N-Heterocyclic carbene catalyzed conjugate umpolung reactions leading to coumarin derivatives†
Yuansong
Jiang
,
Wanzhi
Chen
* and
Weimin
Lu
*
Department of Chemistry, Zhejiang University, Xixi Campus, Hangzhou 310028, People's Republic of China. E-mail: chenwzz@zju.edu.cn or weimlu2000@163.com.; Fax: (+)86-571-88273314; Tel: (+)86-571-88273314
First published on 20th December 2011
Abstract
The NHC-catalyzed selective condensation reactions between cinnamaldehydes and salicylaldehydes viahomoenolate intermediates are described. A number of 3-benzyl-chromen-2-ones could be obtained in good yields. The reactions occurred in high yielding with low catalyst loading.
Introduction
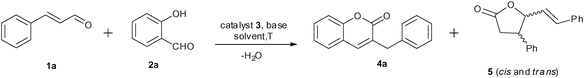
N-Heterocyclic carbene (NHC) catalyzed umpolung reactions have drawn great attention in the past decade and the mechanisms are well documented.1 The adducts of aromatic aldehydes and some NHCs can be used as equivalents of acyl anions to participate in some umpolung reactions, such as nucleophilic addition towards carbon–hetero multi bonds2 (benzoin condensation) and carbon–carbon multi bonds (Stetter Reaction),3 as well as nucleophilic substitution towards the carbon–halogen bond.4 Another type of umpolung reaction is the so-called “conjugate umpolung reaction”.5 As shown in Scheme 1, enamineII as an equivalent of homoenolateIII could be generated by exposing the appropriate NHCs to trans α,β-unsaturated aldehydes such as cinnamaldehyde (1a). Nucleophilic substitution of the homoenolate equivalents towards some electrophiles was used to form carbon–carbon bonds, carbon–nitrogen bonds and carbon–hydrogen bonds affording acyclic esters,5c,6 acyclic amides,7 cyclopantenes,5d,8lactones,5a,9lactams.10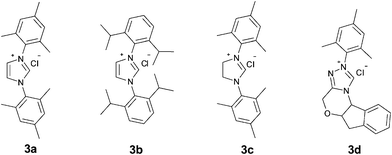 | ||
| Fig. 1 Schematic illustration of the carbene precursors. | ||
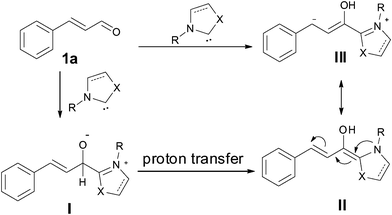 | ||
| Scheme 1 The generation of a homoenolate equivalent from cinnamaldehyde. | ||
Chromen-2-one (known as coumarin) and its derivatives are fundamental building blocks of many bioactive molecules, natural products, and organic materials.11 Bräse and coworkers reported that 3-alkyl-chromen-2-ones could be prepared via an umpolung reaction of cinnamaldehydes,12,13 however, the reaction requires stoichiometric NHCs relative to reactants as the promoters and restricted to electron rich substrates. In 2011, Shi and Bräse reported that catalytic amounts of in situ generated NHC catalyzed a one-pot redox esterification of α,β-unsaturated aldehydes with simultaneous aldol condensation, but it is only efficient for crotonaldehyde.14 Recently, Nair reported that 2H-chromene-3-carboxaldehydes undergo an intramolecular rearrangement mediated by NHC leading to 3-alkyl-chromen-2-ones.15 Herein, we describe an highly efficient synthetic procedure for 3-benzyl-chromen-2-ones using in situ generated NHC as a catalyst and commercially available salicylaldehydes and cinnamaldehydes as starting materials. 3-Ethyl-chromen-2-ones also can be synthesized using the same catalytic system in moderate yields.
Results and discussion
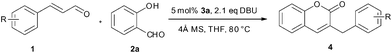
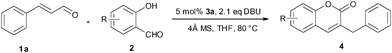
We envisioned that the nucleophilic attack of bulkier NHCs towards cinnamaldehyde would be preferable to that of salicylaldehyde. Bulky NHCs may suppress the formation of 2-hydroxybenzoyl anion derived from salicylaldehyde and promote subsequent “conjugate umpolung” reaction. Thus the condensation of cinnamaldehyde and salicylaldehyde would selectively afford chromen-2-one derivatives. The influences of solvents, bases, temperatures and NHCs on the reaction of cinnamaldehyde (1a) and salicylaldehyde (2a) were studied to optimize the reaction conditions, and the results are listed in Table 1. When the reaction was performed in toluene, the target product 4a could be obtained in a yield of 16% even when up to 20 mol% of 3a was used (Table 1, entry 1). The side product 5 was also observed due to the condensation of the homoenolate equivalent III with cinnamaldehydes (1a) which has already been noted.12 The yield of 4a could be slightly improved when a large excess of 2a was used in the presence of 5 mol% of 3a (entry 2). Higher 2a/1a ratio is believed to suppress the self condensation of 1a. THF was a better solvent in which the reaction took place smoothly at 40 °C affording 4a in 31% yield (entry 3). Other solvents such as DCM, t-BuOH, THF/t-BuOH, toluene, and 1,4-dioxane are much less efficient than THF (entries 7-11). Among the bases examined, DBU (DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene) is more suitable to the generation of NHC than other bases (entry 4). The generation of water was expected in the intermolecular condensation of 1a and 2a, thus the presence of 4 Å molecular sieves as a water absorbent would facilitate the reaction. Actually, addition of 0.5 g of 4 Å molecular sieves sharply raised the yield of 4a to 74% (entry 6). The NHCs derived from the carbene precursors listed in Fig. 1 were all active for the condensation reaction of cinnamaldehyde and salicylaldehyde, but 3b-d showed much lower activity than 3a. Bulkier imidazolium salt 3b was reported to be the best NHC precursor for the condensation reaction of salicylaldehydes and crotonaldehyde, but 3b showed low catalytic activity for other bulky α, β-unsaturated aldehydes.14 In this study, the yield of 4a was also sharply reduced when 3b was used as (entry 16) catalyst precursor. It suggested that bulky homoenolates would require a less steric NHC catalyst. The saturated NHC deprotonated from 3c exhibited the poorest activity (entry 17). Triazolium salt 3d derived NHC was similar to the NHC derived from 3b (entry 18). Raising the temperature to 80 °C could significantly facilitate the reaction, and the yield of the target product was increased to 76% even within 6 h (entry 19).| Entry | 3 (mol%) | Base | Solvent | T/°C | Yield (%)b |
|---|---|---|---|---|---|
| a Reaction conditions: 1a, 1 mmol; 2a, 2 mmol; solvent, 2 mL; 24 h. b Isolated yield. c 1 eq. of 2a was used. d 40 °C, 12 h. e 80 °C, 6 h. | |||||
| 1 | 3a (20) | K2CO3 (3.0 eq) | toluene | 100 | 16c |
| 2 | 3a (5) | K2CO3 (2.1 eq) | toluene | 100 | 29 |
| 3 | 3a (5) | K2CO3 (2.1 eq) | THF | 40 | 31 |
| 4 | 3a (5) | DBU (2.1 eq) | THF | 40 | 52 |
| 5 | 3a (5) | t-BuOK (2.1 eq) | THF | 40 | 6 |
| 6 | 3a (5) | DBU (2.1 eq) | THF + 4 Å MS | 40 | 74 |
| 7 | 3a (5) | DBU (2.1 eq) | DCM + 4 Å MS | 40 | 44 |
| 8 | 3a (5) | DBU (2.1 eq) | t-BuOH + 4 Å MS | 40 | 11 |
| 9 | 3a (5) | DBU (2.1 eq) |
THF/t-BuOH(9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) + 4 Å MS 1) + 4 Å MS |
40 | 50 |
| 10 | 3a (5) | DBU (2.1 eq) | Toluene + 4 Å MS | 40 | 63 |
| 11 | 3a (5) | DBU (2.1 eq) | 1,4-dioxane + 4 Å MS | 40 | 70 |
| 12 | 3a (5) | DBU (6 mol%) | THF + 4 Å MS | 40 | n.r. |
| 13 | 3a (1) | DBU (2.1 eq) | THF + 4 Å MS | 40 | 6 |
| 14 | — | DBU (2.1 eq) | THF + 4 Å MS | 40 | — |
| 15 | 3a (5) | DBU (2.1 eq) | THF + 4 Å MS | 40 | 71d |
| 16 | 3b (5) | DBU (2.1 eq) | THF + 4 Å MS | 40 | 36 |
| 17 | 3c (5) | DBU (2.1 eq) | THF + 4 Å MS | 40 | 7 |
| 18 | 3d (5) | DBU (2.1 eq) | THF + 4 Å MS | 40 | 37 |
| 19 | 3a (5) | DBU (2.1 eq) | THF + 4 Å MS | 80 | 76e |
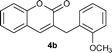
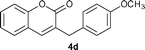
Under the optimized reaction conditions described above, the condensation reaction of salicylaldehyde (2a) with a variety of cinnamaldehydes was investigated to examine the scope of the reaction (Table 2). The cinnamaldehyde derivatives having either electron-withdrawing or -donating groups worked well affording corresponding products in moderate to good yields when 5 mol% of 3a was used. Cinnamaldehydes containing an electron-donating group were more reactive towards salicylaldehyde (entries 1–5) than unsubstituted cinnamaldehyde. The results showed that the electron-donating substituents at meta- or para- positions of cinnamaldehydes play little influence on the reaction, and the yields of corresponding products were comparable to that of 4a. However, 1b having an ortho-methoxyl group gave higher yield of 4b. The electron-withdrawing groups substituted cinnamaldehydes were less reactive. The yield of 4g was 63%, lower than the yield of 4a (entry 6). Cinnamaldehyde containing a strong electron-withdrawing group has proven to be inert in the NHC catalyzed conjugate umpolung condensation reaction between cinnamaldehydes and salicylaldehydes. Actually, 1h containing a trifluoromethyl group showed quite low reactivity and the yield of 4h was only 5% when 5 mol% of 3a was used. Nevertheless, when the catalyst loading was increased to 10 mol%, the reaction could be significantly improved, and the yield was increased to 44%.
The reaction of a series of substituted salicylaldehydes with cinnamaldehyde was also tested under the optimized conditions (Table 3). The results showed that both electron-withdrawing and -donating groups could be tolerated. The electron-donating groups such as methoxyl and methyl on the phenyl ring had little influence on the condensation reaction (entries 1–3 and 9–11). However, the electron-withdrawing groups lowered the yields of corresponding products (entries 6–8). Compared to 4 or 5-substituted salicylaldehydes, 3-substituted salicylaldehydes were less reactive, even 3-methyl salicylaldehyde (2l) was inert. Probably the hindrance of the 3-substituted salicylaldehyde made it difficult to undergo nucleophilic substitution with the active carbonyl speies.6a,6b The optimized reaction conditions had good tolerance towards some functional groups, such as ester groups (entry 12) and hydroxy groups (entry 13), and the corresponding products were suitable for further functionalization.
The reactivity of aliphatic α,β-unsatuated aldehyde was investigated and the results are listed in Scheme 2. The catalytic generation of homoenolate anion was expected to be easier from cinnamaldehydes than from crotonaldehydes as the phenyl ring in cinnamaldehydes could lower the energy of III. Indeed, compared to cinnamaldehyde, crotonaldehyde is not so reactive towards salicylaldehydes. When 5 mol% of 3a was used, we found that the reaction between crotonaldehyde and salicylaldehydes gave 7a in only 5% yield. When the catalyst loading was increased to 30 mol%, 7a could be yielded in 50%. Other salicylaldehydes could also afford corresponding products in moderate yields. 2-Hydroxy-1-naphthaldehyde (2f) reacted as efficiently as 2a. Electron-donating substituents such as methyl and methoxyl could promote the reaction, affording 7b and 7c in 55% and 62% yields, respectively. Salicylaldehydes bearing electron-withdrawing groups such as 2g and 2i reacted less efficiently affording corresponding products in 38% and 43%, respectively.
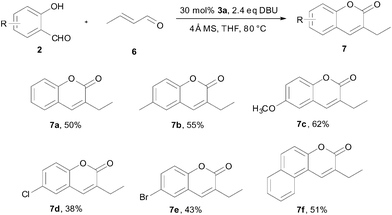 | ||
| Scheme 2 Reaction of salicylaldehydes with crotonaldehyde. | ||
The proposed catalytic cycle is shown in Scheme 3. NHC species generated from deprotonation of IMes·HCl (3a) added to cinnamaldehyde (1a) leading to I. Further proton transfer resulted in the formation of intermediate II. Tautomerization of III would give its enolate form IV. Subsequent nucleophilic addition of IV to the carbonyl group of salicylaldehyde would afford V. Cyclization of V would give VI and release free IMes. Protonization and dehydration would be expected to form the final product 4a. Although γ-lactone 5 as a byproduct would also be expected from the reaction of III with another molecule of cinnamaldehyde. Using 2 equivalent of 2a has significantly suppressed the formation of 5. However, lactone 8, an outcome of III undergoes 1,2-addition to 2a, was not observed.
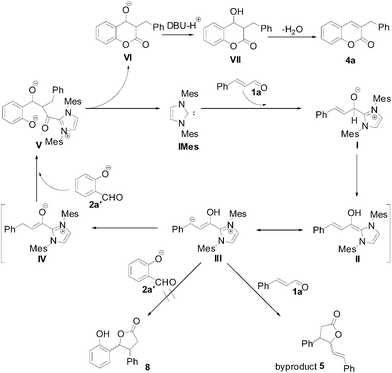 | ||
| Scheme 3 A proposed catalytic cycle. | ||
Conclusion
In summary, we described an NHC catalyzed intermolecular condensation reaction between salicylaldehydes and trans α,β-unsatuated aldehydes leading to 3-benzyl-chromen-2-ones in good to excellent yields. The low catalyst loading, good efficiency and good tolerance towards some functional groups made the procedure a promising way to synthesize chromen-2-one derivatives.Experimental section
General information
All reactions were carried out under a nitrogen atmosphere. THF, 1,4-dioxane and toluene were dried and freshly distilled from sodium/benzophenone. t-BuOH and DCM were dried by distillation from calcium hydride and stored under nitrogen. Petroleum ether and ethyl acetate were distilled. Flash chromatography was carried out with silica gel (silica gel, 300–400 mesh). 1H and 13C NMR spectra were recorded on Bruker AV-400 spectrometers with chloroform, methyl sulfoxide or acetone solutions of the compounds at a temperature of 300 K. Chemical shifts (δ) are expressed in ppm downfield to TMS at δ = 0 ppm and coupling constants (J) are expressed in Hz. IR spectra were recorded on a Bruker Vector-22 spectrophotometer and reported in cm−1. EI mass spectra were recorded on GC-TOF.Cinnamaldehyde and its derivatives were distilled under reduced pressure before use. All the carbene precursors were prepared according to known procedures.16 Other chemicals were used without further purification.
General procedure for NHC catalyzed synthesis of 3-alkyl-2H-chromen-2-one
Under an atmosphere of nitrogen, a mixture of trans α,β-unsaturated aldehyde (1.0 mmol), salicylaldehyde (2.0 mmol), 3a (17.1 mg, 0.05 mmol), DBU (320.0 mg, 2.1 mmol), and 0.5 g of 4 Å molecular sieves in 2 mL of THF was heated at 80 °C for 6 h. After cooling to room temperature the mixture was filtered. The filtrate was concentrated under reduced pressure, and the residue was purified by flash chromatography using petroleum ether/ethyl acetate (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent.
1) as the eluent.
3-Benzyl-2H-chromen-2-one (4a)12
Yield: 76%. White solid. Mp: 105–106 °C. 1H NMR (400 MHz, CDCl3) δ: 7.46 (t, J = 8.0 Hz, 1H), 7.36–7.27 (m, 8H), 7.23 (t, J = 8.0 Hz, 1H), 3.90 (s, 2H); 13C NMR (100 MHz, DMSO-d6) δ: 161.1, 153.1, 140.4, 138.7, 131.5, 129.3, 128.9, 128.7, 128.4, 126.9, 124.9, 119.6, 116.3, 36.4; IR (KBr): 1712, 1608, 1077, 1050, 754, 697 cm−1; HRMS (TOF MS EI+) calcd. for C16H12O2, m/z 236.0837, found 236.0841.3-(2-Methoxybenzyl)-2H-chromen-2-one (4b)14
Yield: 84%. White solid. Mp: 95–96 °C. 1H NMR (400 MHz, CDCl3) δ: 7.44 (dt, J = 7.6 Hz, J = 1.6 Hz, 1H), 7.34–7.26 (m, 4H), 7.20 (m, 2H), 6.96 (dt, J = 7.4 Hz, J = 1.0 Hz, 1H), 6.92 (d, J = 8.0 Hz, 1H), 3.89 (s, 2H), 3.81 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 161.9, 157.7, 153.1, 138.8, 131.4, 130.5, 128.8, 128.4, 127.4, 126.0, 124.2, 120.8, 19.8, 116.4, 110.7, 55.5, 31.0; IR (KBr): 1709, 1607, 1276, 1048, 755 cm−1; HRMS (TOF MS EI+) calcd. for C17H14O3, m/z 266.0943, found 266.0947.3-(3-Methoxybenzyl)-2H-chromen-2-one (4c)
Yield: 73%. White solid. Mp: 92–94 °C. 1H NMR (400 MHz, CDCl3) δ: 7.46 (dt, J = 7.9 Hz, J = 1.4 Hz, 1H), 7.36 (dd, J = 7.8 Hz, J = 1 Hz, 1H), 7.33–7.26 (m, 3H), 7.23 (dt, J = 7.5 Hz, J = 1 Hz, 1H), 6.88 (d, J = 7.6 Hz, 1H), 6.84–6.82 (m, 2H), 3.87 (s, 2H), 3.81 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 161.8, 160.1, 153.3, 139.5, 139.4, 131.0, 129.9, 129.4, 127.6, 124.4, 121.9, 119.6, 116.6, 115.3, 112.3, 55.4, 36.7; IR (KBr): 1707, 1608, 1258, 1151, 1056, 761 cm−1; HRMS (TOF MS EI+) calcd. for C17H14O3, m/z 266.0943, found 266.0948.3-(4-Methoxybenzyl)-2H-chromen-2-one (4d)17
Yield: 75%. White solid. Mp: 119–120 °C. 1H NMR (400 MHz, CDCl3) δ: 7.46 (dt, J = 7.9 Hz, J = 1.4 Hz, 1H), 7.36 (dd, J = 7.8, 1.4 Hz, 1H), 7.32 (d, J = 8.0 Hz, 1H), 7.26–7.20 (m, 4H), 6.90 (d, J = 8.4 Hz, 2H), 3.84 (s, 2H), 3.82 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 161.8, 158.6, 153.2, 139.1, 130.8, 130.5, 129.9, 129.7, 127.5, 124.4, 119.6, 116.5, 114.3, 55.4, 35.8; IR (KBr): 1723, 1608, 1512, 1244, 1166, 1036, 755 cm−1; HRMS (TOF MS EI+) calcd. for C17H14O3, m/z 266.0943, found 266.0948.3-(4-(Dimethylamino)benzyl)-2H-chromen-2-one (4e)
Yield: 77%. Pink solid. Mp: 135–136 °C. 1H NMR (400 MHz, DMSO-d6) δ: 7.70 (s, 1H), 7.62 (d, J = 7.6 Hz, 1H), 7.53 (t, J = 7.8 Hz, 1H), 7.36 (d, J = 8.8 Hz, 1H), 7.30 (t, J = 7.4 Hz, 1H), 7.11 (d, J = 8.0 Hz, 2H), 6.67 (d, J = 8.4 Hz, 2H), 3.67 (s, 2H), 2.84 (s, 6H); 13C NMR (100 MHz, DMSO-d6) δ: 161.2, 153.0, 149.6, 139.6, 131.3, 129.9, 129.7, 128.3, 125.8, 124.8, 119.7, 116.3, 113.0, 40.6, 35.5; IR (KBr): 1707, 1612, 1523, 1357, 1052, 752 cm−1; HRMS (TOF MS EI+) calcd. for C18H17NO2, m/z 279.1259, found 279.1262.3-(4-Methylbenzyl)-2H-chromen-2-one (4f)
Yield: 75%. White solid. Mp: 110 °C. 1H NMR (400 MHz, CDCl3) δ: 7.46 (t, J = 7.3 Hz, 1H), 7.36 (d, J = 7.6 Hz, 1H), 7.31 (d, J = 8.8 Hz, 1H), 7.27 (s, 1H), 7.22 (t, J = 7.6 Hz, 1H), 7.18–7.15 (m, 4H), 3.86 (s, 2H), 2.36 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 161.7, 153.1, 139.2, 136.5, 134.6, 130.8, 129.7, 129.5, 129.3, 127.4, 124.3, 119.6, 116.4, 36.2, 21.1; IR (KBr):1707, 1609, 1511, 1455, 1051, 752 cm−1; HRMS (TOF MS EI+) calcd. for C17H14O2, m/z 250.0994, found 250.0994.3-(4-Chlorobenzyl)-2H-chromen-2-one (4g)
Yield: 63%. White solid. Mp: 127–128 °C. 1H NMR (400 MHz, CDCl3) δ: 7.48 (t, J = 7.6 Hz, 1H), 7.38 (d, J = 7.6 Hz, 1H), 7.33–7.30 (m, 4H), 7.26–7.23 (m, 3H), 3.86 (s, 2H); 13C NMR (100 MHz, CDCl3) δ: 161.6, 153.2, 139.5, 136.3, 132.8, 131.1, 130.8, 129.0, 128.9, 127.5, 124.5, 119.4, 116.5, 36.1; IR (KBr): 1710, 1629, 1608, 1490, 1094, 1068, 1014, 757 cm−1; HRMS (TOF MS EI+) calcd. for C16H11ClO2, m/z 270.0448, found 270.0450.3-(3-(Trifluoromethyl)benzyl)-2H-chromen-2-one (4h)
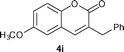
Imidazolium salt 3a (34.2 mg, 0.1 mmol) was used as precatalyst. Yield: 44%. White solid. Mp: 100–101 °C. 1H NMR (400 MHz, CDCl3) δ: 7.55–7.45 (m, 5H), 7.41 (dd, J = 7.8 Hz, J = 1.4 Hz, 1H), 7.36 (s, 1H), 7.33 (d, J = 8.8 Hz, 1H), 7.26 (dt, J = 7.6 Hz, J = 0.8 Hz, 1H), 4.0 (s, 2H); 13C NMR (100 MHz, CDCl3) δ: 161.5, 153.3, 139.8, 139.0, 132.8, 131.2, 131.1 (q, J = 32.1 Hz), 129.3, 128.4, 127.6, 126.0 (q, J = 4.0 Hz), 124.5, 124.2 (q, J = 270.3 Hz), 123.8 (q, J = 3.9 Hz), 119.3, 116.5, 36.6; IR (KBr): 1718, 1609, 1331, 1163, 1121, 1072, 755, 703 cm−1; HRMS (TOF MS EI+) calcd. for C17H11F3O2, m/z 304.0711, found 304.0709.3-Benzyl-6-methoxy-2H-chromen-2-one (4i)12
Yield: 71%. White solid. Mp: 129–130 °C. 1H NMR (400 MHz, CDCl3) δ: 7.38–7.35 (m, 2H), 7.30–7.27 (m, 3H), 7.24 (d, J = 9.2 Hz, 1H), 7.21 (s, 1H), 7.04 (dd, J = 9.6 Hz, J = 2.8 Hz, 1H), 6.79 (d, J = 3.2 Hz, 1H), 3.90 (s, 2H), 3.80 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 161.9, 156.1, 147.5, 139.2, 137.8, 129.8, 129.5, 128.8, 126.9, 119.8, 118.6, 117.4, 109.6, 55.8, 36.6; IR (KBr): 1708, 1577, 1492, 1451, 1284, 1066, 1038, 815, 702 cm−1; HRMS (TOF MS EI+) calcd. for C17H14O3, m/z 266.0943, found 266.0945.3-Benzyl-7-methoxy-2H-chromen-2-one (4j)12
Yield: 72%. White solid. Mp: 106–107 °C. 1H NMR (400 MHz, CDCl3) δ: 7.37–7.33 (m, 2H), 7.30–7.22 (m, 5H), 6.81–6.78 (m, 2H), 3.86 (s, 2H), 3.85 (s, 3H) ; 13C NMR (100 MHz, CDCl3) δ: 162.1, 162.0, 154.8, 139.5, 138.2, 129.4, 128.8, 128.4, 126.8, 125.8, 113.1, 12.4, 100.6, 55.8, 36.5; IR (KBr): 1709, 1616, 1251, 1150, 1051, 1022, 827 cm−1; HRMS (TOF MS EI+) calcd. for C17H14O3, m/z 266.0943, found 266.0947.3-Benzyl-8-methoxy-2H-chromen-2-one (4k)12
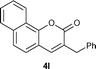
Yield: 62%. White solid. Mp: 86–89 °C. 1H NMR (400 MHz, CDCl3) δ: 7.37–7.34 (m, 2H), 7.30–7.27 (m, 3H), 7.23 (s, 1H), 7.15 (t, J = 8.0 Hz, 1H), 7.01 (dd, J = 8.0 Hz, J = 1.2 Hz, 1H), 6.93 (dd, J = 8.0 Hz, J = 1.2 Hz, 1H), 3.95 (s, 3H), 3.90 (s, 2H) ; 13C NMR (100 MHz, CDCl3) δ: 161.2, 147.1, 142.8, 139.5, 137.8, 129.7, 129.5, 128.8, 126.9, 124.2, 120.2, 119.0, 112.9, 56.3, 36.6; IR (KBr): 1719, 1609, 1580, 1481, 1281, 1110, 1060, 976, 783, 749, 729 cm−1; HRMS (TOF MS EI+) calcd. for C17H14O3, m/z 266.0943, found 266.0945.3-Benzyl-2H-benzo[h]chromen-2-one (4l)18
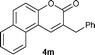
Yield: 68%. White solid. Mp: 129–130 °C. 1H NMR (400 MHz, CDCl3) δ: 8.55–8.53 (m, 1H), 7.86–7.84 (m, 1H), 7.65–7.60 (m, 3H), 7.40–7.28 (m, 7H), 3.97 (s, 2H); 13C NMR (100 MHz, CDCl3) δ: 161.8, 150.0, 140.1, 138.0, 134.4, 129.5, 129.0, 128.9, 128.3, 127.9, 127.1, 127.0, 124.3, 123.6, 123.0, 122.2, 114.9, 36.7; IR (KBr): 1709, 1614, 1061, 815, 761, 700 cm−1; HRMS (TOF MS EI+) calcd. for C20H14O2, m/z 286.0994, found 286.0996.2-Benzyl-3H-benzo[f]chromen-3-one (4m)12
Yield: 76%. White solid. Mp: 156–158 °C. 1H NMR (400 MHz, CDCl3) δ: 8.11 (s, 1H), 8.06 (d, J = 8.4 Hz, 1H), 7.93 (d, J = 8.8 Hz, 1H), 7.89 (d, J = 8.0 Hz, 1H), 7.62 (dt, J = 7.6 Hz, J = 1.2 Hz, 1H), 7.54 (t, J = 7.4 Hz, 1H), 7.47 (d, J = 9.2 Hz, 1H), 7.41–7.29 (m, 5H), 4.01 (s, 2H); 13C NMR (100 MHz, CDCl3) δ: 161.8, 152.5, 138.0, 135.2, 132.1, 130.3, 129.4, 129.0, 128.9, 128.8, 128.5, 128.0, 127.0, 125.9, 121.5, 116.7, 113.4, 37.0; IR (KBr): 1709, 1065 cm−1; HRMS (TOF MS EI+) calcd. for C20H14O2, m/z 286.0994, found 286.0997.3-Benzyl-6-chloro-2H-chromen-2-one (4n)19
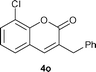
Yield: 50%. White solid. Mp: 143 °C. 1H NMR (400 MHz, CDCl3) δ: 7.40 (dd, J = 8.8 Hz, 2Hz, 1H), 7.39–7.27 (m, 7H), 7.17 (s, 1H), 3.90 (s, 2H); 13C NMR (100 MHz, CDCl3) δ: 161.1, 151.5, 138.0, 137.3, 130.9, 130.7, 129.6, 129.5, 129.0, 127.1, 126.8, 120.6, 117.9, 36.7; IR (KBr): 1722, 1481, 1079, 1048, 849,817, 757, 704 cm−1; HRMS (TOF MS EI+) calcd. for C16H11O2Cl, m/z 270.0448, found 270.0450.3-Benzyl-8-chloro-2H-chromen-2-one (4o)
Yield: 48%. White solid. Mp: 110–113 °C. 1H NMR (400 MHz, CDCl3) δ: 7.52 (dd, J = 8.2 Hz, 1H), 7.38–7.34 (m, 2H), 7.30–7.27 (m, 3H), 7.25–7.24 (m, 2H), 7.16 (t, J = 7.8 Hz, 1H), 3.91 (s, 2H); 13C NMR (100 MHz, CDCl3) δ: 160.7, 148.9, 138.9, 137.4, 131.3, 130.5, 129.5, 129.0, 127.1, 126.0, 124.6, 121.4, 120.8, 36.6; IR (KBr): 1726, 1450, 1073, 1041, 794, 695 cm−1; HRMS (TOF MS EI+) calcd. for C16H11ClO2, m/z 207.0448, found 207.0444.3-Benzyl-6-bromo-2H-chromen-2-one (4p)
Yield: 62%. White solid. Mp: 143–144 °C. 1H NMR (400 MHz, CDCl3) δ: 7.54 (dd, J = 8.8 Hz, J = 2.4 Hz, 1H), 7.49 (d, J = 2.4 Hz, 1H), 7.36 (t, J = 7.2 Hz, 2H), 7.31–7.28 (m, 3H), 7.19 (d, J = 9.2 Hz, 1H), 7.16 (s, 1H), 3.89 (s, 2H); 13C NMR (100 MHz, CDCl3) δ: 161.0, 151.9, 137.9, 137.3, 133.5, 130.9, 129.8, 129.5, 129.0, 127.1, 121.0, 118.2, 116.9, 36.7; IR (KBr): 1720, 1477, 1050, 823, 752, 726, 700 cm−1; HRMS (TOF MS EI+) calcd. for C16H11BrO2, m/z 313.9942, found 313.9943.3-Benzyl-6-methyl-2H-chromen-2-one (4q)18,20
Yield: 68%. White solid. Mp: 123–128 °C. 1H NMR (400 MHz, CDCl3) δ: 7.37–7.34 (m, 2H), 7.30–7.25 (m, 4H), 7.22–7.20 (m, 2H), 7.15 (s, 1H), 3.89 (s, 2H), 2.36 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 161.9, 151.3, 139.3, 137.9, 134.0, 131.8, 129.5, 129.3, 128.8, 127.3, 126.9, 119.2, 116.2, 36.6, 20.8; IR (KBr): 1711, 1492, 1058, 821, 756, 702 cm−1; HRMS (TOF MS EI+) calcd. for C17H14O2, m/z 250.0994, found 250.0998.3-Benzyl-7-methyl-2H-chromen-2-one (4r)
Yield: 69%. White solid. Mp: 103–105 °C. 1H NMR (400 MHz, CDCl3) δ: 7.53 (s, 1H), 7.37–7.27 (m, 6H), 7.16 (d, J = 8.8 Hz, 1H), 7.05 (d, J = 6.8 Hz, 1H), 3.92 (s, 2H), 2.41 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 161.7, 153.7, 138.1, 136.4, 135.6, 130.6, 129.3, 128.8, 128.7, 126.9, 125.7, 118.2, 114.5, 37.0, 18.3; IR (KBr): 1710, 1602, 1462, 1073, 1039, 790, 757, 733, 702 cm−1; HRMS (TOF MS EI+) calcd. for C17H14O2, m/z 250.0994, found 250.0995.Ethyl 3-benzyl-2-oxo-2H-chromene-6-carboxylate (4t)
Yield: 45%. White solid. Mp: 140–142 °C. 1H NMR (400 MHz, CDCl3) δ: 8.13 (dd, J = 8.6 Hz, J = 2.2 Hz, 1H), 8.08 (d, J = 2.0 Hz, 1H), 7.39–7.34 (m, 3H), 7.32–7.28 (m, 4H), 4.38 (q, J = 7.6 Hz, 2H), 3.90 (s, 2H), 1.39 (t, J = 7.6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 165.4, 161.1, 155.9, 139.0, 137.3, 131.9, 130.5, 129.6, 129.5, 129.0, 127.1, 126.9, 119.2, 116.7, 61.5, 36.7, 14.4; IR (neat): 1725, 1705, 1606, 1271, 1242, 1217, 1170, 1101, 1046, 765, 707 cm−1; HRMS (TOF MS EI+) calcd. for C19H16O4, m/z 308.1049, found 308.1047.3-Benzyl-6-hydroxy-2H-chromen-2-one (4u)
4.1 eq of DBU (624 mg, 4.1 mmol) was used. After cooling to room temperature, the reaction mixture was neutralized by dilute hydrochloric acid. The organic layer was separated. Aqueous layer was extracted by ethyl acetate (10 ml × 3). The organic layer was collected and dried over MgSO4. After concentrated under reduced pressure, the residue was purified by flash chromatography using petroleum ether/ethyl acetate (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent. Yield: 61%. Pale yellow solid, Mp: 180–182 °C. 1H NMR (400 MHz, CDCl3) δ: 7.37–7.33 (m, 2H), 7.29–7.26 (m, 3H), 7.20–7.18 (m, 2H), 6.97 (dd, J = 8.6 Hz, J = 3.0 Hz, 1H), 6.79 (d, J = 2.8 Hz, 1H), 5.53 (br, 1H), 3.89 (s, 2H); 13C NMR (100 MHz, Acetone-d6) δ: 161.5, 154.3, 147.4, 139.7, 139.1, 129.8, 129.6, 128.9, 126.9, 120.6, 119.3, 117.3, 112.6, 38.8; IR (neat): 3433, 3199, 1699, 1666, 1580, 1449, 1228, 1143, 975, 715, 697, 589 cm−1; HRMS (TOF MS EI+) calcd. for C16H12O3, m/z 252.0786, found 252.0782.
1) as the eluent. Yield: 61%. Pale yellow solid, Mp: 180–182 °C. 1H NMR (400 MHz, CDCl3) δ: 7.37–7.33 (m, 2H), 7.29–7.26 (m, 3H), 7.20–7.18 (m, 2H), 6.97 (dd, J = 8.6 Hz, J = 3.0 Hz, 1H), 6.79 (d, J = 2.8 Hz, 1H), 5.53 (br, 1H), 3.89 (s, 2H); 13C NMR (100 MHz, Acetone-d6) δ: 161.5, 154.3, 147.4, 139.7, 139.1, 129.8, 129.6, 128.9, 126.9, 120.6, 119.3, 117.3, 112.6, 38.8; IR (neat): 3433, 3199, 1699, 1666, 1580, 1449, 1228, 1143, 975, 715, 697, 589 cm−1; HRMS (TOF MS EI+) calcd. for C16H12O3, m/z 252.0786, found 252.0782.
For 7a–c, 3a (102.3 mg, 0.3 mmol) and DBU (365 mg, 2.4 mmol) were used.
3-Ethyl-2H-chromen-2-one (7a)14
Yield: 50%. White solid. Mp: 72–73 °C. 1H NMR (400 MHz, CDCl3) δ: 7.49–7.44 (m, 3H), 7.32 (d, J = 8.4 Hz, 1H), 7.26 (dt, J = 7.3 Hz, J = 0.8 Hz, 1H), 2.61 (dq, J = 7.5 Hz, J = 1.0 Hz, 2H), 1.27 (t, J = 7.6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 161.8, 153.1, 137.6, 131.4, 130.4, 127.3, 124.3, 119.7, 116.4, 24.0, 12.4; IR (KBr): 1718, 1605, 1175, 1081, 1053, 766, 714 cm−1; HRMS (TOF MS EI+) calcd. for C11H10O2, m/z 174.0681, found 174.0679.3-Ethyl-6-methyl-2H-chromen-2-one (7b)
Yield: 55%. White solid. Mp: 54–55 °C. 1H NMR (400 MHz, CDCl3) δ: 7.43 (s, 1H), 7.27–7.20 (m, 3H), 2.60 (q, J = 7.2 Hz, 2H), 2.4 (s, 3H), 1.25 (t, J = 7.6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 162.1, 151.2, 137.6, 133.9, 131.5, 131.2, 127.1, 119.4, 116.1, 24.0, 20.8, 12.4; IR (KBr): 1710, 1182, 1088, 1053, 818 cm−1; HRMS (TOF MS EI+) calcd. for C12H12O2, m/z 188.0837, found 188.0841.3-Ethyl-6-methoxy-2H-chromen-2-one (7c)
Yield: 62%. White solid. Mp: 106–107 °C. 1H NMR (400 MHz, CDCl3) δ: 7.43 (s, 1H), 7.34 (d, J = 8.4 Hz, 1H), 6.84–6.82 (m, 2H), 3.86 (s, 3H), 5.71 (q, J = 7.5 Hz, 2H), 1.24 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 162.2, 161.9, 154.7, 137.7, 128.1, 127.7, 113.3, 112.3, 100.6, 55.8, 23.8, 12.5; IR (KBr): 1705, 1615, 1506, 1238, 1155, 1087, 1053, 1025 cm−1; HRMS (TOF MS EI+) calcd. for C12H12O3, m/z 204.0786, found 204.0785.6-Chloro-3-ethyl-2H-chromen-2-one (7d)
Yield: 38%. White solid. Mp: 130–131 °C. 1H NMR (400 MHz, CDCl3) δ: 7.43–7.40 (m, 3H), 7.27–7.25 (m, 1H), 2.61 (dq, J = 7.4 Hz, J = 1.1 Hz, 2H), 1.26 (t, J = 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 161.2, 151.5, 136.3, 132.8, 130.5, 129.5, 126.5, 120.8, 117.9, 24.1, 12.3; IR (KBr): 1716, 1631, 1480, 1248, 1180, 1087, 1058, 817 cm−1; HRMS (TOF MS EI+) calcd. for C11H9ClO2, m/z 208.0291, found 208.0294.6-Bromo-3-ethyl-2H-chromen-2-one (7e)14,15
Yield: 43%. White solid. Mp: 113–114 °C. 1H NMR (400 MHz, CDCl3) δ: 7.59 (d, J = 2.0 Hz, 1H), 7.55 (dd, J = 8.8 Hz, J = 2.4 Hz, 1H), 7.40 (s, 1H), 7.20 (d, J = 8.8 Hz, 1H), 2.62 (dq, J = 7.8 Hz, J = 1.1 Hz, 2H), 1.26 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 161.2, 152.0, 136.2, 133.3, 132.8, 129.6, 121.3, 118.2, 116.9, 24.1, 12.3; IR (KBr): 1719, 1091, 1065, 814, 650 cm−1; HRMS (TOF MS EI+) calcd. for C11H9BrO2, m/z 251.9786, found 251.9782.2-Ethyl-3H-benzo[f]chromen-3-one (7f)21
Yield: 51%. White solid. Mp: 108–109 °C. 1H NMR (400 MHz, CDCl3) δ: 8.82 (s, 1H), 8.27 (d, J = 7.2 Hz, 1H), 7.93 (d, J = 8.8 Hz, 1H), 7.91 (d, J = 6.8 Hz, 1H), 7.68 (dt, J = 7.2 Hz, J = 1.2 Hz, 1H), 7.56 (t, J = 7.2 Hz, 1H), 7.47 (d, J = 8.8 Hz, 1H), 2.73 (m, 2H), 1.35 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 161.9, 152.4, 133.3, 131.6, 130.5, 130.3, 129.0, 128.8, 127.9, 125.8, 121.5, 116.8, 113.5, 24.4, 12.6; IR (KBr): 1710, 1576, 1212, 1086, 965, 814 cm−1; HRMS (TOF MS EI+) calcd. for C15H12O2, m/z 224.0837, found 224.0841.Acknowledgements
The authors thank the National Science Foundation of China for financial support through project No. 21072170.References
- (a) K. Zeitler, Angew. Chem., Int. Ed., 2005, 44, 7506 CrossRef CAS; (b) N. Marion, S. Diez-Gonzalez and S. P. Nolan, Angew. Chem., Int. Ed., 2007, 46, 2988 CrossRef CAS; (c) V. Nair, S. Bindu and V. Sreekumar, Angew. Chem., Int. Ed., 2004, 43, 5130 CrossRef CAS; (d) V. Nair, S. Vellalath and B. P. Babu, Chem. Soc. Rev., 2008, 37, 2691 RSC; (e) V. Nair, R. S. Menon, A. T. Biju, C. R. Sinu, R. R. Paul, A. Josea and V. Sreekumar, Chem. Soc. Rev., 2011, 40, 5336 RSC.
- (a) G. Q. Li, L. X. Dai and S. L. You, Chem. Commun., 2007, 852 RSC; (b) Y. Shimakawa, T. Morikawa and S. Sakaguchi, Tetrahedron Lett., 2010, 51, 1786 CrossRef CAS; (c) S. Vedachalam, J. Zeng, B. K. Gorityala, M. Antonio and X. W. Liu, Org. Lett., 2010, 12, 352 CrossRef CAS; (d) L. D. S. Yadav, V. K. Rai, S. Singh and P. Singh, Tetrahedron Lett., 2010, 51, 1657 CrossRef CAS.
- (a) J. L. Moore, M. S. Keff and T. Rovis, Tetrahedron, 2006, 62, 11477 CrossRef CAS; (b) S. C. Cullen and T. Rovis, Org. Lett., 2008, 10, 3141 CrossRef CAS; (c) J. R. de Alaniz, M. S. Kerr, J. L. Moore and T. Rovis, J. Org. Chem., 2008, 73, 2033 CrossRef; (d) Q. Liu, S. Perreault and T. Rovis, J. Am. Chem. Soc., 2008, 130, 14066 CrossRef CAS; (e) A. Orellana and T. Rovis, Chem. Commun., 2008, 730 RSC; (f) D. A. DiRocco, K. M. Oberg, D. M. Dalton and T. Rovis, J. Am. Chem. Soc., 2009, 131, 10872 CrossRef CAS; (g) K. Hirano, A. T. Biju, I. Piel and F. Glorius, J. Am. Chem. Soc., 2009, 131, 14190 CrossRef CAS; (h) Q. Liu and T. Rovis, Org. Lett., 2009, 11, 2856 CrossRef CAS; (i) A. T. Biju, N. E. Wurz and F. Glorius, J. Am. Chem. Soc., 2010, 132, 5970 CrossRef CAS; (j) E. Sanchez-Larios, J. M. Holmes, C. L. Daschner and M. Gravel, Org. Lett., 2010, 12, 5772 CrossRef CAS; (k) T. Jousseaume, N. E. Wurz and F. Glorius, Angew. Chem., Int. Ed., 2011, 50, 1410 CrossRef CAS.
- (a) G. Duvey, F. Nivoliers, P. Rocca, A. Godard, F. Marsais and G. Queguiner, J. Heterocycl. Chem., 2001, 38, 1039 CrossRef CAS; (b) Y. Suzuki, T. Toyota, F. Imada, M. Sato and A. Miyashita, Chem. Commun., 2003, 1314 RSC; (c) Y. Suzuki, S. Ota, Y. Fukuta, Y. Ueda and M. Sato, J. Org. Chem., 2008, 73, 2420 CrossRef CAS; (d) E. Awuah and A. Capretta, J. Org. Chem., 2010, 75, 5627 CrossRef CAS; (e) Y. Suzuki, Y. Fukuta, S. Ota, M. Kamiya and M. Sato, J. Org. Chem., 2011, 76, 3960 CrossRef CAS.
- (a) C. Burstein and F. Glorius, Angew. Chem., Int. Ed., 2004, 43, 6205 CrossRef CAS; (b) L. R. Domingo, R. J. Zaragoza and M. Arno, Org. Biomol. Chem., 2010, 8, 4884 RSC; (c) V. Nair, V. Varghese, B. P. Babu, C. R. Sinu and E. Suresh, Org. Biomol. Chem., 2010, 8, 761 RSC; (d) V. Nair, S. Vellalath, B. P. Babu, V. Varghese, R. R. Paul and E. Suresh, Org. Biomol. Chem., 2010, 8, 4861 RSC.
- (a) A. Chan and K. A. Scheidt, Org. Lett., 2005, 7, 905 CrossRef CAS; (b) S. S. Sohn and J. W. Bode, Org. Lett., 2005, 7, 3873 CrossRef CAS; (c) E. M. Phillips, T. E. Reynolds and K. A. Scheidt, J. Am. Chem. Soc., 2008, 130, 2416 CrossRef CAS; (d) V. Nair, B. P. Babu, S. Vellalath, V. Varghese, A. E. Raveendran and E. Suresh, Org. Lett., 2009, 11, 2507 CrossRef CAS; (e) J. Z. Ma, Y. Huang and R. Y. Chen, Org. Biomol. Chem., 2011, 9, 1791 RSC.
- (a) J. W. Bode and S. S. Sohn, J. Am. Chem. Soc., 2007, 129, 13798 CrossRef CAS; (b) P. C. Chiang, Y. Kim and J. W. Bode, Chem. Commun., 2009, 4566 RSC.
- (a) V. Nair, S. Vellalath, M. Poonoth and E. Suresh, J. Am. Chem. Soc., 2006, 128, 8736 CrossRef CAS; (b) P. C. Chiang, J. Kaeobamrung and J. W. Bode, J. Am. Chem. Soc., 2007, 129, 3520 CrossRef CAS; (c) M. Wadamoto, E. M. Phillips, T. E. Reynolds and K. A. Scheidt, J. Am. Chem. Soc., 2007, 129, 10098 CrossRef CAS; (d) B. Cardinal-David, D. E. A. Raup and K. A. Scheidt, J. Am. Chem. Soc., 2010, 132, 5345 CrossRef CAS; (e) D. T. Cohen, B. Cardinal-David, J. M. Roberts, A. A. Sarjeant and K. A. Scheidt, Org. Lett., 2011, 13, 1068 CrossRef CAS.
- (a) S. S. Sohn, E. L. Rosen and J. W. Bode, J. Am. Chem. Soc., 2004, 126, 14370 CrossRef CAS; (b) V. Nair, S. Vellalath, M. Poonoth, R. Mohan and E. Suresh, Org. Lett., 2006, 8, 507 CrossRef CAS; (c) J. Kaeobamrung and J. W. Bode, Org. Lett., 2009, 11, 677 CrossRef CAS; (d) K. Zeitler and C. A. Rose, J. Org. Chem., 2009, 74, 1759 CrossRef CAS; (e) K. Takaki, K. Shiraishi, K. Okinaga, S. Takahashi and K. Komeyama, RSC Adv., 2011, 1, 1799 RSC.
- (a) M. He and J. W. Bode, Org. Lett., 2005, 7, 3131 CrossRef CAS; (b) M. He, J. R. Struble and J. W. Bode, J. Am. Chem. Soc., 2006, 128, 8418 CrossRef CAS; (c) A. Chan and K. A. Scheidt, J. Am. Chem. Soc., 2007, 129, 5334 CrossRef CAS; (d) A. Chan and K. A. Scheidt, J. Am. Chem. Soc., 2008, 130, 2740 CrossRef CAS; (e) M. He and J. W. Bode, J. Am. Chem. Soc., 2008, 130, 418 CrossRef CAS; (f) M. Rommel, T. Fukuzumi and J. W. Bode, J. Am. Chem. Soc., 2008, 130, 17266 CrossRef CAS.
- (a) A. S. Douglas, Brit. Med. Bull., 1955, 11, 39 CAS; (b) P. Zboril, J. Holasova and V. Dadak, Collect. Czech. Chem. C., 1970, 35, 2983 CAS; (c) P. W. Lequesne, J. Am. Chem. Soc., 1983, 105, 6536 Search PubMed; (d) Y. Y. Stoilov and S. P. Bazhulin, Kvantovaya Elektronika, 1986, 13, 633 CAS; (e) G. J. Zlabinger, C. Nohammer, G. A. Bohmig and J. E. Menzel, J. Cancer Res. Clin. Oncol., 1994, 120, S17 CrossRef CAS; (f) L. Santana, E. Uriarte, F. Roleira, N. Milhazes and F. Borges, Curr. Med .Chem., 2004, 11, 3239 CAS; (g) F. Borges, F. Roleira, N. Milhazes, L. Santana and E. Uriarte, Curr. Med. Chem., 2005, 12, 887 CrossRef CAS.
- J. Torang, S. Vanderheiden, M. Nieger and S. Brase, Eur. J. Org. Chem., 2007, 943 CrossRef CAS.
- A. Behrenswerth, N. Volz, J. Torang, S. Hinz, S. Brase and C. E. Muller, Bioorg. Med. Chem., 2009, 17, 2842 CrossRef CAS.
- M. Shi, U. Gross, P. J. Gross and S. Brase, Synlett, 2011, 635 CrossRef.
- V. Nair, C. R. Sinu, R. Rejithamol, K. C. S. Lakshmi and E. Suresh, Org. Biomol. Chem., 2011, 9, 5511 CAS.
- (a) A. J. Arduengo, R. Krafczyk, R. Schmutzler, H. A. Craig, J. R. Goerlich, W. J. Marshall and M. Unverzagt, Tetrahedron, 1999, 55, 14523–14534 CrossRef CAS; (b) J. R. Struble and J. W. Bode, Org. Synth., 2010, 87, 362 CAS.
- K. C. Majumdar, G. H. Jana, S. K. Ghosh and S. Saha, Monatsh. Chem., 1997, 128, 641 CrossRef CAS.
- N. Britto, G. G. Vinayak, R. S. Mali and A. C. Ranade, Synth. Commun., 1989, 19, 1899 CrossRef CAS.
- T. V. Krishnamoorthy, K. Rajagopalan and K. K. Balasubramanian, Tetrahedron Lett., 1985, 26, 1747 CrossRef CAS.
- Y. Deshmukh Sanjay, L. Kelkar Shriniwas and S. Wadia Murzban, Synth. Commun., 1990, 20, 855 CrossRef.
- (a) T. Nakabayashi, E. Hori and N. Okamura, Yakugaku Zasshi, 1954, 74, 250 CAS; (b) R. Adams, L. O. Binder and F. C. McGrew, J. Am. Chem. Soc., 1942, 64, 1791 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c1ra00916h/ |
| This journal is © The Royal Society of Chemistry 2012 |