires. Specifically, the Co3O4 nanowires not only provide new OH– hosts but also increase the specific surface area, resulting in higher capacitance. Moreover, the larger degree of porosity will enhance the electrolyte/Co3O4 contact area and the open space between neighboring NWs allow for easy diffusion of the electrolyte which may lead to high power applications when the sample is charged or discharged at high current,38 in other words, better rate capability can be achieved. On the other hand, the reasons for that the capacitive behavior is still better than Co3O4 NWAs are as following: as schematically demonstrated in Fig. 7, Co3O4 nanowires on nanosheets have an increased portion of exposed surfaces compared to pure Co3O4 NWAs (Fig. 3B) by using Co3O4 NSAs as a roughness substrate. Meanwhile, Co3O4 NSAs play an important role in promoting the conductivity of NSWAs, which improve the utilization of Co3O4 nanowires in the electrochemical process, especially in regions far away from the current collector (e.g. tips). As a result, the composite electrode exhibits higher capacitive performance compared to pure Co3O4 NSAs and NWAs.
 | ||
| Fig. 7 Schematic image of the electron transmission in hierarchical structure. | ||
The unique hierarchical porous architecture is another important reason for achieving high capacitance, good rate capability and cycling stability. Firstly, the Ni foam substrate with micro holes and zigzag flow channels, result in an excellent mass transport property and large surface area per unit area of the electrode, as seen in Fig. 1.20 Secondly, each nanosheet, acting as the base of the hierarchical structure, has its own electric contact with the current collector and thus can ensure that all individuals participate in the electrochemical reaction, which enhances the utilization of the active materials and thus the need for binders or conducting additives, which add extra contact resistance or weight, is eliminated. Meanwhile, the nanosheet provides a 3D scaffold to support the Co3O4 nanowires growth, preventing the aggregation during the growth process and electrochemical test. Thus, the nanosheet presents an efficient template for hierarchical hybrid array growth. Thirdly, compared with conventional nanowires arrays, open space between neighboring NWs on the nanosheet is much larger which allows for easy diffusion of the electrolyte into the inner region of the electrode, resulting in a high utilization of materials.38 Fourthly, the synergistic effect of nanosheet and nanowire is critical to high capacitive performance as mentioned above. Additionally, the hierarchical array possesses a favorable morphological and phase stability as seen in Fig. 6, which helps to alleviate the structure or phase damage caused by volume expansion and redox reaction during the cycling process.
Conclusions
In summary, three-ordered Co3O4 NSWAs were synthesized by a facile and cost-effective strategy for supercapacitor applications. The unique architecture shows excellent electrochemical performance (5.44 F cm−2 or 715 F g−1 at 5 mA cm−2, 69% maintained at high current density and 100% retention after more than 1000 cycles), much better than the individual parts (Co3O4 NSAs and NWAs). This work is important in the development of a practical supercapacitor electrode because it offers a material with a combination of ultra-high specific capacitance, high power capacitance, and high stability, together with low cost, simple procedures, and high reproducibility. The electrode design concept can also direct the fabrication of multifunctional hybrid nano- and microstructures, which will be promising for a large spectrum of device applications.Acknowledgements
This work was financially supported by the NSFC, the Beijing Natural Science Foundation, the Foundation for Authors of National Excellent Doctoral Dissertations of P. R. China, the Program for New Century Excellent Talents in Universities, and the 973 Program (No. 2011CBA00503, 2011CB932403).References
- X. Lang, A. Hirata, T. Fujita and M. Chen, Nat. Nanotechnol., 2011, 6, 232–236 CrossRef CAS.
- Y. Zhu, S. Murali, M. D. Stoller, K. Ganesh, W. Cai, P. J. Ferreira, A. Pirkle, R. M. Wallace, K. A. Cychosz and M. Thommes, Science, 2011, 332, 1537–1541 CrossRef CAS.
- C. Liu, F. Li, L. P. Ma and H. M. Cheng, Adv. Energy. Mater., 2010, 22, E28–E62 Search PubMed.
- E. Frackowiak and F. Beguin, Carbon, 2001, 39, 937–950 CrossRef CAS.
- H. G. Zhang, X. D. Yu and P. Braun, Nat. Nanotechnol., 2011, 6, 277–281 CrossRef CAS.
- X. Du, C. Wang, M. Chen, Y. Jiao and J. Wang, J. Phys. Chem. C, 2009, 113, 2643–2646 CrossRef CAS.
- C. Liu, Z. Yu, D. Neff, A. Zhamu and B. Z. Jang, Nano Lett., 2010, 10, 4863–4868 CrossRef CAS.
- M. Kaempgen, C. K. Chan, J. Ma, Y. Cui and G. Gruner, Nano Lett., 2009, 9, 1872–1876 CrossRef CAS.
- K. H. An, W. S. Kim, Y. S. Park, J. M. Moon, D. J. Bae, S. C. Lim, Y. S. Lee and Y. H. Lee, Adv. Funct. Mater., 2001, 11, 387–392 CrossRef CAS.
- J. W. Lang, L. B. Kong, W. J. Wu, Y. C. Luo and L. Kang, Chem. Commun., 2008, 4213–4215 RSC.
- X. Xia, J. Tu, X. Wang, C. Gu and X. Zhao, J. Mater. Chem., 2010, 21, 671–679 Search PubMed.
- H. L. Wang, H. S. Casalongue, Y. Liang and H. J. Dai, J. Am. Chem. Soc., 2010, 132, 7472–7477 CrossRef CAS.
- G. W. Yang, C. L. Xu and H. L. Li, Chem. Commun., 2008, 48, 6537–6539 Search PubMed.
- H. Wang, Y. Liang, T. Mirfakhrai, Z. Chen, H. S. Casalongue and H. Dai, Nano Res., 2011, 4, 729–736 CrossRef CAS.
- Y. Gao, S. Chen, D. Cao, G. Wang and J. Yin, J. Power Sources, 2010, 195, 1757–1760 CrossRef CAS.
- H. Cheng, Z. G. Lu, J. Q. Deng, C. Chung, K. Zhang and Y. Y. Li, Nano Res., 2010, 3, 895–901 Search PubMed.
- J. Yan, E. Khoo, A. Sumboja and P. S. Lee, ACS Nano, 2010, 4, 4247–4255 CrossRef CAS.
- H. Zhang, G. Cao, Z. Wang, Y. Yang, Z. Shi and Z. Gu, Nano Lett., 2008, 8, 2664–2668 CrossRef CAS.
- R. Liu, J. Duay and S. B. Lee, ACS Nano, 2010, 4, 4299–4307 CrossRef CAS.
- X. Qing, S. Liu, K. Huang, K. Lv, Y. Yang, Z. Lu, D. Fang and X. Liang, Electrochim. Acta, 2011, 56, 4985–4991 CrossRef CAS.
- X. H. Xia, J. P. Tu, X. L. Wang, C. D. Gu and X. B. Zhao, Chem. Commun., 2011, 47, 5786–5788 RSC.
- Y. Li, K. Huang, Z. Yao, S. Liu and X. Qing, Electrochim. Acta, 2010, 56, 2140–2144 Search PubMed.
- G. Wang, H. Liu, J. Horvat, B. Wang, S. Qiao, J. Park and H. Ahn, Chem.–Eur. J., 2010, 16, 11020–11027 CrossRef CAS.
- J. Xu, L. Gao, J. Cao, W. Wang and Z. Chen, Electrochim. Acta, 2010, 56, 732–736 CrossRef CAS.
- S. Xiong, C. Yuan, X. Zhang, B. Xi and Y. Qian, Chem.–Eur. J., 2009, 15, 5320–5326 CrossRef CAS.
- W. Zhou, C. Cheng, J. Liu, Y. Y. Tay, J. Jiang, X. Jia, J. Zhang, H. Gong, H. H. Hng and T. Yu, Adv. Funct. Mater., 2011, 21, 2439–2445 CrossRef CAS.
- X. Xiao, L. Wang, D. Wang, X. He, Q. Peng and Y. Li, Nano Res., 2009, 2, 923–930 CrossRef CAS.
- X. Xiao, J. Lu and Y. Li, Nano Res., 2010, 3, 733–737 CrossRef CAS.
- J. Liu, J. Jiang, C. Cheng, H. Li, J. Zhang, H. Gong and H. J. Fan, Adv. Mater., 2011, 23, 2075–2080 CrossRef.
- L. Bao, J. Zang and X. Li, Nano Lett., 2011, 11, 1215–1220 CrossRef CAS.
- J. Liu, C. Cheng, W. Zhou, H. Li and H. J. Fan, Chem. Commun., 2011, 47, 3436–3438 RSC.
- Z. Y. Lu, Z. Chang, W. Zhu and X. M. Sun, Chem. Commun., 2011, 47, 9651–9653 RSC.
- Z. Y. Lu, Z. Chang, J. F. Liu and X. M. Sun, Nano Res., 2011, 4, 658–665 CrossRef CAS.
- Y. Yuan, X. Xia, J. Wu, J. Yang, Y. Chen and S. Guo, Electrochim. Acta, 2010, 56, 2627–2632 Search PubMed.
- J. Huang, V. Meunier, B. G. Sumpter, P. M. Ajayan, J. J. Yoo, K. Balakrishnan, A. Srivastava, M. Conway, A. L. M. Reddy and J. Yu, Nano Lett., 2011, 11, 1423–1427 CrossRef CAS.
- J. Jiang, J. Liu, X. Huang, Y. Li, R. Ding, X. Ji, Y. Hu, Q. Chi and Z. Zhu, Cryst. Growth Des., 2009, 10, 70–75 Search PubMed.
- S. Kandalkar, Mater. Res. Bull., 2010, 46, 48–51 Search PubMed.
- Y. Li, B. Tan and Y. Wu, Nano Lett., 2008, 8, 265–270 CrossRef CAS.
Footnotes |
| † Electronic Supplementary Information (ESI) available: capacitance calculation equation, XRD pattern of the precursor, Electrochemical characterization of the Co3O4 NSAs and NWAs. See DOI: 10.1039/c1ra01008e/ |
| ‡ contributed equally to this work |
| This journal is © The Royal Society of Chemistry 2012 |
