Chlorhexidine-loaded calcium phosphate nanoparticles for dental maintenance treatment: combination of mineralising and antibacterial effects
Anna
Kovtun
a,
Diana
Kozlova
a,
Kathirvel
Ganesan
a,
Caroline
Biewald
b,
Nadine
Seipold
b,
Peter
Gaengler
b,
Wolfgang H.
Arnold
b and
Matthias
Epple
a
aInstitute of Inorganic Chemistry and Center for Nanointegration Duisburg-Essen (CeNIDE), University of Duisburg-Essen, Universitaetsstrasse 5-7, 45117, Essen, Germany. E-mail: matthias.epple@uni-due.de
bFaculty of Health, School of Dentistry, University of Witten/Herdecke, Alfred-Herrhausen-Strasse 50, 58448, Witten, Germany
First published on 22nd November 2011
Abstract
One of the main problems in dental medicine is the growth of bacterial biofilms on tooth surfaces which cause caries and periodontitis. We have developed a new system for oral hygiene and dental treatment that consists of either a paste or a rinsing solution containing calcium phosphate nanoparticles, functionalized with the antibacterial agent chlorhexidine. As calcium phosphate is the natural component of tooth mineral, it can lead to the remineralization of damaged enamel, while chlorhexidine prevents the colonization of the tooth surface by bacteria. In the form of a paste, a bifunctional system with both mineralizing and antibacterial properties is obtained. The nanoparticles may also stick to open dentin tubules at the root surface due to their coating with carboxymethyl cellulose. In vitro studies on teeth show that the paste sticks well to the root surface and closes dentin tubules.
Introduction
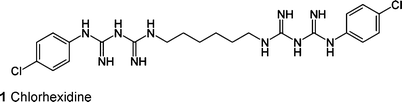
Bioceramics based on nanocrystalline hydroxyapatite have been widely used for many years in biomedicine and tissue engineering due to their high biocompatibility. Hydroxyapatite is a natural component of bone and teeth,1,2 possesses good mechanical properties and is fully biodegradable if present in nanocrystalline form.3–7 Nanoparticulate hydroxyapatite was proposed as carrier for biomolecules,8–10drugs,11,12nucleic acids,13,14 and porphyrins.15,16Calcium phosphate nanoparticles are also used in toothpastes to enhance the remineralization of teeth enamel and were also proposed to occlude open dentin tubules.17,18Chlorhexidine (1) is very common in dental medicine as an antibacterial agent against gram-positive and gram-negative bacteria and fungi.19 It is used to treat periodontal diseases20 in the form of pastes, sprays or mouth rinses.21–23. However, chlorhexidine is toxic at high concentrations for eukaryotic cells because of its interaction with membrane proteins, e.g.Na+-K+-ATPase.24 Babich et al. showed a toxic effect of chlorhexidine at high concentrations on fibroblasts after 1, 24 and 72 h.25
Here, we have loaded calcium phosphate nanoparticles with chlorhexidine to combine mineralization ability and antibacterial effect. To achieve an optimal loading with chlorhexidine and to enhance the adhesion properties on the tooth surface, the nanoparticles must be modified by adhesive polymers to make them more sticky. For this, we used carboxymethyl cellulose (CMC). This leads to a good adhesion on dental surfaces, remineralization, and occlusion of dentin tubules and bactericidal effect. The nanoparticles can be applied either as dispersion (“mouth rinse”) or as paste.
Experimental
Chemicals
The following chemicals were used: Carboxymethyl cellulose (CMC; Aldrich, molecular weight 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1; degree of substitution, D.S., 0.7), calcium lactate (p.a.; Fluka) and diammonium hydrogenphosphate (p.a.; Fluka), chlorhexidine (Aldrich, Steinheim, Germany), and fluoresceine (Riedel-de Haen, Seelze, Germany). Ultrapure water (Purelab ultra instrument from ELGA) was used for all preparations.
000 g mol−1; degree of substitution, D.S., 0.7), calcium lactate (p.a.; Fluka) and diammonium hydrogenphosphate (p.a.; Fluka), chlorhexidine (Aldrich, Steinheim, Germany), and fluoresceine (Riedel-de Haen, Seelze, Germany). Ultrapure water (Purelab ultra instrument from ELGA) was used for all preparations.
Synthesis of chlorhexidine-loaded carboxymethyl cellulose-func tionalized calcium phosphate nano particles (CaP-CMC-CHX)
Calcium phosphate nanoparticles were continuously prepared by mixing aqueous solutions of calcium lactate (18 mM, pH 9) and diammonium hydrogen phosphate (10.8 mM, pH 9) with peristaltic pumps in a tube reactor (internal tube diameter: 2.54 mm). The in situ-prepared calcium phosphate nanoparticles were functionalized with carboxymethyl cellulose (2 g L−1) to prevent the agglomeration of the particles and further loaded with chlorhexidine (2.7 mM, pH 8). Each solution was pumped with a flow rate of 10 mL min−1. The hydrodynamic residence time was 1.5 s from mixing the calcium and phosphate solutions to the addition of CMC, then 10 s until the addition of CHX, and a further 0.8 s until the outlet. This continuous procedure leads to standardized synthesis conditions and a constant product quality, being very important for a biomedical application.The resulting dispersion was centrifuged at 2540 g (z300 instrument from Hermle Labortechnik GmbH, Wehingen, Germany) to remove the counter ions and a possible excess of organic additives, then redispersed in ultrapure water and centrifuged again. The obtained wet product had the consistency of a paste (i.e. a highly viscous, toothpaste-like material) and a content of solid of 22 ± 4 wt% as measured by thermogravimetry. The preparation of the nanoparticulate paste is schematically shown in Fig. 1.
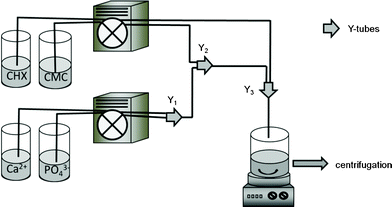 | ||
| Fig. 1 Schematic diagram of the preparation of a paste of CaP-CMC-CHX nanoparticles. Y1, Y2 and Y3 are tube connectors. The distances between two connections were: Y1 to Y2 = 10 cm; Y2 to Y3 = 100 cm. | ||
The release of chlorhexidine from the nanoparticles was studied by dissolution experiments. 1 g of the nanoparticulate paste (i.e. including 78 wt% of water) was redispersed in 200 mL of artificial saliva (83 mL H2O, 15 mL KCl (1 M), 1 mL CaCl2 (150 mM), 1 mL KH2PO4 (90 mM) in 100 mL solution, pH 7) and stirred for 0, 1, 2 or 3 days. Then the particles were collected by centrifugation (2540 g) and subjected to thermogravimetric and elemental analysis.
Characterisation methods
Scanning electron microscopy of the particles was performed with a FEI Quanta 400 ESEM instrument in high vacuum. Sputtering was performed with gold–palladium for 30 s. Thermogravimetric analysis (TGA) and infrared spectroscopy (IR) were carried out with a Netzsch STA 409 PC instrument (dynamic oxygen atmosphere; 50 ml min−1; heating rate 2.5 K min−1; open alumina crucible). The typical sample mass for TGA was 20 mg. The elemental composition of the obtained paste was determined by atomic absorption spectroscopy (AAS; graphite tube furnace; Thermo Electron Corporation, M-Series) and UV-visible spectroscopy (Varian Cary WinUV spectrophotometer in 1 cm quartz cuvettes). The contents of carbon, hydrogen, nitrogen and sulphur were determined by standard combustion analysis with an EA 1110 (CE Instruments) instrument. X-Ray powder diffraction (XRD) was carried out with a STOE transmission diffractometer STADI P 2003-10 (Cu Kα radiation, λ = 1.54 Å). Emission and excitation spectra were measured at ambient temperature with a J&M spectrofluorometer (Analytische Mess- und Regeltechnik FL3095-500) equipped with a diode array polychromator and a 75 W xenon lamp.Scanning electron microscopic studies of nanoparticles on teeth
Extracted human teeth with no root caries lesions were cut into two halves. On the enamel and root surfaces, 3.3 mm2 windows (i.e. areas to study the application of the nanoparticles) were prepared. One half served as control and remained untreated; the other half exhibited experimental windows on the enamel and root dentin surface. The teeth were macerated in 5% KOH for one week. All windows in the teeth were then etched with 30% H3PO4 for 60 s to simulate clinical erosion of enamel and exposed root dentin. The controls were left untreated and stored in 0.9% NaCl solution for 72 h. The nanoparticulate paste was applied onto the experimental windows with a rotating brush for 3 min at 2.0 N. The teeth were then stored in remineralizing solution (artificial saliva) for 72 h. After washing with distilled water, all samples were dehydrated in a graded acetone row and critically point-dried with a CPD 30 instrument (Baltec, Liechten stein). The samples were sputtered with gold–palladium and investigated with an XL 30 FEG scanning electron microscope (Philips, Eindhoven, The Netherlands) at 20 kV acceleration voltage. The samples were then fractured through the middle of the windows and the edges were again investigated by SEM.Results and discussion
We have synthesized particles using carboxymethyl cellulose (CMC) to prevent the growth of the nanoparticles, to achieve a maximum loading with chlorhexidine (CHX), and to enhance the adhesion on the tooth surface. Positively charged chlorhexidine strongly adsorbs onto the negatively charged CMC-functionalized calcium phosphate nanoparticles, due to the electrostatic interaction between the biguanide groups of chlorhexidine and the carboxylate groups of CMC.For chemical analysis, the paste was dried in air at room temperature for 24 h. The resulting particles had a spherical morphology, a diameter around 150–200 nm, and a negative zeta potential (−37 ± 6 mV) due to the outer shell of CMC, whereas unfunctionalized calcium phosphate particles had a zeta potential around zero.26Fig. 2 shows a representative scanning electron micrograph. The particles were stable as a colloidal dispersion in water for only about an hour before sedimentation occurred, a fact that points to ongoing agglomeration of the primary nanoparticles. However, for the projected application as tooth remineralisation material, an application as a paste is convenient, and therefore we prepared the particles in the form of a paste by partial removal of water by centrifugation (content of solid about 20–25 wt%). During the application, agglomerates will be redispersed during the mechanical action of a toothbrush or a polishing cup.
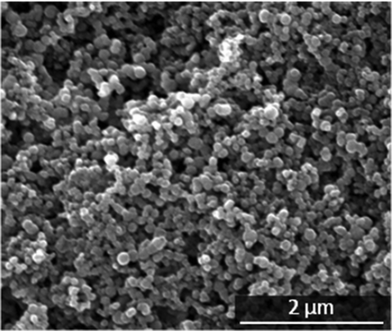 | ||
| Fig. 2 Scanning electron micrograph of the primary CaP-CMC-CHX nanoparticles. | ||
The IR spectra of the nanoparticles showed vibration bands of both CHX and CMC (Fig. 3). The adsorbed water molecules gave a weak absorption band around 1645 cm−1 (bending) and a broad absorption band around 3400 cm−1 (stretching vibration). The weak C–O bending vibration of carbonate group was observed at 1385 cm−1. The characteristic vibration bands of hydroxyapatite (1038, 604, and 565 cm−1) were clearly observed for the functionalized nanoparticles.
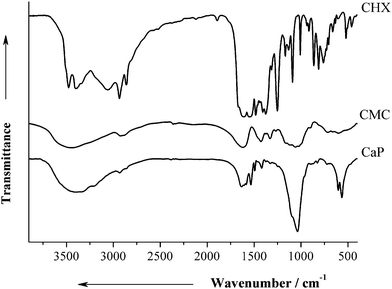 | ||
| Fig. 3 IR data of pure CHX, pure CMC, and air-dried CaP-CMC-CHX nanoparticles (CaP). | ||
X-Ray powder diffraction data of calcium phosphate-CMC-CHX nanoparticles are shown in Fig. 4. The broad diffraction peaks show that the calcium phosphate nanoparticles consist of poorly crystalline hydroxyapatite in accordance to literature spectra.27
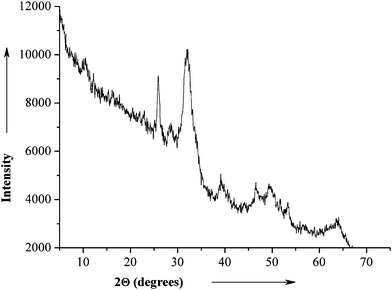 | ||
| Fig. 4 X-Ray powder diffractogram of air-dried CaP-CMC-CHX nanoparticles. | ||
The composition of the nanoparticles was determined by thermogravimetric (Fig. 5) and elemental analysis. The first decomposition step of 5.7 wt% corresponds to the loss of residual water. The second and third steps (13.4 wt% and 12.9 wt%, respectively) correspond to the combustion of organic components (CMC and CHX). The fourth step (1.3 wt%) corresponds to the loss of CO2 from carbonated apatite. Note that the given numbers for the mass loss are not exact as the steps are overlapping. They were selected from visual inspection of the TG curve, taking into account the inflection points of the TG curve.
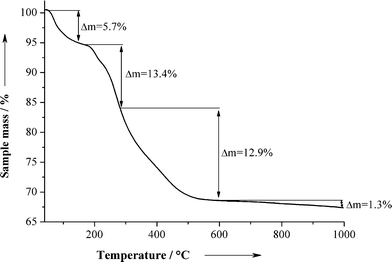 | ||
| Fig. 5 Thermogravimetric analysis of the air-dried CaP-CMC-CHX nanoparticles. | ||
Table 1 shows the elemental analysis data of CaP-CMC-CHX nanoparticles. The nanoparticles contained about 48.6 ± 4.1 wt% of calcium phosphate (sum of calcium and phosphate; note that the exact stoichiometry of the calcium phosphate phase is unknown). The content of chlorhexidine was calculated from the nitrogen content to 13.1 ± 2.2 wt% according to eqn 1 with M(CHX) = 505.46 g mol−1 (C22H30Cl2N10):
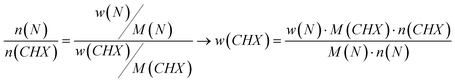 | (1) |
| C/wt% | H/wt% | N/wt% | Ca/wt% | PO43−/wt% | Chlorhexidine/wt% (calculated) | Calcium phosphate/wt% (calculated) | Ca/PO43− molar ratio |
|---|---|---|---|---|---|---|---|
| 18.5 ± 4.4 | 3.3 ± 0.8 | 3.4 ± 0.4 | 19.9 ± 2.9 | 28.7 ± 2.9 | 13.1 ± 2.2 | 48.6 ± 4.1 | 1.56 |
The content of chlorhexidine of 13.1 wt% in the dried particles corresponds to about 3 wt% in the wet paste which contains 78 ± 4 wt% water. This loading is high enough to achieve the desired bactericidal effect. According to Mohammadi et al., a bacteriostatic effect is obtained at a chlorhexidine concentration of 0.2% and a bacteriotoxic effect occurs at 2% of chlorhexidine.28 However, whereas in mouth rinses chlorhexidine is used at low concentrations of 0.1–0.2%, in tooth varnishes much higher concentrations are used, up to 40% without being toxic for humans.29
We determined the release kinetics of chlorhexidine and the dissolution of the particles under physiological conditions by dispersion of the paste in artificial saliva for 3 days and then subjected it to thermogravimetry and elemental analysis (Fig. 6 and Table 2). The particles released the chlorhexidine almost completely during the first day. The nanoparticles still contained carbon which indicates the presence of CMC; however, the amount of calcium phosphate increased from 48.6 to 72.0 wt% in two days. That leads to the conclusion that the nanoparticles will release the chlorhexidine during the first day in a clinical application, but will still attach to the tooth surface due to the presence of the sticky CMC. This will lead to the occlusion of dentin tubules and the remineralization of eroded teeth.30
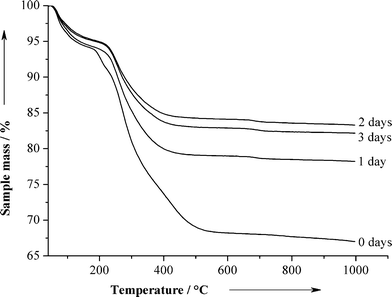 | ||
| Fig. 6 Thermogravimetry of CaP-CMC-CHX nanoparticles after dispersion for several days. | ||
| Time/days | C/wt% | H/wt% | N/wt% | Ca/wt% | Chlorhexidine/wt% (calculated) | Calcium phosphate/wt% (calculated) |
|---|---|---|---|---|---|---|
| 0 | 18.5 | 3.3 | 3.4 | 19.9 | 13.1 | 48.6 |
| 1 | 7.2 | 1.6 | 0.7 | 26.9 | 2.4 | 67.0 |
| 2 | 5.1 | 1.2 | 0.0 | 28.9 | 0.0 | 72.0 |
| 3 | 5.7 | 1.5 | 0.0 | 28.9 | 0.0 | 72.0 |
To follow the nanoparticles after application in the mouth, we additionally loaded them with fluorescein (40.97 mg L−1) which was added during the synthesis to the CMC solution. The addition of fluorescein had no significant influence on the morphology or size of the particles. The fluorescence spectrum of the fluorescein-loaded particles is shown in Fig. 7. At the excitation wavelength of 306 nm, the isolated dried powder showed a strong fluorescence at 551 nm (yellow-green colour).
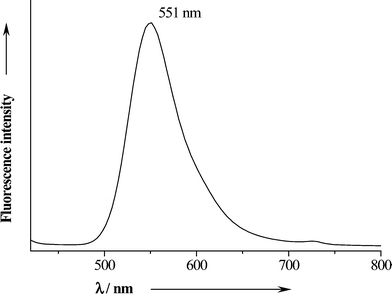 | ||
| Fig. 7 Fluorescence emission spectrum of solid fluorescein-labelled air-dried CaP-CMC-CHX nanoparticles. | ||
The application of the fluorescein-labelled paste to teeth demonstrated that the nanoparticles stuck well onto the tooth surface, especially at the cervical and proximal areas (Fig. 8).
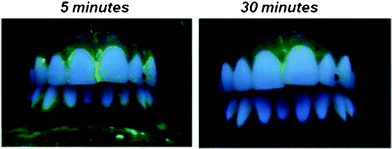 | ||
| Fig. 8 Photograph of the fluorescein-labelled nanoparticulate paste on the tooth surface, 5 and 30 min after application. The green fluorescence indicates adsorbed nanoparticles. The paste was predominantly located at the cervical and proximal tooth surface. After 30 min it was still visible. | ||
We tested the antimicrobial activity of the nanoparticles at different concentrations of CaP-CMC-CHX paste against bacteria (Fig. 9). For this test, the paste was diluted to 2 wt% to obtain a dispersion by ultrasonication for 10 min. After further dilution by factors 10, 102, 103, 104 times, respectively, in ultrapure water, these dispersions were tested on the gram-negative strain Escherichia coli and the gram-positive strain Lactobacillus casei. The results showed an efficient inhibition of bacterial growth even after a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 dilution, in good agreement with the literature data.28 However, more extensive studies are required to prove the activity against bacterial colonies (biofilms) occurring in the mouth.31
100 dilution, in good agreement with the literature data.28 However, more extensive studies are required to prove the activity against bacterial colonies (biofilms) occurring in the mouth.31
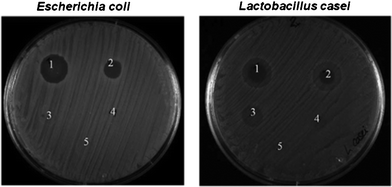 | ||
| Fig. 9 Effect of 10 μL of CaP-CMC-CHX nanoparticles at different concentrations against Escherichia coli and Lactobacillus casei. The original dispersion of 2 wt% nanoparticles present in dispersion was diluted by factors of 10 (1), 102 (2), 103 (3), 104 (4). (5) shows a control experiment without chlorhexidine. | ||
In vitro experiments on human teeth showed the adsorption of the particles on the dental surfaces (Fig. 10 to 12). All control windows on enamel and root dentin surfaces showed the typical erosion patterns, i.e. prismatic demineralization of enamel, and open, empty dentin tubules.32,33 After gentle polishing; the treated windows showed well-adsorbed layers of CaP-CMC-CHX nanoparticles which were completely covering erosive niches, the prismatic demineralization of enamel as well as dentin tubule entrances. These features of surface SEM were confirmed by SEM of fractured samples (Fig. 13).
 | ||
| Fig. 10 Scanning electron micrograph of nanoparticle-treated enamel. Left: Overview of the surface. The nanoparticulate paste completely covers the surface. Right: Fractured sample with a dense cover of nanoparticles on the enamel surface. | ||
 | ||
| Fig. 11 Scanning electron micrograph of the eroded enamel surface of a control sample (left) and of an equivalent sample in which the surface was completely covered by the nanoparticulate paste after the application (right). | ||
 | ||
| Fig. 12 Scanning electron micrograph showing the eroded root surface of a control sample with open dentin tubules (left) and the root surface of an equivalent sample that was completely covered by the nanoparticles after application (right). | ||
 | ||
| Fig. 13 Scanning electron micrograph of the fractured window of a control sample and experimental sample. In the control window, the demineralized surface can be seen as a dark surface layer. In the experimental sample, the demineralized surface layer is covered by the nanoparticles. Small tags can be seen at the openings of the dentin tubules. | ||
Conclusions
The functionalized nanoparticles showed a clear ability to adsorb on tooth surfaces (enamel and dentin) and also to close open dentin tubules. It can be stated that chlorhexidine-loaded CMC-functionalized calcium phosphate nanoparticles represent a very promising tool to improve oral hygiene and dental treatment in cases of common enamel and/or dentin erosion, dentin hypersensitivity, gingivitis and marginal periodontitis. They are expected to combat biofilm formation and to remineralize the tooth surface by biomimetic tooth sealing demineralised surface defects on crowns and roots.Acknowledgements
We thank Prof. B. Siebers and Dr J. Marrero Coto for help with the bacterial tests. The study was supported by Hager&Werken, Duisburg, Germany.References
- H. A. Lowenstam and S. Weiner, On biomineralization, Oxford University Press, New York, 1989 Search PubMed.
- S. V. Dorozhkin and M. Epple, Angew. Chem., Int. Ed., 2002, 41, 3130–3146 CrossRef CAS.
- V. Uskokovic and D. P. Uskokovic, J. Biomed. Mater. Res., Part B, 2011, 96B, 152–191 CrossRef CAS.
- M. Epple, K. Ganesan, R. Heumann, J. Klesing, A. Kovtun, S. Neumann and V. Sokolova, J. Mater. Chem., 2010, 20, 18–23 RSC.
- Y. Cai and R. Tang, J. Mater. Chem., 2008, 18, 3775–3787 RSC.
- C. Rey, C. Combes, C. Drouet, A. Lebugle, H. Sfihi and A. Barroug, Materialwiss. Werkstofftech., 2007, 38, 996–1002 CrossRef CAS.
- C. Capuccini, P. Torricelli, E. Boanini, M. Gazzano, R. Giardino and A. Bigi, J. Biomed. Mater. Res., Part A, 2009, 89A, 594–600 CrossRef CAS.
- Y. Mizushima, T. Ikoma, J. Tanaka, K. Hoshi, T. Ishihara, Y. Ogawa and A. Ueno, J. Controlled Release, 2006, 110, 260–265 CrossRef CAS.
- T. Matsumoto, M. Okazaki, M. Inoue, S. Yamaguchi, T. Kusunose, T. Toyonaga, Y. Hamada and J. Takahashi, Biomaterials, 2004, 25, 3807–3812 CrossRef CAS.
- A. des Rieux, V. Fievez, M. Garinot, Y. J. Schneider and V. Préat, J. Controlled Release, 2006, 116, 1–27 CrossRef CAS.
- E. Boanini, M. Gazzano, K. Rubini and A. Bigi, Adv. Mater., 2007, 19, 2499–2502 CrossRef CAS.
- B. Palazzo, M. Iafisco, M. Laforgia, N. Margiotta, G. Natile, C. L. Bianchi, D. Walsh, S. Mann and N. Roveri, Adv. Funct. Mater., 2007, 17, 2180–2188 CrossRef CAS.
- V. Sokolova and M. Epple, Angew. Chem., Int. Ed., 2008, 47, 1382–1395 CrossRef CAS.
- A. Maitra, Expert Rev. Mol. Diagn., 2005, 5, 893–905 CrossRef CAS.
- K. Ganesan, A. Kovtun, S. Neumann, R. Heumann and M. Epple, J. Mater. Chem., 2008, 18, 3655–3661 RSC.
- J. Schwiertz, A. Wiehe, S. Gräfe, B. Gitter and M. Epple, Biomaterials, 2009, 30, 3324–3331 CrossRef CAS.
- N. Roveri, E. Battistella, C. L. Bianchi, I. Foltran, E. Foresti, M. Iafisco, M. Lelli, A. Naldoni, B. Palazzo and L. Rimondini, J. Nanomater., 2009, 2009, 746383 CrossRef.
- A. Peetsch and M. Epple, Mater.-Wiss. u. Werkstofftechn., 2011, 42, 131–135 CrossRef CAS.
- K. S. Lim and P. C. Kam, Anaesth. Intensive Care, 2008, 36, 502–512 Search PubMed.
- D. Russell and M. J. Day, J. Hosp. Infect., 1993, 25, 229–238 CrossRef.
- D. Steinberg and M. Rothman, Diagn. Microbiol. Infect. Dis., 1996, 26, 109–115 CrossRef CAS.
- M. E. Frank, J. F. Gent and T. P. Hettinger, Physiol. Behav., 2001, 74, 85–99 CrossRef CAS.
- N. J. Medlicott, D. W. Holborow, M. J. Rathbone, D. S. Jones and I. G. Tucker, J. Controlled Release, 1999, 61, 337–343 CrossRef CAS.
- K. Helgeland, G. Heyden and G. Rolla, Scand. J. Dent. Res., 1971, 79, 209–215 CAS.
- H. Babich, B. J. Wurzburger, Y. L. Rubin, M. C. Sinensky and L. Blau, Cell Biol. Toxicol., 1995, 11, 79–88 CrossRef CAS.
- V. Sokolova, O. Prymak, W. Meyer-Zaika, H. Cölfen, H. Rehage, A. Shukla and M. Epple, Materialwiss. Werkstofftech., 2006, 37, 441–445 CrossRef CAS.
- H. Urch, M. Vallet-Regi, L. Ruiz, J. M. Gonzalez-Calbet and M. Epple, J. Mater. Chem., 2009, 19, 2166–2171 RSC.
- Z. Mohammadi and P. V. Abbott, Int. Endod. J., 2009, 42, 288–302 CrossRef CAS.
- M. Puig-Silla, J. M. Montiel-Company and J. M. Almerich-Silla, Med. Oral Patol. Oral Cir. Bucal., 2008, 13, E257–E260 Search PubMed.
- F. Lippert, D. M. Parker and K. D. Jandt, J. Colloid Interface Sci., 2004, 280, 442–448 CrossRef CAS.
- M. Hannig and C. Hannig, Nat. Nanotechnol., 2010, 5, 565–569 CrossRef CAS.
- W. H. Arnold, V. Bietau, P. O. Renner and P. Gaengler, Arch. Oral Biol., 2007, 52, 591–597 CrossRef.
- W. H. Arnold, A. Dorow, S. Langenhorst, Z. Gintner, J. Banoczy and P. Gaengler, BMC Oral Health, 2006, 6, 8 CrossRef.
| This journal is © The Royal Society of Chemistry 2012 |