The low-temperature (400 °C) coating of few-layer graphene on porous Li4Ti5O12viaC28H16Br2 pyrolysis for lithium-ion batteries†
Zelang
Jian
ab,
Liang
Zhao
b,
Rui
Wang
b,
Yong-Sheng
Hu
*b,
Hong
Li
b,
Wen
Chen
*a and
Liquan
Chen
b
aState Key Laboratory of Advanced Technology for Materials Synthesis and Processing, School of Materials Science and Engineering, Wuhan University of Technology, Wuhan 430070, China. E-mail: chenw@whut.edu.cn; Fax: +86 27 87864580
bBeijing National Laboratory for Condensed Matter Physics, Institute of Physics, Chinese Academy of Sciences, Beijing 100190, China. E-mail: yshu@aphy.iphy.ac.cn; Fax: +86 10 82649808
First published on 10th January 2012
Abstract
Porous Li4Ti5O12 coated with few-layer graphene was prepared via the low-temperature pyrolysis of C28H16Br2 at 400 °C. The coating layer was very thin and uniform. The coated sample shows superior Li storage performance compared with the as-prepared sample. Capacities of 131 and 104 mA h g−1 can be reached at current rates of 5 and 10 C, respectively. Moreover, cyclic performance is significantly improved after coating. The capacity decreases from 144.6 to 124.4 mA h g−1 after 2400 cycles at a current rate of 2 C in a half cellversusLi/Li+, with high capacity retention of 86%.
Lithium-ion batteries (LIBs) have been considered one of the most promising power sources for various types of electric vehicles and large-scale energy storage because of their high energy density, high power density, and environmental friendly features.1–3 Thus, the search for electrode materials with high power density, long cycle life, and good safety features has become a key issue in the past several years. Spinel Li4Ti5O12 has attracted increasing attention as an anode material for LIBs because of the minimal change in its lattice parameter and relatively high voltage during Li+ insertion/extraction; these attributes lead to potentially good cyclability and high safety.4–12 However, pure Li4Ti5O12 has limited applications in electric vehicles and large-scale storage because of its low electronic conductivity and moderate Li+ diffusion coefficient.13–15
Strategies such as decreasing particle size and carbon coating have been proposed to solve the problems of pure Li4Ti5O12. Carbon coating can enhance the surface electrical conductivity of electrode materials, and decreasing particle size can reduce the Li+ diffusion length; both are effective ways of improving rate performance.16–19 Typically, carbon coating of electrode materials is conducted at over 700 °C.8,11,20–23 At such a high temperature, some electrode materials, including lithium transition metal oxides, may be reduced or may become unstable during the carbon coating process.24 Thus, few reports on the carbon coating of lithium transition metal oxides have been published. Furthermore, high temperature pyrolysis means more energy consumption, which is contrary to our aim of energy saving. Our group has recently reported porous Li4Ti5O12 coated with N-doped carbon derived from ionic liquid at 600 °C, which significantly improved the high rate and cyclic performance of the sample.25 However, this working temperature is still too high for the coating of some lithium transition metal oxides. The low-temperature coating of a highly conductive surface layer has been an essential technology for optimizing poorly conductive and less thermally stable anode and cathode materials for LIBs.
Graphene, a two-dimensional one-atom thick sheet of carbon, has attracted considerable attention in the field of nanoscience because of its high surface area of over 2600 m2 g−1, excellent thermal and mechanical properties, and superior electrical conductivity.26–30 Recently, many graphene based composites have been widely investigated as electrode materials for LIBs and supercapacitors.31–33 However, most of them are mechanical mixtures of graphene and the active materials or are prepared in a complicated way.
10,10′-Dibromo-9,9′-bianthryl (C28H16Br2) has a highly symmetrical structure and high carbon content (up to 65.66%). Its molecular structure is shown in Fig. 1. Recently, Cai et al.34 reported the use of C28H16Br2 as a precursor for the preparation of graphene on the (111) surface of Au and Ag at 400 °C, in which Au and Ag were used as substrates. These may also act as catalysts. As shown in Fig. 1, the precursor undergoes dehalogenation to form single covalent C–C bonds between each monomer to create polymer chains at around 200 °C. Then, the polymer chains are subject to cyclodehydrogenation to form graphene nanoribbons at around 400 °C. Here, we extended this approach to coating the electrode materials with few-layer graphene at a rather low temperature of 400 °C. The rough surfaces of the electrode materials (e.g., Li4Ti5O12) were successfully coated with few-layer graphene using C28H16Br2 as a precursor.
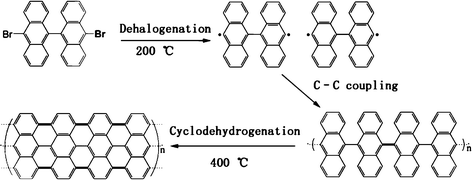 | ||
| Fig. 1 Scheme for the pyrolysis process of the precursor. | ||
The porous Li4Ti5O12 microspheres were prepared by spray drying.35 The primary particle size was about 50 nm, and these nanoparticles aggregated to form porous microspheres (the morphologies of the as-prepared Li4Ti5O12 are shown in Fig. S1†). Then, the porous Li4Ti5O12 powder was mixed with appropriate proportions of the C28H16Br2 solution. After the evaporation of the organic solvent, the mixture of C28H16Br2 and Li4Ti5O12 was heat-treated at 400 °C to obtain the resultant composite. The experimental details of the synthetic approach and its characterizations are described in the experimental section in the ESI†.
The powder X-ray diffraction (XRD) patterns of the as-prepared Li4Ti5O12 and Li4Ti5O12 samples coated with few-layer graphene (Li4Ti5O12/graphene) are shown in Fig. S2†. All the peaks of both samples can be indexed in accordance with those of pure Li4Ti5O12 (JCPDS card no. 880673). No difference in the patterns between the as-prepared Li4Ti5O12 and Li4Ti5O12/graphene samples is observed, indicating that the crystalline structure of Li4Ti5O12 is maintained after coating via the low-temperature approach at 400 °C.
High resolution transmission electron microscopy (HRTEM) and Raman measurements were carried out to investigate the distribution and composition of the coating layer on the Li4Ti5O12 surface. The HRTEM images of the Li4Ti5O12/graphene sample are shown in Fig. 2, which clearly reflects that the resultant material is polycrystalline. The crystalline planes of Li4Ti5O12 (111) and (400) with crystal spacings of 0.482 and 0.209 nm can be clearly observed. This result further confirms that the crystalline structure is maintained after coating. Interestingly, an ultrathin coating layer is observed on the surface of the Li4Ti5O12 sample. The thickness of the coating layer is about 1 nm and the space between the two graphene coating layers is 0.35 nm, which is approximately equal to the plane spacing of graphite. Raman spectroscopy is a powerful tool for detecting carbon and investigating its degree of crystallization. Fig. 3a shows the Raman spectra of the as-prepared Li4Ti5O12 and Li4Ti5O12/graphene samples. For the Li4Ti5O12/graphene sample, some new bands appear while some weaken compared with the as-prepared Li4Ti5O12 sample. The new bands located at 1352 and 1614 cm−1 correspond to the D and G bands, respectively, which are typical bands of graphitic materials. The G band is a typical zone center vibration mode of graphite crystallites, corresponding to the ordered sp2 bonded carbon, whereas the D band is an edge vibration mode or disorder layer.36–38 The sharp bands and narrow full width at half maximum of the G and D bands indicate a high degree of graphitization in the carbon coating layer, which is impossible to achieve with other carbon precursors (e.g., sugar) pyrolyzed at such a low-temperature. The inset in Fig. 3a shows the Raman spectrum of the Li4Ti5O12/graphene sample at the range of 2000–3000 cm−1. One peak located at 2703 cm−1 corresponds to the 2D band, which is a characteristic band of graphene. On the basis of the Raman spectroscopy and HRTEM results, we can conclude that the coating layer is few-layer graphene. Other new bands, such as those located at 1269 and 1225 cm−1, are in good agreement with those reported by Cai et al.34 The Raman bands of Li4Ti5O12 become so weak in the Li4Ti5O12/graphene sample because the conductive graphene coating layer reduces the penetration depth of the incident laser. Thermogravimetric/differential thermal analysis (TG/DTA) was performed to estimate the carbon content of the Li4Ti5O12/graphene sample. Fig. 3b shows the TG/DTA curves of the Li4Ti5O12/graphene sample under an oxygen atmosphere. The exothermic peak located at 460 °C corresponds to the oxidation of carbon. The TG curve shows that the carbon content is 5.91 wt%, which approximates to the theoretical value.
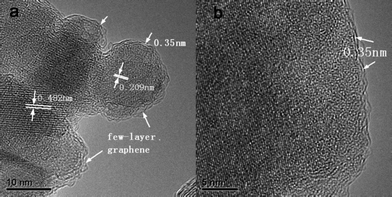 | ||
| Fig. 2 HRTEM images of the Li4Ti5O12/graphene sample. | ||
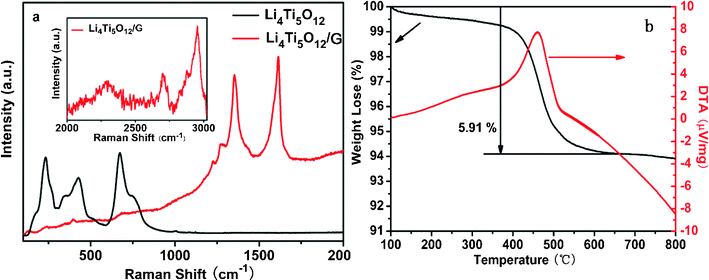 | ||
| Fig. 3 (a) Raman spectra of the as-prepared Li4Ti5O12 and Li4Ti5O12/graphene samples, the inset is the Raman spectrum of the Li4Ti5O12/graphene sample at range of 2000–3000 cm−1; (b) TG/DTA curves of the Li4Ti5O12/graphene sample. | ||
The rate capability and long cyclic performance of the Li4Ti5O12/graphene sample were investigated and compared with those of the as-prepared Li4Ti5O12 sample. As shown in Fig. 4a, the Li4Ti5O12/graphene sample exhibits a slightly lower capacity at a low current rate than the Li4Ti5O12 sample. However, the capacity of the as-prepared Li4Ti5O12 sample rapidly decreases with increasing current rate. Although the capacity of the Li4Ti5O12/graphene sample is 153 mA h g−1 at a current rate of 0.5 C (0.5 C means 3 mol Li insertion into Li4Ti5O12 in 2 h), this capacity can be maintained at 134 and 104 mA h g−1 at current rates of 5 and 10 C, respectively. In contrast, the capacities of the Li4Ti5O12 sample are only 61 and 30 mA h g−1 at current rates of 5 and 10 C, respectively. The Li4Ti5O12/graphene sample with a 3.51 wt.% carbon content (Fig. S3†) also shows improved rate performance, whose capacities at 5 and 10 C are 134.6 and 77.6 mA h g−1, respectively (Fig. S4†). The long cyclic performance is shown in Fig. 4b. After 2400 cycles, a 124.4 mA h g−1 capacity can be maintained for the Li4Ti5O12/graphene sample at a current rate of 2 C, with the capacity retention of 86% and coulombic efficiency of 100% in all cycles except the first cycle. On the other hand, the capacity of the as-prepared Li4Ti5O12 sample decreases to 100 mA h g−1 at the same current rate after 120 cycles. Electrochemical impedance spectroscopy measurements were carried out to compare the charge transfer resistances of the as-prepared Li4Ti5O12 and Li4Ti5O12/graphene samples. Fig. 5 shows the Nyquist plots of both electrodes at a fully charged state of 2.2 V and their fitting results, determined using the equivalent circuit. The experimental values (circles) fit well with the calculated values (lines), and the Rct values (charge transfer resistance) of the as-prepared Li4Ti5O12 and Li4Ti5O12/graphene samples extracted from the fitting results are 1048 and 172 Ω, respectively. Therefore, the improved rate performance of Li4Ti5O12/graphene can be attributed to the decreased resistance after few-layer graphene coating on the surface of Li4Ti5O12. The graphene coating layer enhances the surface/interface electrical conductivity of the Li4Ti5O12 particles and the electrical contact among particles. After this, the layer facilitates the Li insertion/extraction process, as demonstrated by the decreased charge transfer resistance of the coated sample. In addition, the uniformly coated graphene layers prevent direct contact between the Li4Ti5O12 particles and the electrolyte, which may also solve the well-known problem of gas-release in Li4Ti5O12 based batteries. Both factors are favorable to achieving excellent long cyclic performance.
 | ||
| Fig. 4 (a) Discharge and charge capacities of the as-prepared Li4Ti5O12 and Li4Ti5O12/graphene samples at different current rates (△ Li4Ti5O12 discharge, ▲ Li4Ti5O12 charge, □ Li4Ti5O12/graphene discharge and ■ Li4Ti5O12/graphene charge); (b) cyclic performance of Li4Ti5O12/graphene, the inset is the cyclic performance of the as-prepared Li4Ti5O12 sample. | ||
 | ||
| Fig. 5 Nyquist plots for the Li4Ti5O12 and Li4Ti5O12/graphene samples, as well as the fitting results (line) using an equivalent circuit shown in the lower inset. The higher inset is the enlarged plot. | ||
Furthermore, few-layer graphene coated on Li2MnO3 was also prepared using the same approach (see the HRTEM image in Fig. S5†). Preliminary results show that the crystalline structure and chemical state of Mn remain unchanged after few-layer graphene coating. The investigation of the Li storage behavior of the coated sample is still ongoing and will be discussed in a forthcoming paper. The extension of the proposed approach to future work indicates that it is a versatile technique.
In conclusion, we have demonstrated that the graphene resulting from the C28H16Br2 precursor can also be formed on the rough surfaces of lithium transition metal oxides at a temperature as low as 400 °C (e.g., Li4Ti5O12), not only on the (111) surface of Au or Ag.34 The rate performance and long cyclic performance of the Li4Ti5O12 sample coated with few-layer graphene are quite promising. The process of this coating approach is relatively simple, and, more importantly, the pyrolysis temperature of the used precursor can be as low as 400 °C. In addition, this new graphene coating approach could easily be extended to other active electrode materials for electrochemical devices.
Acknowledgements
We thank the invaluable discussions with Prof. Michel Armand. This work was supported by funding from “863” Project (2009AA033101), “973” Projects (2010CB833102), NSFC (50972164), Science and Technology Planning Project of Guangdong Province (2010A090602001), CAS project (KJCX2-YW-W26), the 100 Talent Project of the Chinese Academy of Sciences and Scientific Research Key Project Fund of Ministry of Education (109111).References
- V. Etacheri, R. Marom, R. Elazar, G. Salitra and D. Aurbach, Energy Environ. Sci., 2011, 4, 3243–3262 CAS.
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS.
- Y. Wang and G. Z. Cao, Adv. Mater., 2008, 20, 2251–2269 CrossRef CAS.
- A. S. Arico, P. Bruce, B. Scrosati, J. M. Tarascon and W. Van Schalkwijk, Nat. Mater., 2005, 4, 366–377 CrossRef CAS.
- T. Ohzuku, A. Ueda and N. Yamamoto, J. Electrochem. Soc., 1995, 142, 1431–1435 CrossRef CAS.
- M. M. Rahman, J. Z. Wang, M. F. Hassan, D. Wexler and H. K. Liu, Adv. Energy Mater., 2011, 1, 212–220 CrossRef CAS.
- Q. Wang, S. M. Zakeeruddin, I. Exnar and M. Gratzel, J. Electrochem. Soc., 2004, 151, A1598–A1603 CrossRef CAS.
- H.-G. Jung, S.-T. Myung, C. S. Yoon, S.-B. Son, K. H. Oh, K. Amine, B. Scrosati and Y.-K. Sun, Energy Environ. Sci., 2011, 4, 1345–1351 CAS.
- E. A. Davis and N. F. Mott, Philos. Mag., 1970, 22, 903–913 CrossRef CAS.
- L. Cheng, J. Yan, G. N. Zhu, J. Y. Luo, C. X. Wang and Y. Y. Xia, J. Mater. Chem., 2010, 20, 595–602 RSC.
- L. F. Shen, C. Z. Yuan, H. J. Luo, X. G. Zhang, K. Xu and Y. Y. Xia, J. Mater. Chem., 2010, 20, 6998–7004 RSC.
- Y. Wang, H. Liu, K. Wang, H. Eiji, Y. Wang and H. Zhou, J. Mater. Chem., 2009, 19, 6789–6795 RSC.
- M. Wagemaker, E. R. H. van Eck, A. P. M. Kentgens and F. M. Mulder, J. Phys. Chem. B, 2009, 113, 224–230 CrossRef CAS.
- C. Ouyang, Z. Zhong and M. Lei, Electrochem. Commun., 2007, 9, 1107–1112 CrossRef CAS.
- G.-N. Zhu, H.-J. Liu, J.-H. Zhuang, C.-X. Wang, Y.-G. Wang and Y.-Y. Xia, Energy Environ. Sci., 2011, 4, 4016–4022 CAS.
- W. J. H. Borghols, M. Wagemaker, U. Lafont, E. M. Kelder and F. M. Mulder, J. Am. Chem. Soc., 2009, 131, 17786–17792 CrossRef CAS.
- J. Kim and J. Cho, Electrochem. Solid-State Lett., 2007, 10, A81–A84 CrossRef CAS.
- A. S. Prakash, P. Manikandan, K. Ramesha, M. Sathiya, J. M. Tarascon and A. K. Shukla, Chem. Mater., 2010, 22, 2857–2863 CrossRef CAS.
- E. Kang, Y. S. Jung, G. H. Kim, J. Chun, U. Wiesner, A. C. Dillon, J. K. Kim and J. Lee, Adv. Funct. Mater., 2011, 21, 4349–4357 CrossRef CAS.
- L. Cheng, X. L. Li, H. J. Liu, H. M. Xiong, P. W. Zhang and Y. Y. Xia, J. Electrochem. Soc., 2007, 154, A692–A697 CrossRef CAS.
- Z. Ding, L. Zhao, L. Suo, Y. Jiao, S. Meng, Y.-S. Hu, Z. Wang and L. Chen, Phys. Chem. Chem. Phys., 2011, 13, 15127–15133 RSC.
- A. Q. Pan, J. Liu, J. G. Zhang, W. Xu, G. Z. Cao, Z. M. Nie, B. W. Arey and S. Q. Liang, Electrochem. Commun., 2010, 12, 1674–1677 CrossRef CAS.
- R. Liu, S. M. Mahurin, C. Li, R. R. Unocic, J. C. Idrobo, H. J. Gao, S. J. Pennycook and S. Dai, Angew. Chem., Int. Ed., 2011, 50, 6799–6802 CrossRef CAS.
- Q. Cao, H. P. Zhang, G. J. Wang, Q. Xia, Y. P. Wu and H. Q. Wu, Electrochem. Commun., 2007, 9, 1228–1232 CrossRef CAS.
- L. Zhao, Y.-S. Hu, H. Li, Z. Wang and L. Chen, Adv. Mater., 2011, 23, 1385–1388 CrossRef CAS.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, M. I. Katsnelson, I. V. Grigorieva, S. V. Dubonos and A. A. Firsov, Nature, 2005, 438, 197–200 CrossRef CAS.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS.
- Y. EunJoo, J. Kim, E. Hosono, Z. Hao-shen, T. Kudo and I. Honma, Nano Lett., 2008, 8, 2277–2282 CrossRef.
- J. J. Yoo, K. Balakrishnan, J. Huang, V. Meunier, B. G. Sumpter, A. Srivastava, M. Conway, A. L. M. Reddy, J. Yu, R. Vajtai and P. M. Ajayan, Nano Lett., 2011, 11, 1423–1427 CrossRef CAS.
- C. Li, L. Gu, J. Tong and J. Maier, ACS Nano, 2011, 5, 2930–2938 CrossRef CAS.
- L. Yang and L. Gao, J. Alloys Compd., 2009, 485, 93–97 CrossRef CAS.
- D. Wang, D. Choi, J. Li, Z. Yang, Z. Nie, R. Kou, D. Hu, C. Wang, L. V. Saraf, J. Zhang, I. A. Aksay and J. Liu, ACS Nano, 2009, 3, 907–914 CrossRef CAS.
- D. H. Wang, R. Kou, D. Choi, Z. G. Yang, Z. M. Nie, J. Li, L. V. Saraf, D. H. Hu, J. G. Zhang, G. L. Graff, J. Liu, M. A. Pope and I. A. Aksay, ACS Nano, 2010, 4, 1587–1595 CrossRef CAS.
- J. Cai, P. Ruffieux, R. Jaafar, M. Bieri, T. Braun, S. Blankenburg, M. Muoth, A. P. Seitsonen, M. Saleh, X. Feng, K. Müllen and R. Fasel, Nature, 2010, 466, 470–473 CrossRef CAS.
- K. C. Hsiao, S. C. Liao and J. M. Chen, Electrochim. Acta, 2008, 53, 7242–7247 CrossRef CAS.
- A. C. Ferrari and J. Robertson, Phys. Rev. B: Condens. Matter, 2000, 61, 14095–14107 CrossRef CAS.
- Y. Wang and H. Zhou, Energy Environ. Sci., 2011, 4, 1704–1707 CAS.
- V. Lee, L. Whittaker, C. Jaye, K. M. Baroudi, D. A. Fischer and S. Banerjee, Chem. Mater., 2009, 21, 3905–3916 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: SEM images for the as-prepared Li4Ti5O12 sample; XRD patterns for the as prepared Li4Ti5O12 and Li4Ti5O12/graphene samples; TG curve and rate performance for the Li4Ti5O12/graphene sample with a carbon content of 3.51 wt%; HRTEM image for the Li2MnO3/graphene. See DOI: 10.1039/c2ra01263d |
| This journal is © The Royal Society of Chemistry 2012 |
