A new outlook on solubility of carbohydrates and sugar alcohols in ionic liquids
Lucinda J. A.
Conceiçao
ab,
Ewa
Bogel-Łukasik
b and
Rafał
Bogel-Łukasik
*a
aLaboratório Nacional de Energia e Geologia, I.P., Unit of Bioenergy, Estrada do Paço do Lumiar 22, 1649-038, Lisboa, Portugal. E-mail: rafal.lukasik@lneg.pt; Fax: +351217163636; Tel: +351210924600 ext. 4224
bDepartamento de Química, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, 2829-516, Caparica, Portugal
First published on 4th January 2012
Abstract
Ionic liquids are innovative media characterised by an easy tunability of physical and chemical properties with the potential for broad usefulness in a wide spectrum of chemical applications. One example of such an application is a complex biorefinery concept leading to the exploitation of the biomass for the production of energy (heat, power, fuel) and value added products. Until now ionic liquids have proven their feasibility to dissolve selectively biomass as a whole as well as individual carbohydrates. This work demonstrates the solubility of carbohydrates and value added products—sugar alcohols—which can be obtained from biomass through the biorefinery concept. The novelty of our investigation includes either solubility studies of sugar alcohols or an involvement of unexplored ionic liquids yet. In this research, a variety of ionic liquids constituted by imidazolium, pyridinium and phosphonium cations was applied. Furthermore, anions of ionic liquids, attractive from a point of view of a broad application, such as: 2-(2-methoxyethoxy)ethylsulfate, hydrogen sulfate, thiocyanate, tricyanomethanide, tetrachloroferrate, perfluorobutane sulfonate and tosylate were investigated. In this work, it was discovered that solubility of carbohydrates and sugar alcohols can exceed even 75 wt% at an easily achievable temperature depending on the choice of the ionic liquid.
Introduction
Carbohydrates and their derivatives are the most abundant organic compounds in nature. Depending on their molecular weight, carbohydrates can be divided between low molecular carbohydrates, such as mono-and disaccharides, and more complex, high molecular weight oligo-and polysaccharides. Carbohydrates are important constituents of animal and human's world because they are among the most important building blocks of cell walls, exoskeletons and regulators of the human body's functions. They are major constituents of the plant kingdom. Due to a general presence of carbohydrates in nature, recently a focus has been dedicated to the processing of carbohydrates to a variety of products. The methodology leading to this transformation, called biorefinery, aims at a maximal exploitation of the carbohydrate-rich raw material to produce energy (fuel, heat, power) and the high value added products (e.g. chemicals).1Sugar alcohols are among high value added products originated in the biorefinery of the carbohydrate biomass. The most popular sugar alcohols are xylitol and mannitol. Xylitol is an artificial sweetener which is adsorbed slowly without increasing a sugar level in the blood that makes it an alternative sugar for diabetes.2 Due to this feature, xylitol is extensively used in the “sugar-free” chewing gums, dietary drinks and foods. Xylitol is also a “toothfriendly” sugar substitute because of a plaque-reducing effect.3 Furthermore, xylitol is recognised as a building block from the biomass biorefinery that serves for the synthesis of many chemicals.4 The second sugar alcohol mentioned, mannitol, is broadly used e.g. in medicine as a drug carrier to the brain and to other organs of the human body.5Mannitol, similarly to xylitol, is used as a sweetener and as a booster of a cooling effect in mint candies and chewing gums.6
From the numerous applications of carbohydrates and their products e.g.sugar alcohols, it can be concluded that the efficient methods of processing of these compounds are crucial for their successful application. One of the initial steps of each process is dissolution of the reagent(s) in the defined solvent. Unfortunately, carbohydrates and their derivatives such as sugar alcohols are soluble in water and rather poorly soluble in most of organic solvents. Water is not the best solvent for the majority of the organic compounds, thus, this obstacle hinders its application in specific processes. Therefore, novel solvents for carbohydrates and organic chemicals are required. Ionic liquids (ILs) seem to be one of the “aurea mediocritas” as they reveal a large solvating capacity and are selective solvents for either various organic chemicals7–9 or carbohydrates.10 ILs are salts which facilitate more sustainable applications in reactions8,11 and separations12 mostly due to their unique properties, such as a high thermal stability13,14 and great solvent power.7,9,15,16 Furthermore, a very low vapour pressure of ILs reduces a risk of exposure that is a clear advantage over the use of classical volatile solvents.17 ILs are compounds composed solely of ions with immeasurable combinations of anions and cations. They possess widely tuneable properties, such as hydrophobicity,18 polarity and miscibility with other solvents.19,20 The comprehensive utilisation of environmentally preferable solvents, such as ionic liquids and renewable feedstocks (e.g. lignocellulosic biomass) enrols in principles of green chemistry.21
The first literature report about the application of ionic liquids, at that time called molten salts, in the dissolution of cellulose is dated in 1934. For the dissolution of cellulose Graenacher used earlier prepared or in situ formed pyridinium ionic liquids.22 Only in the last two decades, the interest in the application of ionic liquids in the dissolution of carbohydrates and biomass has revived.10,23
In this work we report the solubility (solid–liquid and liquid–liquid phase equilibria) of a variety of carbohydrates, such as: xylose, glucose and sucrose, as well as sugar alcohols, such as xylitol and mannitol, in ionic liquids in a broad range of compositions (up to 77.3 wt% of solute) and temperatures (15.78 °C–149.02 °C). This research involves unexplored ionic liquids yet to boost a spectrum of the potentially interesting solvents for carbohydrates and sugar origin compounds. That is why ionic liquids containing anions for the latently appealing application in catalysis, reactions with supercritical fluids, or hydrolysis were studied. Particularly ionic liquids with imidazolium, phosphonium and pyridinium cations and 2-(2-methoxyethoxy)ethylsulfate, hydrogen sulfate, thiocyanate, tricyanomethanide, tetrachloroferrate, perfluorobutane sulfonate, tosylate anions were tested. The chemical structures of ionic liquids, carbohydrates and sugar alcohols studied in this work are presented in Fig. 1 and 2, respectively.
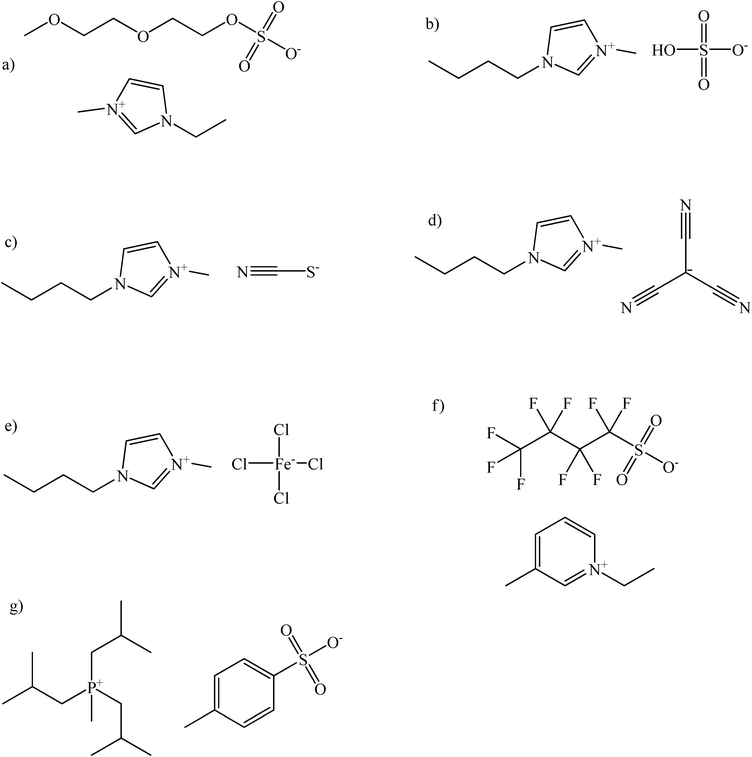 | ||
| Fig. 1 The ionic liquids used in this study: a) 1-ethyl-3-methylimidazolium 2-(2-methoxyethoxy)ethylsulfate; b) 1-butyl-3- methylimidazolium hydrogen sulfate; c) 1-butyl-3-methylimidazolium thiocyanate; d) 1-butyl-3-methylimidazolium tricyanomethanide; e) 1-butyl-3-methylimidazolium tetrachloroferrate (III); f) 1-ethyl-3-methylpyridinium perfluorobutane sulfonate; g) triisobutylmethylphosphonium tosylate. | ||
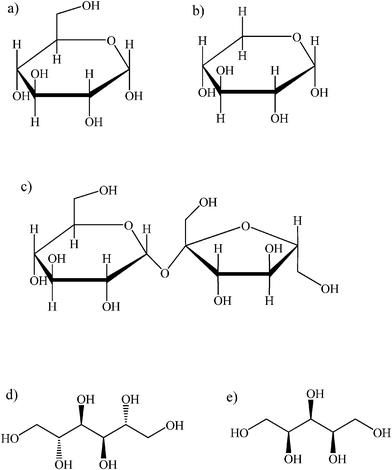 | ||
| Fig. 2 Carbohydrates and sugar alcohols investigated in this work: a) D-(+)-glucose; b) D-(+)-xylose; c) D-(+)-sucrose; d) D-mannitol; e) D-xylitol. | ||
The state of the art in research devoted to solubilities of carbohydrates or sugar alcohols in ILs is characterised by a common ignorance about the water content in both compounds.
The present work highlights the critical aspects of halide and water content in the above mentioned groups of compounds, among which water is a main impurity of the studied solutes. Water is recognised as one of chemicals influencing the most physico-chemical properties of any mixture in measurements of solubility.14,24 In this paper, we sought to provide the water and halide content data for all the investigated solutes and solvents after their treatment under vacuum.
Experimental section
Chemicals
For the purpose of this investigation, the following ionic liquids were selected: 1-ethyl-3-methylimidazolium 2-(2-methoxyethoxy)ethylsulfate [emim][MeOEtOEtSO4] (purity > 98 wt%), 1-butyl-3-methylimidazolium hydrogen sulfate [bmim][HSO4] (purity > 99 wt%), 1-butyl-3-methylimidazolium thiocyanate [bmim][SCN] (purity > 98 wt%), 1-butyl-3-methylimidazolium tricyanomethanide [bmim][C(CN)3] (purity > 98 wt%), 1-butyl-3-methylimidazolium tetrachloroferrate [bmim][FeCl4] (purity > 97 wt%), 1-ethyl-3-methylpyridinium perfluorobutane sulfonate [empy][C4F9SO3] (purity > 98 wt%) and triisobutylmethylphosphonium tosylate [(i-Bu)3MeP][TsO] (purity > 95 wt%). The [emim][MeOEtOEtSO4] ionic liquid was purchased from Solvent Innovation GmbH, Cologne, Germany. The [bmim][HSO4], [bmim][FeCl4] and [(i-Bu)3MeP][TsO] ionic liquids were obtained from Io-Li-Tec GmbH, Heilbronn, Germany. The [bmim][SCN] and [bmim][C(CN)3] ionic liquids were acquired from Solchemar, Lisbon, Portugal and [empy][C4F9SO3] from Merck KGAA, Darmstadt, Germany. The halide content in all ionic liquids was <100 ppm according to suppliers' information. All ionic liquids were pre-treated before the experiments by being degassed, dried and freed from any traces of volatile compounds and water by applying a vacuum (0.1 Pa) at moderate temperature (60 °C) for minimum 24 h prior to use. The anhydrous carbohydrates (D-(+)-glucose, D-(+)-xylose, D-(+)-sucrose) and sugar alcohols (D-mannitol, D-xylitol) were used in this study. Xylitol was bought from Sigma with the stated purity > 99 wt%. D-(+)-glucose (Merck art. 8337), D-(+)-xylose (Merck art. 8689) and D-mannitol (Merck art. 5987) were purchased from Merck USA with the HPLC grade (purity > 99 wt%). D-(+)-sucrose with a purity > 99 wt% was bought from Scharlab S.L., Barcelona, Spain. Carbohydrates and sugar alcohols were kept under vacuum (0.1 Pa) at moderate 60 °C for at least 24 h prior to use.All the studied compounds underwent drying procedures as described previously and the fresh samples of each chemical were used to prepare solutions, always immediately prior to the phase diagram determination.
The water content of each IL as well as solutes was determined by a volumetric Karl–Fischer titration. The Karl–Fischer titrations revealed the water contents as follows: [emim][MeOEtOEtSO4]—2320 ppm; [bmim][HSO4]—5530 ppm; [bmim][SCN]—8170 ppm; [bmim][C(CN)3]—3380 ppm; [bmim][FeCl4]—5030 ppm; [empy][C4F9SO3]—6110 ppm; [(i-Bu)3MeP][TsO]—4890 ppm; (D-(+)-glucose—2460 ppm; D-(+)-xylose—4310 ppm; D-(+)-sucrose—880 ppm; D-mannitol—7840 ppm; D-xylitol—11890 ppm.
Experimental procedure
Solid–liquid and liquid–liquid equilibrium (SLE) phase envelopes of the studied systems were obtained at ambient pressure of 0.1 MPa and at temperature range starting from 15 up to 140 °C using a dynamic (synthetic) method described elsewhere.7 The solutions were prepared in Pyrex conical 5 mL flasks (Supelco) by weighing the pure components with an accuracy of 10−4 g. The mixture of solute (carbohydrate or sugar alcohol) and solvent (ionic liquid) was heated very slowly (at less 2 Kh−1 near the equilibrium temperature) with continuous stirring inside a Pyrex glass cell, placed in a thermostat. The concentration of the solute in the IL studied was in the range of 1.0 to 77.3 wt% of solute. In some particular cases, the concentration was lower either due to the limited solubility or technical problems mostly coming from the high viscosity of the mixture which obstructed observation of the last crystal (SLE) or two phases (LLE) disappearance. The solubility measurements were confirmed by the visual observation of the solution under the microscope (200× magnification) and no sugar crystals' presence in the IL was noticed.The silicon oil was used as a thermostatic fluid. The last crystal disappearance temperatures, detected visually, were measured with a calibrated DOSTMANN electronic P600 thermometer equipped in a Pt 100 probe totally immersed in the thermostating liquid. The uncertainty of the temperature measurements was ±0.1 K and that of the mass fraction did not exceed ±0.1.
Results
Carbohydrates solubilities in ILs
Solubility of glucose, xylose and sucrose in various ILs was investigated. The tabulated data of the solubility of carbohydrates in ionic liquids are compiled in Table 1.| Carbohydrate | Ionic liquid | T/°C | wt (%) | Carbohydrate | Ionic liquid | T/°C | wt (%) | Carbohydrate | Ionic liquid | T/°C | wt (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| D-(+)-glucose | [emim][MeOEtOEtSO4] | 55.18 | 2.4 | D-(+)-xylose | [emim][MeOEtOEtSO4] | 51.85 | 2.1 | (+)-sucrose | [emim][MeOEtOEtSO4] | 70.45 | 2.1 |
| 61.30 | 5.0 | 60.81 | 5.1 | 81.79 | 5.5 | ||||||
| 83.68 | 15.0 | 83.33 | 15.0 | 107.52 | 15.1 | ||||||
| 120.22 | 33.9 | 114.11 | 29.9 | 137.48 | 25.3 | ||||||
| [bmim][HSO4] | 77.75 | 2.0 | [bmim][HSO4] | 76.43 | 2.0 | [bmim][HSO4] | 85.59 | 2.0 | |||
| 80.53 | 4.9 | 83.29 | 11.6 | 89.90 | 5.0 | ||||||
| 82.47 | 7.8 | 89.08 | 20.0 | 95.12 | 10.1 | ||||||
| 86.64 | 15.1 | 96.15 | 30.1 | 99.30 | 15.1 | ||||||
| 89.70 | 20.1 | 98.63 | 35.0 | 106.98 | 25.9 | ||||||
| 93.02 | 25.1 | 104.10 | 45.0 | 115.35 | 35.0 | ||||||
| 101.53 | 35.1 | 107.03 | 50.0 | [bmim][SCN] | 42.05 | 2.0 | |||||
| 108.21 | 45.3 | [bmim][SCN] | 15.78 | 2.0 | 51.86 | 5.2 | |||||
| 111.65 | 50.2 | 35.55 | 10.5 | 66.63 | 10.0 | ||||||
| [bmim][SCN] | 27.62 | 2.0 | 54.58 | 19.9 | 96.05 | 20.0 | |||||
| 41.11 | 10.6 | 105.24 | 40.5 | 126.77 | 30.0 | ||||||
| 64.33 | 20.0 | 114.93 | 45.1 | 138.52 | 35.3 | ||||||
| 108.11 | 40.0 | [bmim][C(CN)3] | 78.42 | 3.1 | [bmim][C(CN)3] | 108.05 | 1.0 | ||||
| 133.02 | 50.0 | 84.63 | 5.0 | 118.23 | 2.1 | ||||||
| [bmim][C(CN)3] | 89.03 | 2.0 | 98.02 | 10.2 | 133.49 | 5.3 | |||||
| 106.31 | 5.2 | 116.89 | 20.0 | [(i-Bu)3MeP][TsO] | 105.82 | 3.1 | |||||
| 120.12 | 10.1 | [empy][C4F9SO3] | 109.69 | 2.0 | 115.58 | 5.3 | |||||
| 131.45 | 15.0 | 121.03 | 5.0 | 128.98 | 10.1 | ||||||
| [empy][C4F9SO3] | 127.98 | 2.0 | 135.35 | 10.5 | |||||||
| 149.02 | 5.0 | [(i-Bu)3MeP][TsO] | 77.01 | 1.8 | |||||||
| [(i-Bu)3MeP][TsO] | 92.14 | 2.0 | 90.54 | 5.1 | |||||||
| 98.10 | 5.0 | 102.84 | 9.9 | ||||||||
| 109.89 | 10.0 | 115.42 | 15.8 | ||||||||
| 119.74 | 15.4 | 122.31 | 20.0 |
The investigation was focused on the solubility of monosaccharides, such as glucose (hexose) and xylose (pentose) in seven different ionic liquids. It was found that the carbohydrates were not soluble in 1-butyl-3-methylimidazolium tetrachloroferrate ionic liquid in the range of the composition and temperature studied. The other imidazolium based ionic liquids exhibited a limited solubility strongly dependent on the ionic liquid as well as on a particular carbohydrate. For example, both monosaccharides were well soluble in 1-butyl-3-methylimidazolium thiocyanate (>47.0 wt% of carbohydrate at 120 °C); however, xylose exhibited a slightly higher solubility than glucose in [bmim][SCN] as depicted in Fig. 3a.
![The solubility of glucose (•) and xylose (○) expressed in wt% in a) [bmim][SCN]; b) [bmim][HSO4]; c) [emim][MeOEtOEtSO4]; d) [bmim][C(CN)3]; e) [empy][C4F9SO3]; f) [(i-Bu)3MeP][TsO] ionic liquid.](/image/article/2012/RA/c1ra01006a/c1ra01006a-f3.gif) | ||
| Fig. 3 The solubility of glucose (•) and xylose (○) expressed in wt% in a) [bmim][SCN]; b) [bmim][HSO4]; c) [emim][MeOEtOEtSO4]; d) [bmim][C(CN)3]; e) [empy][C4F9SO3]; f) [(i-Bu)3MeP][TsO] ionic liquid. | ||
Considering the solubility of both monocarbohydrates in [bmim][HSO4] (Fig. 3b), similar conclusions can be drawn. At a temperature significantly lower than 120 °C, solubilities were above 50.0 wt% of the solute and accordingly glucose or xylose were well soluble in the mentioned IL. In addition, the difference in solubilities was hardly detectable but it can be noticed that xylose exhibits still favourable solubility comparing with glucose.
Xylose and glucose were found to have a slightly lower solubility in [emim][MeOEtOEtSO4] ionic liquid as illustrated in Fig. 3c. In general, both carbohydrates were equally well soluble in this ionic liquid and for a temperature of 120 °C, the solubility was above 30.0 wt% of the monosaccharide.
The results reached for [bmim][C(CN)3] are the opposite to the above mentioned data. The solubility data for the examined monocarbohydrates in [bmim][C(CN)3] show that at 120 °C, more than a 20.0 wt% of xylose and a 10.0 wt% of glucose can be dissolved in this IL (Fig. 3d). A similar segregation in solubilities of glucose and xylose was observed in the pyridinium ionic liquid. In the case of the ionic liquid containing a highly perfluorinated anion, the solubility of xylose at 120 °C was almost 5 times higher than the solubility of glucose (0.8 wt% vs. 4.3 wt%). Regardless of the difference in solubilities, it is important to underline that the solubility of both monocarbohydrates in the tested ionic liquid was much lower than in the aforementioned imidazolium ionic liquids. Even at a significantly higher temperature of 140 °C, the solubility in the pyridinium IL was only moderate (3.7 wt% for glucose and 12.0 wt% for xylose as shown in Fig. 3e).
Contrary to the pyridinium ionic liquid, the triisobutylmethylphosphonium tosylate ionic liquid showed a significantly larger affinity towards the examined carbohydrates. At 120 °C, the solubility of either glucose or xylose was far above 15.0 wt%. Similarly to all the previously mentioned ionic liquids, xylose was found to be more soluble than glucose in [(i-Bu)3MeP][TsO] (Fig. 3f).
The solubility of disaccharides was investigated, for example that of sucrose in four imidazolium and one phosphonium based ionic liquids. In particular, in solubility tests the following ILs were examined: [emim][MeOEtOEtSO4], [bmim][HSO4], [bmim][SCN], [bmim][C(CN)3], [(i-Bu)3MeP][TsO]. The selection of these five ionic liquids was based on results of the solubility of glucose in ILs. The obtained data allow to state that generally sucrose is a less soluble carbohydrate than the corresponding monosaccharide—glucose. The comparison of these two carbohydrates is illustrated in Fig. 4a–e.
![Comparison of the solubility of glucose (•) and sucrose (○) expressed in wt% in a) [bmim][SCN]; b) [bmim][HSO4]; c) [emim][MeOEtOEtSO4]; d) [bmim][C(CN)3]; e) [(i-Bu)3MeP][TsO] ionic liquid.](/image/article/2012/RA/c1ra01006a/c1ra01006a-f4.gif) | ||
| Fig. 4 Comparison of the solubility of glucose (•) and sucrose (○) expressed in wt% in a) [bmim][SCN]; b) [bmim][HSO4]; c) [emim][MeOEtOEtSO4]; d) [bmim][C(CN)3]; e) [(i-Bu)3MeP][TsO] ionic liquid. | ||
After the analysis of the obtained solubility data for disaccharide (sucrose) and monosaccharide (glucose) it can be concluded that sucrose exhibits a noticeably lower solubility than glucose in the corresponding ionic liquids. The largest differences in solubilities of glucose and sucrose were observed in the case of the [bmim][SCN], [bmim][HSO4] and [emim][MeOEtOEtSO4] ionic liquids. The difference surpassed a 15.0 wt% of carbohydrate at temperatures above 120 °C as presented in Fig. 4a–c. For example the solubility of glucose in [bmim][SCN] was 45.0 wt% while sucrose was soluble only in 28.0 wt%. In [bmim][HSO4] and [emim][MeOEtOEtSO4], the solubility of glucose was 60.5 wt% and 34.0 wt%, respectively, while in such ionic liquids sucrose was soluble only in 41.5 wt% and 19.0 wt%, respectively. The solubility of sucrose in [bmim][C(CN)3] was also found to be lower than the solubility of glucose; however, the difference was only slightly smaller and equal to 10.0 wt% at 130 °C (Fig. 4d). The analogous difference in solubilities between sucrose and glucose was observed for [(i-Bu)3MeP][TsO] solvent (Fig. 4e). For this ionic liquid, the solubility of sucrose was still lower than the solubility of glucose at the investigated temperature range, nevertheless, the difference is close to 10.0 wt% at 120 °C as glucose was found to be soluble in the IL in 16.0 wt% and sucrose in 6.5 wt%.
Solubility of sugar alcohols
The obtained solubility data of sugar alcohols in ionic liquids are presented in Table 2.| Sugar alcohol | Ionic liquid | T/°C | wt (%) | Sugar alcohol | Ionic liquid | T/°C | wt (%) |
|---|---|---|---|---|---|---|---|
| a Liquid–liquid equilibria. | |||||||
| D-mannitol | [emim][MeOEtOEtSO4] | 57.07 | 2.2 | D-xylitol | [emim][MeOEtOEtSO4] | 32.57 | 2.0 |
| 67.88 | 5.4 | 51.54 | 10.7 | ||||
| 92.67 | 10.4 | 65.16 | 20.1 | ||||
| 122.37 | 19.9 | 76.14 | 30.6 | ||||
| [bmim][HSO4] | 64.26 | 2.1 | 86.89 | 40.7 | |||
| 79.64 | 4.9 | 89.98 | 60.0 | ||||
| 88.70 | 10.0 | 102.35 | 60.0a | ||||
| 101.55 | 15.1 | [bmim][HSO4] | 52.20 | 2.2 | |||
| 106.14 | 22.8 | 65.92 | 10.0 | ||||
| [bmim][SCN] | 31.94 | 1.8 | 70.44 | 15.4 | |||
| 57.50 | 7.5 | 78.22 | 26.7 | ||||
| 64.73 | 10.1 | 84.04 | 35.2 | ||||
| 77.18 | 12.6 | 90.00 | 50.0 | ||||
| 83.51 | 15.2 | 92.37 | 50.0a | ||||
| 99.38 | 20.1 | [bmim][SCN] | 21.29 | 2.0 | |||
| [bmim][C(CN)3] | 107.76 | 2.0 | 34.54 | 10.1 | |||
| 116.23 | 3.2 | 46.68 | 20.3 | ||||
| 131.60 | 5.1 | 73.15 | 40.9 | ||||
| 138.27 | 7.5 | 86.93 | 60.0 | ||||
| [(i-Bu)3MeP][TsO] | 96.20 | 1.9 | 90.01 | 77.3 | |||
| 101.73 | 4.8 | 100.19 | 77.3a | ||||
| 109.47 | 7.9 | [bmim][C(CN)3] | 60.74 | 2.0 | |||
| 113.83 | 9.4 | 80.39 | 10.2 | ||||
| 126.19 | 14.8 | 90.00 | 20.1 | ||||
| 90.12 | 20.1 | ||||||
| 90.00 | 30.6 | ||||||
| 98.76 | 30.6a | ||||||
| 90.05 | 40.4 | ||||||
| 106.98 | 40.4a | ||||||
| [(i-Bu)3MeP][TsO] | 65.94 | 2.1 | |||||
| 75.13 | 10.0 | ||||||
| 90.00 | 20.0 | ||||||
| 90.57 | 20.0a | ||||||
| 90.00 | 30.5 | ||||||
| 99.46 | 30.5a | ||||||
| 90.04 | 40.0 | ||||||
| 103.64 | 40.0a | ||||||
| 90.08 | 50.1 | ||||||
| 106.54 | 50.1a | ||||||
| 90.12 | 60.1 | ||||||
| 111.00 | 60.1a | ||||||
The solubility of sugar alcohols, such as xylitol and mannitol in ionic liquids was investigated as well. Both sugar alcohols were studied in systems containing the same ionic liquids as used in investigations with sucrose. Following the obtained data presented in Fig. 5a–e it can be undoubtedly stated that xylitol is much more soluble in the investigated ionic liquids than mannitol. In the extreme case, the solubility of mannitol in [bmim][SCN] was lower than that of xylitol by almost 60.0 wt% at 100 °C because mannitol was soluble in only 20.0 wt% while xylitol was in 77.3 wt% (Fig. 5a). The only slightly smaller difference in solubilities were noticed in [bmim][HSO4], [emim][MeOEtOEtSO4], [bmim][C(CN)3] and [(i-Bu)3MeP][TsO], in which at 100 °C the solubility of xylitol was 67.0, 57.0, 31.5 and 34.0 wt%. In this particular set of ILs, mannitol exhibited the solubility of 15.8, 13.0, 1.8 and 4.0 wt%. The interesting seems to be the fact that among all the examined systems only xylitol with the investigated ionic liquids exhibited the solid–liquid phase equilibria, and at temperatures above 90 °C the miscibility gap was observed for the more concentrated solutions.
![The solubility of mannitol (•) and xylitol (○) expressed in wt% in a) [bmim][SCN]; b) [bmim][HSO4]; c) [emim][MeOEtOEtSO4]; d) [bmim][C(CN)3]; e) [(i-Bu)3MeP][TsO] ionic liquid. In graph e) fields correspond to 1: solid (xylitol)–liquid (IL), 2: liquid (xylitol)–liquid (IL) and 3: one liquid phase.](/image/article/2012/RA/c1ra01006a/c1ra01006a-f5.gif) | ||
| Fig. 5 The solubility of mannitol (•) and xylitol (○) expressed in wt% in a) [bmim][SCN]; b) [bmim][HSO4]; c) [emim][MeOEtOEtSO4]; d) [bmim][C(CN)3]; e) [(i-Bu)3MeP][TsO] ionic liquid. In graph e) fields correspond to 1: solid (xylitol)–liquid (IL), 2: liquid (xylitol)–liquid (IL) and 3: one liquid phase. | ||
Discussion
Among large number of publications about ionic liquids over a few decades only a few represent relevant works in relation to carbohydrates or biomass.25 Thus, this field is still in its infancy; however, it is considered that in the next few years the interest in ionic liquids in combination with biomass will be increasing especially in the applicability of ionic liquids in the biomass conversion process. Nevertheless, the major problem in any chemical process is solubility of both compounds; therefore, it is extremely important to examine simple as well as complex systems containing ionic liquids and carbohydrates. As an example of the importance of these kind of research the conversion of carbohydrates to 5-hydroxymethylfurfural can be given. In the recent review about the methodology of conversion of simple monocarbohydrates (pentoses and hexoses) or biomass to the interesting building block (5-HMF) a large progress has been highlighted.26 It can be learnt from this work that the ionic liquid plays a double role in the process. In the first instance, the IL is a solvent for carbohydrate material and in the second instance, works as co-catalyst helping to complex metal halides and dissolve in the carbohydrate-rich matrix. Thus, it is clearly presented that the fundamental research about the solubility of carbohydrates in new ionic liquids is extremely important because allows to design complex processes guiding to the production of the high value products.Among the numerous ionic liquids theoretically available, only limited numbers of them have been used until now in the various applications including the solubility studies.10 In this work, particularly new, never or rarely used ionic liquids were investigated in order to boost a spectrum of the potentially interesting solvents for carbohydrates and sugar origin compounds. For this reason, ionic liquids containing anions for the latently appealing application in catalysis ([FeCl4] ionic liquid), reactions with supercritical fluids (perfluorinated ionic liquid), or hydrolysis (acidic [HSO4] ionic liquid), etc. were studied. However, as this is a pioneer application of the aforementioned ILs, it is difficult to draw clear conclusions coming from their application in solubilities of sugars and sugar alcohols. Additionally, in order to show a complexity of the analysis of the solubility data, it is crucial to underline that the collected data for carbohydrates are incomparable with the literature available but unfortunately mostly unreliable data. As the example of such incomplete data might serve the fact that results of solubility are often not accompanied by the information related to impurities present either in solute or in the solvent. Such impurities are known to affect strongly the solubility. One of the common impurities in case of the ionic liquid is water. Nevertheless, the elemental data about the water content in both ionic liquid and carbohydrate are permanently omitted (see tabulated data in the review10) that makes data on solubilities incomparable or might lead to false conclusions and statements.
Carbohydrate dissolutions
Until now, the solubilities of glucose as cellulose have been studied in series of ionic liquids at different conditions. Contrary to glucose, xylose was studied extremely rarely and only three single solubility data were reported for this carbohydrate.10,27,28The results presented here show that in all the studied ionic liquids except [bmim][FeCl4], both, glucose and xylose exhibited moderate to good solubility. In general, xylose was more soluble than glucose and the difference in solubilities reached 11.0 wt% of carbohydrate at 120 °C in [bmim][C(CN)3] (Fig. 3d). The exception from this (Fig. 3a–c) is almost equal solubility of both carbohydrates found in case of [bmim][SCN], [bmim][HSO4] and [emim][MeOEtOEtSO4]. The highest solubility of both carbohydrates was observed in [bmim][HSO4] at 120 °C as it was about 50.0 wt% of solute. The only slightly lower solubility of carbohydrates was noticed in the [bmim][SCN] ionic liquid, in which glucose and xylose were soluble in more than 47.0 wt% at 120 °C. The fact that both carbohydrates have a high solubility in either [bmim][HSO4] or [bmim][SCN] ionic liquid can be explained by a large affinity of anions towards monosaccharides. In particular, it can be caused by: 1) a highly acidic effect of [HSO4] which acts as strong hydrogen bond donor, 2) [SCN] responsibility for the strong hydrogen bond accepting23 interactions due to a high polarizability of the anion and the specific structure stabilised by the resonance. The solubility of carbohydrates in [emim][MeOEtOEtSO4] was found to be lower than in the two aforementioned ionic liquids. Such solubility obtained might be an effect of the shorter alkyl chain in the cation comparing with the previously mentioned ionic liquids (ethylvs.butyl) and undoubtedly it can be affected by the anion. Considering the structure of [HSO4] and [MeOEtOEtSO4] it can be expected that the solubility of both should not be significantly different due to the presence of the sulfate group. Nevertheless, the results obtained showed at least a 30% lower solubility in the case of the [MeOEtOEtSO4] anion at 120 °C. This difference might be explained by substitution of hydrogen by the methoxyethoxy group, thus, the anion from the proton donor as in the case of [HSO4] became the proton acceptor due to the lack of the acidic hydrogen and the presence of several oxygen atoms. All the alterations in the anion led apparently to weaker interactions between [MeOEtOEtSO4] ionic liquid and carbohydrate which could affect the lower solubility of carbohydrates in 1-ethyl-3-methylimidazolium 2-(2-methoxyethoxy)ethylsulfate than in 1-butyl-3-methylimidazolium hydrogen sulfate. Analysing different studied imidazolium based ionic liquids it can be stated that a structural similarity of [C(CN)3] and [SCN] allows to expect that tricyanomethanide ionic liquid would be the worst solvent for carbohydrates. It is probably due to the presence of three cyanomethanide groups which makes the structure less polarized, thus, the ability to form hydrogen bonds with the carbohydrates skeleton could be reduced. Furthermore, comparing the obtained data for monocyano anion ([SCN]) and tricyano anion [C(CN)3] with the solubility data of glucose in dicyanamide ionic liquids published in the literature,29,30 it can be concluded that solubility of glucose in [N(CN)2] IL is between solubility of carbohydrate in ionic liquids containing mono-([SCN]) and tricyano-([C(CN)3]) anion. This means that in general the monocyano anion is a stronger hydrogen bond acceptor than a more complex multicyano anion, thus, the solubility of carbohydrates can be higher in [SCN] than in [N(CN)2] or even in [C(CN)3] ionic liquid. The other important issue considered in this study is an effect of the heteroatom located in the anion. Due to a similar Pauling electronegativity of sulphur, nitrogen or carbon, a central heteroatom could not be rather an influencing factor guiding to such drastic differences in solubilities.
The obtained data for other studied ionic liquids demonstrate that both pyridinium and phosphonium ionic liquids are rather poor solvents for both monosaccharides. The equally poor solubilities of other carbohydrates such as cellulose in both types of ILs were reported earlier;31–33 however, the strong influence of anions (perfluorinated and tosylate) has not been examined yet. Nevertheless, basing on the literature data for pyridinium and phosphonium ionic liquids it can be noticed that the obtained results fit well in the expected range of solubility for these ionic liquids regardless of a type of the studied anions.
Sucrose was selected for this study as representative of disaccharides. Data presented here illustrated that sucrose is less soluble in ionic liquids than the corresponding monosaccharide–glucose. The differences were significant and reached at least 10.0 or even 15.0 wt% at 120 °C in all the studied ionic liquids (Fig. 4a–e). Nonetheless, it is important to realise that sucrose is disaccharide constituted by the two hexoses (glucose and fructose). Furthermore, as the solubility data are presented in weight fraction, thus, it can be appropriate and interesting to consider sucrose as the two hexose units and then compare the solubility data. In such a case it can be evidently seen that sucrose (two hexose units) is pretty much equally soluble to glucose in the same ionic liquid. As an example it can be given the comparison of the solubility of glucose and sucrose in [bmim][SCN] presented in Fig. 6.
![The solubility of sucrose (○) and glucose (•) and sucrose—counted as the two hexose units—(□) expressed in wt% in [bmim][SCN] ionic liquid.](/image/article/2012/RA/c1ra01006a/c1ra01006a-f6.gif) | ||
| Fig. 6 The solubility of sucrose (○) and glucose (•) and sucrose—counted as the two hexose units—(□) expressed in wt% in [bmim][SCN] ionic liquid. | ||
A shift in the solubilities between sucrose and sucrose as the two hexose units expressed in wt% is clearly visible, although, sucrose is still less soluble than glucose in the tested ionic liquids. Such a small difference between solubilities of glucose and sucrose presented as double glucose might be caused by an entropic effect. A large entropic effect guided to the negative deviation from the ideal solubility that might explain why a smaller glucose is more soluble than sucrose (even when is considered as the two hexose molecules for better reflection in wt% in Fig. 6) in the [SCN] ionic liquid.34
Solubility of sugar alcohols
To the best of our knowledge, the data of solubility of sugar alcohols in ILs presented in this work are the first published in this field, except the scarce date concerning only solubility of xylitol in a few ionic liquids.35 Payne and Kerton found that xylitol was insoluble in tetradecyl(trihexyl)phosphonium dodecylbenzenesulfonate. They also showed that xylitol in the concentration of 20 mg g−1 at 100 °C was soluble in 1-butyl-3-methylimidazolium chloride, 1-butyl-3-methylimidazolium hexafluorophosphate as well as in the two eutectic mixtures of choline chloride with oxalic or citric acid.Data covering xylitol solubilities presented here are more comprehensive due to wider range of the concentration of xylitol and broader temperature scale examined. Additionally, the other popular sugar alcohol, mannitol, was studied here and the obtained data were compared with those gained for xylitol. Analysing the solubility results it can be concluded that mannitol is significantly less soluble in the scanned ionic liquids than xylitol. The responsibility for such a difference can have a considerable difference in the melting points and enthalpies of melting of both sugar alcohols. This might lead to tremendous dissimilarities in their solubilities in ILs. The melting point and enthalpy of melting of mannitol is 165.95 °C and 56.1 kJ mol−1,36 while xylitol exhibits the phase transition at only 92.55 °C with a noticeably lower enthalpy of melting at the level of 37.4 kJ mol−1.36 The consequences of the relatively low melting point of xylitol and lower enthalpy of the phase transition might cause such a difference in solubilities and guide to the existence of (liquid–liquid phase equilibria) for systems containing the mentioned sugar alcohol and the IL for solutions richer in the sugar alcohol. Such an effect is observed as miscibility gap presented in Fig. 5 for temperatures higher than 90 °C.
Considering the obtained results it can be stated that xylitol is soluble in triisobutylmethylphosphonium tosylate ionic liquid contrary to the tetradecyl(trihexyl)phosphonium dodecylbenzenesulfonate ionic liquid reported.35 This dissimilarity confirms that even for the same type of ionic liquid (phosphonium), the alkyl chain substituents as well as anion are important and play a significant role in affecting the solubility.
Another interesting observation is a comparison of the solubility of sugar alcohols and the corresponding carbohydrates in ionic liquids. In the case of the solubility of pentose and xylitol, sugar alcohol was found to be always more soluble in the studied ionic liquids due to a low melting point of xylitol. The only difference was observed for a very low concentration of solute (<5.0 wt%) in 1-butyl-3-methylimidazolium thiocyanate for which solubility of xylose was insignificantly higher as presented in Fig. 7.
![Comparison of the solubility of xylose (○) and xylitol (•) expressed in wt% in [bmim][SCN] ionic liquid.](/image/article/2012/RA/c1ra01006a/c1ra01006a-f7.gif) | ||
| Fig. 7 Comparison of the solubility of xylose (○) and xylitol (•) expressed in wt% in [bmim][SCN] ionic liquid. | ||
The comparison of solubility results of mannitol and glucose in the IL shows that contrary to the pair of xylitol and xylose, glucose is much more soluble than mannitol. One of the explanations of this effect might be a difference in the melting points between mannitol (165.95 °C)36 and glucose (Tm = 147 °C)37 which is though moderate but accompanied by a high enthalpy of melting for mannitol might lead to such a lower solubility of mannitol in the ionic liquid in comparison with glucose.
Conclusions
The use of ionic liquids in the specific application is important because among the solvents they are ones of the most selective. Moreover, as organic solvents, they allow performing reactions with organic chemicals contrary to water as a medium. Ionic liquids are ones among not many groups that are easily tailored; however, still very narrow selection of the ILs has been tested. That is why a boost in the number of the studied ionic liquids is a key issue. Furthermore, a careful analysis of the investigated compounds towards i.e. impurities is fundamental as it is the only way to make data reliable as well as useful for others. The expansion of the application of the ILs in new fields such as carbohydrates and chiefly in sugar alcohols is vital because the biorefinery concept as well as green and sustainable chemistry has become the needs of the current and future processes. The obtained results confirm the aforementioned conclusions because by tailoring the ionic liquid, the solubility of carbohydrate and sugar alcohol can be easily modified from the extremely high solubility (77.3 wt% of xylitol in [bmim][SCN] at 100 °C) to the negligible one (1.8 wt% of xylose in [empy][C4F9SO3] at the same conditions).Acknowledgements
This work was supported by the Fundação para a Ciência e a Tecnologia (FCT, Portugal) through grants SFRH/BPD/26356/2006, PEst-C/EQB/LA0006/2011, and by the European Commission for the financial support of the PROETHANOL2G Project (FP7-ENERGY-2009-BRAZIL; Grant agreement: 251151).References
- www.siadeb.org.
- K. Lang, Klin. Wochenschr., 1971, 49, 233–245 CrossRef CAS
.
- J. M. Tanzer, Int. Denat. J., 1995, 45, 65–76 CAS
.
-
US Department of Energy Report; ed. T. Werpy and G. Petersen; Top Value Added Chemicals From Biomass. 2004 Search PubMed
.
- M. Ikeda, A. K. Bhattacharjee, T. Kondoh, T. Nagashima and N. Tamaki, Biochem. Biophys. Res. Commun., 2002, 291, 669–674 CrossRef CAS
.
-
M. W. Kearsley and R. C. Deis, Ames: Oxford, 2006; 249–261
.
- U. Domańska, E. Bogel-Łukasik and R. Bogel-Łukasik, J. Phys. Chem. B, 2003, 107, 1858–1863 CrossRef
.
- E. Bogel-Łukasik, S. Santos, R. Bogel-Łukasik and M. Nunes da Ponte, J. Supercrit. Fluids, 2010, 54, 210–217 CrossRef
.
- C. A. S. Trindade, Z. P. Visak, R. Bogel-Łukasik, E. Bogel-Łukasik and M. Nunes da Ponte, Ind. Eng. Chem. Res., 2010, 49, 4850–4857 CrossRef CAS
.
- M. E. Zakrzewska, E. Bogel-Łukasik and R. Bogel-Łukasik, Energ. Fuel., 2010, 24, 737–745 CrossRef CAS
.
- J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508–3576 CrossRef CAS
.
- C. I. Melo, R. Bogel-Łukasik and E. Bogel-Łukasik, J. Supercrit. Fluids, 2012, 61, 191–198 CrossRef CAS
.
- H. L. Ngo, K. LeCompte, L. Hargens and A. B. McEwen, Thermochim. Acta, 2000, 357, 97–102 CrossRef
.
- U. Domańska and R. Bogel-Łukasik, J. Phys. Chem. B, 2005, 109, 12124–12132 CrossRef
.
- J. M. Crosthwaite, S. N. V. K. Aki, E. J. Maginn and J. F. Brennecke, J. Phys. Chem. B, 2004, 108, 5113–5119 CrossRef CAS
.
-
U. Domańska, A. Marciniak and R. Bogel-Łukasik, in: Ionic Liquids IIIA: Fundamentals, Progress, Challenges, and Opportunities Properties and Structure, ed. R. D. Rogers and K. R. Seddon, ACS Symposium Series 901, American Chemical Society, Washington D.C., 2005, 256–269 Search PubMed
.
- Y. U. Paulechka, G. J. Kabo, A. V. Blokhin, O. A. Vydrov, J. W. Magee and M. Frenkel, J. Chem. Eng. Data, 2003, 48, 457–462 CrossRef CAS
.
- J. G. Huddleston, A. E. Visser, W. M. Reichert, H. D. Willauer, G. A. Broker and R. D. Rogers, Green Chem., 2001, 3, 156–164 RSC
.
- C. Chiappe and D. Pieraccini, J. Phys. Org. Chem., 2005, 18, 275–297 CrossRef CAS
.
- U. Domańska and E. Bogel-Łukasik, Ind. Eng. Chem. Res., 2003, 42, 6986–6992 CrossRef
.
-
P. Anastas, and J. Warner, ed. Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998 Search PubMed
.
-
C. Greanacher, US Patent 1934/1943176 Search PubMed
.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D.; Rogers, J. Am. Chem. Soc., 2002, 124, 4974–4975 CrossRef CAS
.
- U. Domańska, E. Bogel-Łukasik and R. Bogel-Łukasik, Chem.–Eur. J., 2003, 9, 3033–3041 CrossRef
.
- The number of records for “ionic liquid*” as TOPIC is 48851 while for “biomass*” OR “lignocellulos*” AND “ionic liquid*” as TOPIC is 398 ISI Web of Knowledge v. 5.3 on 15th of November of 2011.
- M. E. Zakrzewska, E. Bogel-Łukasik and R. Bogel-Łukasik, Chem. Rev., 2011, 111, 397–417 CrossRef CAS
.
-
S. K. Spear, A. E. Visser and R. D. Rogers, in: Proceedings of the Sugar Processing Research Institute (SPRI); 2002 Conference on Sugar
Processing Research, New Orleans, LA, 2002; 336–340 Search PubMed
.
-
L. Moens and N. Khan, in: Ionic Liquids: Industrial Applications to Green Chemistry, ed. R. D. Rogers and K. R.Seddon, American Chemical Society, Washington, D.C., 2002; 360–372 Search PubMed
.
- S. H. Lee, D. T. Dang, S. H. Ha, W. J. Chang and Y. M. Koo, Biotechnol. Bioeng., 2008, 99, 1–8 CrossRef CAS
.
- Q. Liu, M. H. A. Janssen, F. van Rantwijk and R. A. Sheldon, Green Chem., 2005, 7, 39–42 RSC
.
- T. Heinze, K. Schwikal and S. Barthel, Macromol. Biosci., 2005, 5, 520–525 CrossRef CAS
.
- H. Zhao, G. A. Baker, Z. Song, O. Olubajo, T. Crittle and D. Peters, Green Chem., 2008, 10, 696–705 RSC
.
- M. Zavrel, D. Bross, M. Funke, J. Buchs and A. C. Spiess, Bioresour. Technol., 2009, 100, 2580–2587 CrossRef CAS
.
- R. Bogel-Łukasik, D. Matkowska, E. Bogel-Łukasik and T. Hofman, Fluid Phase Equilib., 2010, 293, 168–174 CrossRef
.
- S. M. Payne and F. M. Kerton, Green Chem., 2010, 12, 1648–1653 RSC
.
- G. Barone, G. Della Gatta, D. Ferro and V. Piacente, J. Chem. Soc., Faraday Trans., 1990, 86, 75–79 RSC
.
- L. Kofler and H. Sitte, Monatsh. Chem., 1950, 81, 619–626 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2012 |
