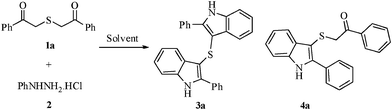
Solvent based selectivity in the synthesis of di(2-aryl-1H-3-indolyl) sulfides and 1-aryl-2-[(2-aryl-1H-3-indolyl)sulfanyl]-1-ethanones†
Selvam
Chitra
a,
Nidhin
Paul
b,
Shanmugam
Muthusubramanian
*b and
Paramasivam
Manisankar
*a
aDepartment of Industrial Chemistry, Alagappa University, Karaikudi—630 003, Tamil Nadu, India. E-mail: muthumanian2001@yahoo.com
bDepartment of Organic Chemistry, School of Chemistry, Madurai Kamaraj University, Madurai 625 021, India. E-mail: pms11@rediffmail.com
First published on 15th December 2011
Abstract
The commendable product selectivity exhibited by the solvents during the reaction of 2-[(2-oxo-2-arylethyl)sulfanyl]-1-aryl-1-ethanones with phenylhydrazine hydrochloride yielding exclusively 1-aryl-2-[(2-aryl-1H-3-indolyl)sulfanyl]-1-ethanones in THF and di(2-aryl-1H-3-indolyl) sulfides in ethanol is described.
Introduction
Indole nucleus is found in many medicinal compounds and hence is considered to be a very important heterocyclic moiety.1Indole and its derivatives possess a wide spectrum of biological activities including anti-inflammatory,2 antimicrobial,3 antibacterial,4anticonvulsant,5 cardiovascular6 and HIV-integrase inhibitor7 characteristics.The Fischer indolization of carbonyl synthons continues to maintain its prominent role as a route to indoles8,9 and in synthetic combinatorial chemistry.10 Though the Fischer method is the most widely used protocol for the synthesis of indoles, it suffers from low yields,11 formation of side products and low regioselectivity in the case of unsymmetrical ketones.11a–b,12 In contrast, the work described in the present investigation has achieved a remarkable selectivity yielding either mono- or bisindoles with the reaction medium determining the selectivity. It can be seen that protic solvents tend to favour the formation of bisindole, while aprotic solvents prefer to yield monoindole. Acetonitrile seems to be non selective.
Results and discussion
In continuation of our effort on the synthesis of diverse heterocyclic compounds13 of biological significance, we herein report a very simple and highly selective method for the synthesis of di(2-aryl-1H-3-indolyl) sulfides and 1-aryl-2-[(2-aryl-1H-3-indolyl)sulfanyl]-1-ethanones by the reaction of 2-[(2-oxo-2-arylethyl)sulfanyl]-1-aryl-1-ethanones with phenylhydrazine hydrochloride in ethanol and THF respectively (Scheme 1). It is interesting that the natural product Echinosulfone A, a sulfone from the marine sponge, Echinodictyum1e,14 has a bisindole core similar to that of di(2-aryl-1H-3-indolyl) sulphides generated during the present investigation.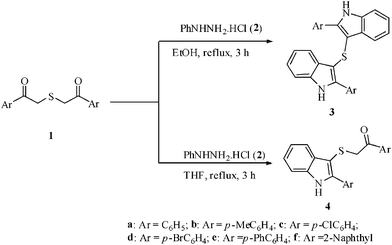 | ||
| Scheme 1 Synthesis of mono- and bisindoles. | ||
A mixture of 2-[(2-oxo-2-phenylethyl)sulfanyl]-1-phenyl-1-ethanone 1a (1 mmol) and phenylhydrazine hydrochloride 2 (2.5 mmol) when refluxed in ethanol (7 ml) gave di(2-phenyl-1H-3-indolyl) sulphide (3a) in 85% yield (Table 1). It is noteworthy that whatever be the mole ratio between 2-[(2-oxo-2-phenylethyl)sulfanyl]-1-phenyl-1-ethanone 1a and phenylhydrazine hydrochloride 2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1
1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5; 1
1.5; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; 1
2; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5), only the diheteroaryl sulphide and no mono indole was obtained. The best yield was obtained with a 1
2.5), only the diheteroaryl sulphide and no mono indole was obtained. The best yield was obtained with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 ratio (Table 1). Under these optimized conditions for the generation of 3, various substituted diketones 1 were selected to react with phenylhydrazine hydrochloride 2 to give different di(2-aryl-1H-3-indolyl) sulfides 3 in high yields (76–85%) within three hours (Table 2). The reaction proceeded efficiently, tolerating both electron donating and withdrawing substituents on the aromatic ring. When the reaction was performed in methanol, 2-propanol and ethylene glycol, the yield of 3a decreased considerably (Table 1). When solvents like toluene, chloroform, dichloromethane and DMF were used, either a viscous mass with no recognisable products was obtained or the starting materials were recovered unchanged. A notable observation is that the reaction has led to a mixture of 3a and 4a, when the reaction was investigated in acetonitrile (Table 1).
2.5 ratio (Table 1). Under these optimized conditions for the generation of 3, various substituted diketones 1 were selected to react with phenylhydrazine hydrochloride 2 to give different di(2-aryl-1H-3-indolyl) sulfides 3 in high yields (76–85%) within three hours (Table 2). The reaction proceeded efficiently, tolerating both electron donating and withdrawing substituents on the aromatic ring. When the reaction was performed in methanol, 2-propanol and ethylene glycol, the yield of 3a decreased considerably (Table 1). When solvents like toluene, chloroform, dichloromethane and DMF were used, either a viscous mass with no recognisable products was obtained or the starting materials were recovered unchanged. A notable observation is that the reaction has led to a mixture of 3a and 4a, when the reaction was investigated in acetonitrile (Table 1).
| Entry | Solvent | Mole ratio, 1a:2 | Time (h) | Yield of 3a (%)a | Yield of 4a (%)a |
|---|---|---|---|---|---|
| a Isolated yield after purification by column chromatography. | |||||
| 1 | EtOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
3 | 85 | 0 |
| 2 | EtOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0 |
3 | 76 | 0 |
| 3 | EtOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
3.5 | 54 | 0 |
| 4 | MeOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
3 | 72 | 0 |
| 5 | 2-Propanol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
3 | 64 | 0 |
| 6 | Ethylene glycol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
3 | 61 | 0 |
| 7 | DMF | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
5 | 0 | 0 |
| 8 | Toluene | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
5 | 0 | 0 |
| 9 | CH3CN | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
5 | 21 | 32 |
| 10 | CHCl3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
5 | 0 | 0 |
| 11 | CH2Cl2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
5 | 0 | 0 |
| 12 | THF | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
3 | 0 | 72 |
| 13 | THF | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
3 | 0 | 82 |
| 14 | THF | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0 |
3 | 0 | 81 |
| 15 | THF | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
3.5 | 0 | 82 |
| Entry | Ar | in EtOH | in THF | ||
|---|---|---|---|---|---|
| Time (h) | Yield of 3 (%)a | Time (h) | Yield of 4 (%)a | ||
| a Isolated yield after purification by recrystallisation from ethyl acetate. | |||||
| a | C6H5 | 3 | 85 | 3 | 82 |
| b | p-MeC6H4 | 3 | 81 | 3 | 79 |
| c | p-ClC6H4 | 1 | 82 | 2.5 | 85 |
| d | p-BrC6H4 | 1.5 | 79 | 2 | 82 |
| e | p-PhC6H4 | 2 | 81 | 4 | 78 |
| f | 2-Naphthyl | 1.5 | 76 | 3 | 79 |
Interestingly, when this reaction between 1a and 2 was performed in THF in different mole ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1
1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5; 1
1.5; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5), only the monoindole, 1-phenyl-2-[(2-phenyl-1H-3-indolyl)sulfanyl]-1-ethanone 4a was obtained in good yield (Table 1).
2.5), only the monoindole, 1-phenyl-2-[(2-phenyl-1H-3-indolyl)sulfanyl]-1-ethanone 4a was obtained in good yield (Table 1).
The cleanest conversion and highest yield of 4a was achieved when 1.5 equiv of the phenylhydrazine hydrochloride for 1.0 equivalent of 2-[(2-oxo-2-phenylethyl)sulfanyl]-1-phenyl-1-ethanone 1a was used. This protocol for 4a was used to generate a range of monoindoles 4a–f in 78–85% isolated yield (Table 2).
The structures of the isolated products bisindoles 3 and monoindoles 4 were deduced on the basis of IR, mass, 1H NMR and 13C NMR spectral studies. The structure of the symmetrical bisindoles 3 is in accord with the NMR spectroscopic data as illustrated for di[2-(4-methylphenyl)-1H-3-indolyl] sulphide 3b (Fig. 1).
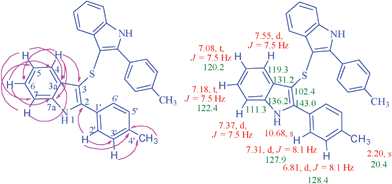 | ||
| Fig. 1 Selected HMBCs and 1H and 13C chemical shifts in compound 3b. | ||
The 1H NMR spectrum of 3b has two triplets at 7.08 and 7.18 ppm (J = 7.5 Hz) which are assignable to H-5 and H-6 of the indole ring respectively. These protons show C,H-COSY correlation with C-5 at 120.2 and C-6 at 122.4 ppm. Both H-5 and H-6 protons further show HMBCs with C-3a at 131.2, C-7 at 111.3 ppm and C-7a at 136.1, C-4 at 119.3 ppm respectively. The H-4 hydrogen gives a doublet at 7.55 ppm (J = 7.5 Hz), which shows C,H-COSY correlation with the signal at 119.3 ppm assignable to C-4 and HMBCs with C-3 at 102.4, C-3a at 131.2, C-7a at 136.2, C-6 at 122.4 ppm (Fig. 1). The doublet at 7.37 ppm (J = 7.5 Hz) is due to H-7 hydrogen and is having HMBC correlation with C-3a at 131.2 and C-5 at 120.2 ppm. The H-7 further gives C,H-COSY correlation with the signal at 111.3 ppm due to C-7. The NH proton appeared as a singlet at 10.68 ppm. Bisindole 3b shows absorption at 3374 cm−1 in its IR spectrum. There is neither the presence of carbonyl absorption band in IR nor any carbonyl signal in 13C NMR spectrum.
The 1H NMR spectrum of 1-(4-bromophenyl)-2-[2-(4-bromophenyl)-1H-3-indolyl]sulfanyl-1-ethanone (Fig. 2) 4d, the H-5 and H-6 protons of the indole ring appeared as triplet of doublets at 7.15 and 7.22 ppm (J = 7.8, 1.2 Hz) respectively. These protons show C,H-COSY correlation with C-5 at 120.8 ppm and C-6 at 123.3 ppm respectively and they further show HMBCs with C-3a at 131.3 ppm, C-7 at 112.1 ppm and C-4 at 119.4 ppm, C-7a at 136.8 ppm. The H-4 proton appears as a doublet at 7.67 ppm (J = 7.8 Hz) which shows C,H-COSY correlation with the signal at 119.4 ppm assignable to C-4 and HMBCs with C-6 at 123.3, C-7a at 136.8 ppm (Fig. 2). The multiplet between 7.45 and 7.47 ppm is due to H-7 hydrogen. The NH proton appears as a singlet at 10.99 ppm. The formation of monoindole is confirmed by the CH2 proton singlet at 4.00 ppm, which shows (i) C,H-COSY correlation with carbon signal at 41.4 ppm, due to C-2′′, (ii) HMBCs with C-3 at 100.8, carbonyl carbon at 193.4 ppm and C-1′′ at 134.9 ppm. Monoindole 4d shows absorptions at 3345 (NH), 3051 (C–H) and 1656 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1 indicating the presence of one indole ring and one carbonyl functionality. The mass spectrum of 4a displayed the molecular ion [M-1] peak at m/z = 342.0 [calcd. 342.1].
O) cm−1 indicating the presence of one indole ring and one carbonyl functionality. The mass spectrum of 4a displayed the molecular ion [M-1] peak at m/z = 342.0 [calcd. 342.1].
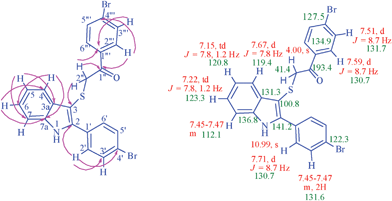 | ||
| Fig. 2 Selected HMBCs and 1H and 13C chemical shifts in compound 4d. | ||
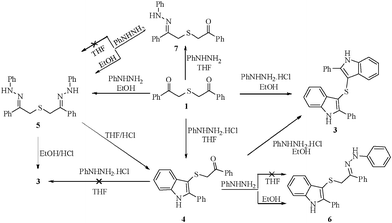
The product selectivity of the reaction has been further explored by the experiments depicted in Scheme 2. When diphenacyl sulfide 1a was allowed to react with phenylhydrazine in ethanol under reflux conditions for 30 min, it afforded bis(2-phenyl-2-(2-phenylhydrazono) ethyl)sulfane 5a.15Bishydrazone 5a subsequently reacted with con. HCl in ethanol yielding (82%) the bisindole 3a. However, when 5a was treated with HCl in THF medium, only the monoindole 4a was obtained in 85% yield. It can be noticed that (i) the cyclization has occurred at one end and (ii) the phenylhydrazo group was hydrolyzed to ketone at the other end (Scheme 2). In a separate experiment, 4 was allowed to react with phenylhydrazine in ethanol, which yielded 6. The second indolization of monoindole 4 can be effected in a facile manner with phenylhydrazine hydrochloride in ethanol medium (Table 3).
| Entry | Ar | Yield of 3 with PhNHNH2.HCl (%)a | Yield of 6 with PhNHNH2 (%)a |
|---|---|---|---|
| a Isolated yield after purification by recrystallisation from ethyl acetate. | |||
| a | p-MeC6H4 | 79 | 81 |
| b | p-ClC6H4 | 85 | 83 |
| c | p-BrC6H4 | 83 | 80 |
It is again interesting that the reaction of 1 with phenylhydrazine in THF yielded only the mono hydrazone 7, (Table 4) which on further treatment with another mole of phenylhydrazine in ethanol, provided the bishydrazone 5. But in THF medium, even after prolonged heating for 8 h, the mono hydrazone 7 did not react with another mole of phenylhydrazine (Scheme 2).
| Entry | Ar | Yield of 5 in EtOH (%)a | Yield of 7 in THF (%)a |
|---|---|---|---|
| a Isolated yield after purification by recrystallisation from ethanol. | |||
| a | C6H5 | 86 | 89 |
| b | p-MeC6H4 | 87 | 92 |
| c | p-ClC6H4 | 89 | 95 |
| d | p-BrC6H4 | 89 | 93 |
| e | p-OMeC6H4 | 86 | 90 |
It is pertinent to note that simple 1,5-diketone 8, on reaction with phenylhydrazine in ethanol, yielded mono and bis-indole derivatives depending on the proportion of reagents.16 In the present investigation, the reaction of 8 with phenylhydrazine was attempted in THF medium. But the reaction has not given any desirable products (Scheme 3). Obviously the selectivity is more pronounced only in diaroyl sulphides, not in other 1,5-diketones.
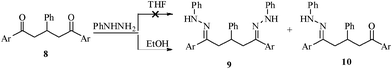 | ||
| Scheme 3 Effect of solvent on the reaction of phenylhydrazine with 1,5-diketone 8. | ||
The striking difference between the protic and aprotic solvents in dictating the course of the above reaction makes to believe that the reason for the observed selectivity is related to hydrogen bonding, assisted by sulfur. Probably in THF, the molecules prefer to have intermolecular hydrogen bonding between the carbonyl of one unit and the NH of the other, thus explaining the preferential formation of 4 or 7. Hence in THF, the reactivity of carbonyl is reduced/prevented. In ethanol, this intermolecular hydrogen bonding may not be there, as ethanol can solvate the molecules. Thus in ethanol, the second carbonyl is as reactive as the first one as evident from the formation of 3, 5 and 6.
Conclusion
In conclusion, it is shown that solvent plays a vital role in deciding the course of the reaction between diphenacyl sulfide and phenylhydrazine/phenylhydrazine hydrochloride. The exclusive formation of either one or two indole rings with THF and ethanol illustrates the dramatic selectivity by the solvents in Fisher indole synthesis.Experimental section
All melting points reported in this work were measured in open capillaries. The 1H and 13C NMR spectra have been measured at 300 and 75 MHz respectively using Bruker 300 MHz (Avance) instrument in CDCl3 using tetramethylsilane (TMS) as internal standard. Chemical shifts are reported as δ values (ppm). All one- and two-dimensional NMR spectra were obtained using standard Bruker software throughout. Elemental analyses were performed on a Perkin Elmer 2400 Series II Elemental CHNS analyzer. IR spectra were recorded on a JASCO FT IR instrument (KBr pellet).General procedure for di(2-aryl-1H-3-indolyl) sulphide (3)
A mixture of 2-[(2-oxo-2-arylethyl)sulfanyl]-1-aryl-1-ethanone 1 (1 mmol) and phenylhydrazine hydrochloride 2 (2.5 mmol) in ethanol (7 ml) was refluxed for 3 h. After completion of the reaction, monitored by TLC, the mixture was poured into ice cold water and the solid separated was purified by recrystallisation from ethyl acetate.Di(2-phenyl-1H-3-indolyl) sulphide (3a)
Isolated as colorless solid; m.p. 132–133 °C; IR (KBr): 3380 (NH), 3054 (C–H) cm−1; 1H NMR (300 MHz,CDCl3) δH: 6.99 (t, 4H, J = 7.5 Hz, Ar–H), 7.09–7.27 (m, 12H, Ar–H), 7.57 (d, 2H J = 7.8 Hz, Ar–H), 8.10 (s, 2H, NH); 13C NMR (75 MHz, CDCl3) δC: 104.0, 110.8, 120.0, 120.9, 123.1, 127.8, 127.9, 128.0, 130.7, 130.9, 135.4, 142.3. Anal. Calcd for C28H20N2S: C, 80.74; H, 4.84; N, 6.73%. Found C, 80.69; H, 4.80; N, 6.77%.Di[2-(4-methylphenyl)-1H-3-indolyl] sulphide (3b)
Isolated as colorless solid; m.p. 126–127 °C; IR (KBr): 3374 (NH), 3052 (C–H) cm−1; 1H NMR (300 MHz, Acetone-d6) δH: 2.20 (s, 6H, CH3), 6.81 (d, 4H, J = 8.1 Hz, Ar–H), 7.08 (t, 2H, J = 7.5 Hz, Ar–H), 7.18 (t, 2H , J = 7.5 Hz, Ar–H), 7.31 (d, 4H, J = 8.1 Hz, Ar–H), 7.37 (d, 2H, J = 7.5 Hz, Ar–H), 7.55 (d, 2H, J = 7.5 Hz, Ar–H), 10.68 (s, 2H, NH); 13C NMR (75 MHz, Acetone-d6) δC: 20.4, 102.4, 111.3, 119.3, 120.2, 122.4, 127.9, 128.3, 128.4, 131.2, 136.2, 137.6, 143.0. Anal. Calcd for C30H24N2S: C, 81.05; H, 5.44; N, 6.30%. Found C, 81.00; H, 5.41; N, 6.35%.Di[2-(4-chlorophenyl)-1H-3-indolyl] sulphide (3c)
Isolated as colorless solid; m.p. 132–133 °C; IR (KBr): 3378 (NH), 3060 (C–H) cm−1; 1H NMR (300 MHz,CDCl3) δH: 6.92 (d, 4H, J = 8.4 Hz, Ar–H), 7.11 (d, 4H, J = 8.4 Hz, Ar–H), 7.15–7.22 (m, 2H , Ar–H), 7.26–7.27 (m, 4H, Ar–H), 7.62 (d, 2H, J = 8.1 Hz, Ar–H), 8.15 (s, 2H, NH); 13C NMR (75 MHz, CDCl3) δC: 104.4, 110.9, 120.0, 121.2, 123.6, 128.1, 128.6, 129.0, 130.7, 134.0, 135.4, 140.9. Anal. Calcd for C28H18Cl2N2S: C, 69.28; H, 3.74; N, 5.77%. Found C, 69.25; H, 3.69; N, 5.81%.Di[2-(4-bromophenyl)-1H-3-indolyl] sulphide (3d)
Isolated as colorless solid; m.p. 179–180 °C; IR (KBr): 3378 (NH), 3058 (C–H) cm−1; 1H NMR (300 MHz, CDCl3) δH: 7.06–7.26 (m, 14H, Ar–H), 7.62 (d, 2H, J = 7.8 Hz, Ar–H), 8.03 (s, 2H, NH); 13C NMR (75 MHz, CDCl3) δC: 104.6, 111.0, 120.0, 121.3, 122.3, 123.7, 128.9, 129.5, 130.7, 131.0, 135.5, 140.8. Anal. Calcd for C28H18Br2N2S: C, 58.56; H, 3.16; N, 4.88%. Found C, 58.52; H, 3.13; N, 4.91%.Di[2-(biphenyl)-1H-3-indolyl] sulphide (3e)
Isolated as colorless solid; m.p. 166–167 °C; IR (KBr): 3384 (NH), 3058 (C–H) cm−1; 1H NMR (300 MHz, Acetone-d6) δH: 7.08–7.19 (m, 4H, Ar–H), 7.28 (d, 4H, J = 7.5 Hz, Ar–H), 7.35 (d, 4H , J = 7.2 Hz, Ar–H), 7.44 (t, 4H, J = 7.2 Hz, Ar–H), 7.50–7.62 (m, 6H, Ar–H), 7.69–7.71 (m, 2H, Ar–H), 8.08 (d, 2H, J = 7.2 Hz, Ar–H), 10.79 (s, 2H, NH); 13C NMR (75 MHz, Acetone-d6) δC: 102.9, 111.4, 119.3, 120.3, 122.7, 126.0, 126.5, 126.6, 127.3, 128.3, 128.6, 128.8, 130.0, 131.2, 136.3, 140.1, Anal. Calcd for C40H28N2S: C, 84.47; H, 4.96; N, 4.93%. Found C, 84.42; H, 4.92; N, 4.98%.Di[2-(2-naphthyl)-1H-3-indolyl] sulphide (3f)
Isolated as colorless solid; m.p. 182-183 °C; IR (KBr): 3378 (NH), 3058 (C–H) cm−1; 1H NMR (300 MHz, Acetone-d6) δH: 7.13–7.25 (m, 6H, Ar–H), 7.30–7.41(m, 6H, Ar–H), 7.49 (d, 2H, J = 8.7 Hz, Ar–H), 7.60 (dd, 2H, J = 8.4, 1.5 Hz, Ar–H), 7.68–7.42 (m, 4H, Ar–H), 7.90 (s, 2H, Ar–H), 10.86 (s, 2H, NH); 13C NMR (75 MHz, Acetone-d6) δC: 103.2, 111.5, 119.4, 120.5, 122.8, 125.7, 125.8, 126.1, 127.1, 127.2, 127.3, 128.2, 128.5, 131.3, 132.8, 132.9, 136.4, 142.7. Anal. Calcd for C36H24N2S: C, 83.69; H, 4.68; N, 5.42%. Found C, 83.64; H, 4.65; N, 5.46%.General procedure for 1-aryl-2-[(2-aryl-1H-3-indolyl)sulfanyl]-1-ethanones (4)
A mixture of 2-[(2-oxo-2-arylethyl)sulfanyl]-1-aryl-1-ethanone 1 (1 mmol) and phenylhydrazine hydrochloride 2 (1.5 mmol) in THF (10 ml) was refluxed for 3 h. After completion of the reaction, monitored by TLC, the mixture was filtered to remove phenyl hydrazine hydrochloride and the filtrate was poured into ice cold water and the solid separated was purified by recrystallisation from ethyl acetate. Spectroscopic data for 4 are given below:1-Phenyl-2-[(2-phenyl-1H-3-indolyl)sulfanyl]-1-ethanone (4a)
Isolated as colorless solid; m.p. 160–161 °C; IR (KBr): 3345 (NH), 3054 (C–H), 1654 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), cm−1; 1H NMR (300 MHz, Acetone-d6) δH: 4.09 (s, 2H, CH2), 7.12 (td, 1H, J = 8.1, 1.2 Hz, Ar–H), 7.20 (td, 1H, J = 8.1, 1.2 Hz, Ar–H), 7.31–7.40 (m, 5H, Ar–H), 7.46 (d, 1H, J = 7.8 Hz, Ar–H), 7.51–7.56 (m, 1H, Ar–H), 7.65 (d, 1H, J = 7.8 Hz, Ar–H), 7.78 (dd, 2H, J = 8.1, 1.8 Hz, Ar–H), 7.86 (dd, 2H, J = 8.1, 1.8 Hz, Ar–H), 10.96 (s, 1H, NH); 13C NMR (75 MHz, Acetone-d6) δC: 41.5, 100.0, 111.5, 118.9, 120.1, 122.5, 128.0, 128.1, 128.2, 128.3, 128.4, 131.0, 131.7, 132.7, 135.6, 136.1, 141.5, 194.1. m/z 342.0 [M-1] calcu. 342.1 [M-1]. Anal. Calcd for C22H17NOS: C, 76.94; H, 4.99; N, 4.08%. Found C, 76.91; H, 4.95; N, 4.13%.
O), cm−1; 1H NMR (300 MHz, Acetone-d6) δH: 4.09 (s, 2H, CH2), 7.12 (td, 1H, J = 8.1, 1.2 Hz, Ar–H), 7.20 (td, 1H, J = 8.1, 1.2 Hz, Ar–H), 7.31–7.40 (m, 5H, Ar–H), 7.46 (d, 1H, J = 7.8 Hz, Ar–H), 7.51–7.56 (m, 1H, Ar–H), 7.65 (d, 1H, J = 7.8 Hz, Ar–H), 7.78 (dd, 2H, J = 8.1, 1.8 Hz, Ar–H), 7.86 (dd, 2H, J = 8.1, 1.8 Hz, Ar–H), 10.96 (s, 1H, NH); 13C NMR (75 MHz, Acetone-d6) δC: 41.5, 100.0, 111.5, 118.9, 120.1, 122.5, 128.0, 128.1, 128.2, 128.3, 128.4, 131.0, 131.7, 132.7, 135.6, 136.1, 141.5, 194.1. m/z 342.0 [M-1] calcu. 342.1 [M-1]. Anal. Calcd for C22H17NOS: C, 76.94; H, 4.99; N, 4.08%. Found C, 76.91; H, 4.95; N, 4.13%.
1-(4-Methylphenyl)-2-[2-(4-methylphenyl)-1H-3-indolyl]sulfanyl-1-ethanone (4b)
Isolated as colorless solid; m.p. 153–154 °C; IR (KBr): 3344 (NH), 3052 (C–H), 1654 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), cm−1; 1H NMR (300 MHz, CDCl3) δH: 2.32 (s, 3H, CH3), 2.35 (s, 3H, CH3), 3.91 (s, 2H, CH2), 7.03 (d, 2H, J = 8.1 Hz, Ar–H), 7.07 (d, 2H, J = 8.1 Hz, Ar–H), 7.12–7.16 (m, 2H, Ar–H), 7.18–7.23 (m, 1H, Ar–H), 7.52 (d, 2H, J = 8.1 Hz, Ar–H), 7.57 (d, 2H, J = 8.1 Hz, Ar–H), 7.64–7.67 (m, 1H, Ar–H), 8.65 (s, 1H, NH); 13C NMR (75 MHz, CDCl3) δC: 21.2, 21.6, 41,9, 100.5, 111.2, 119.2, 120.6, 122.8, 128.1, 128.5, 128.7, 128.9, 129.0, 131.0, 133.0, 135.5, 138.1, 141.7, 143.6, 194.7. m/z 372.1 [M + 1] calcu. 372.1 [M + 1]. Anal. Calcd for C24H21NOS: C, 77.59; H, 5.70; N, 3.77%. Found C, 77.55; H, 5.65; N, 3.81%.
O), cm−1; 1H NMR (300 MHz, CDCl3) δH: 2.32 (s, 3H, CH3), 2.35 (s, 3H, CH3), 3.91 (s, 2H, CH2), 7.03 (d, 2H, J = 8.1 Hz, Ar–H), 7.07 (d, 2H, J = 8.1 Hz, Ar–H), 7.12–7.16 (m, 2H, Ar–H), 7.18–7.23 (m, 1H, Ar–H), 7.52 (d, 2H, J = 8.1 Hz, Ar–H), 7.57 (d, 2H, J = 8.1 Hz, Ar–H), 7.64–7.67 (m, 1H, Ar–H), 8.65 (s, 1H, NH); 13C NMR (75 MHz, CDCl3) δC: 21.2, 21.6, 41,9, 100.5, 111.2, 119.2, 120.6, 122.8, 128.1, 128.5, 128.7, 128.9, 129.0, 131.0, 133.0, 135.5, 138.1, 141.7, 143.6, 194.7. m/z 372.1 [M + 1] calcu. 372.1 [M + 1]. Anal. Calcd for C24H21NOS: C, 77.59; H, 5.70; N, 3.77%. Found C, 77.55; H, 5.65; N, 3.81%.
1-(4-Chlorophenyl)-2-[2-(4-chlorophenyl)-1H-3-indolyl]sulfanyl-1-ethanone (4c)
Isolated as colorless solid; m.p. 153–154 °C; IR (KBr): 3346 (NH), 3050 (C–H), 1656 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), cm−1; 1H NMR (300 MHz, Acetone-d6) δH: 4.02 (s, 2H, CH2), 7.15 (t, 1H, J = 7.5 Hz, Ar–H), 7.22 (t, 1H, J = 7.5 Hz, Ar–H), 7.32–7.36 (m, 5H, Ar–H), 7.56 (d, 1H, J = 7.5 Hz, Ar–H), 7.67 (d, 2H, J = 8.4 Hz, Ar–H), 7.78 (d, 2H, J = 8.4 H), 10.93 (s, 1H, NH); 13C NMR (75 MHz, Acetone-d6) δC: 40.9, 100.1, 111.6, 118.9, 120.4, 122.8, 128.1, 128.2, 128.3, 129.9, 130.1, 130.3, 130.8, 133.5, 133.9, 138.3, 140.7, 192.7. Anal. Calcd forC22H15Cl2NOS: C, 64.08; H, 3.67; N, 3.40%. Found C, 64.04; H, 3.62; N, 3.43%.
O), cm−1; 1H NMR (300 MHz, Acetone-d6) δH: 4.02 (s, 2H, CH2), 7.15 (t, 1H, J = 7.5 Hz, Ar–H), 7.22 (t, 1H, J = 7.5 Hz, Ar–H), 7.32–7.36 (m, 5H, Ar–H), 7.56 (d, 1H, J = 7.5 Hz, Ar–H), 7.67 (d, 2H, J = 8.4 Hz, Ar–H), 7.78 (d, 2H, J = 8.4 H), 10.93 (s, 1H, NH); 13C NMR (75 MHz, Acetone-d6) δC: 40.9, 100.1, 111.6, 118.9, 120.4, 122.8, 128.1, 128.2, 128.3, 129.9, 130.1, 130.3, 130.8, 133.5, 133.9, 138.3, 140.7, 192.7. Anal. Calcd forC22H15Cl2NOS: C, 64.08; H, 3.67; N, 3.40%. Found C, 64.04; H, 3.62; N, 3.43%.
1-(4-Bromophenyl)-2-[2-(4-bromophenyl)-1H-3-indolyl]sulfanyl-1-ethanone (4d)
Isolated as colorless solid; m.p. 164–165 °C; IR (KBr): 3345 (NH), 3051 (C–H), 1656 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), cm−1; 1H NMR (300 MHz, Acetone-d6) δH: 4.00 (s, 2H, CH2), 7.15 (td, 1H, J = 7.8, 1.2 Hz, Ar–H), 7.22 (td, 1H, J = 7.8, 1.2 Hz, Ar–H), 7.45–7.47 (m, 3H, Ar–H), 7.51(d, 2H, J = 8.7 Hz, Ar–H), 7.59 (d, 2H, J = 8.7 Hz, Ar–H), 7.67 (d, 1H, J = 7.8 Hz, Ar–H), 7.71 (d, 2H, J = 8.7 Hz, Ar–H), 10.99 (s, 1H, NH); 13C NMR (75 MHz, Acetone-d6) δC: 41.4, 100.8, 112.1, 119.4, 120.9, 122.2, 123.3, 127.5, 130.7 (2C), 131.1, 131.4, 131.6, 131.7, 135.0, 136.7, 141.2, 193.4. Anal. Calcd for C22H15Br2NOS: C, 52.72; H, 3.02; N, 2.79%. Found C, 52.69; H, 3.00; N, 2.84%.
O), cm−1; 1H NMR (300 MHz, Acetone-d6) δH: 4.00 (s, 2H, CH2), 7.15 (td, 1H, J = 7.8, 1.2 Hz, Ar–H), 7.22 (td, 1H, J = 7.8, 1.2 Hz, Ar–H), 7.45–7.47 (m, 3H, Ar–H), 7.51(d, 2H, J = 8.7 Hz, Ar–H), 7.59 (d, 2H, J = 8.7 Hz, Ar–H), 7.67 (d, 1H, J = 7.8 Hz, Ar–H), 7.71 (d, 2H, J = 8.7 Hz, Ar–H), 10.99 (s, 1H, NH); 13C NMR (75 MHz, Acetone-d6) δC: 41.4, 100.8, 112.1, 119.4, 120.9, 122.2, 123.3, 127.5, 130.7 (2C), 131.1, 131.4, 131.6, 131.7, 135.0, 136.7, 141.2, 193.4. Anal. Calcd for C22H15Br2NOS: C, 52.72; H, 3.02; N, 2.79%. Found C, 52.69; H, 3.00; N, 2.84%.
1-(Biphenyl)-2-[2-(biphenyl)-1H-3-indolyl]sulfanyl-1-ethanone (4e)
Isolated as colorless solid; m.p. 204–205 °C; IR (KBr): 3344 (NH), 3051 (C–H), 1660 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), cm−1; 1H NMR (300 MHz, Acetone-d6) δH: 4.07 (s, 2H, CH2), 6.88 (td, 2H, J = 8.1, 0.9 Hz, Ar–H), 7.06 (td, 2H, J = 8.1, 0.9 Hz, Ar–H), 7.30–7.63 (m, 10H, Ar–H), 7.70–7.78 (m, 6H, Ar–H), 8.09 (d, 2H, J = 8.7 Hz, Ar–H), 10.79 (s, 1H, NH); 13C NMR (75 MHz, Acetone-d6) δC: 41.3, 104.2, 111.3, 111.6, 119.0, 119.3, 119.8, 120.4, 122.3, 122.7, 126.1, 126.6, 126.7, 127.0, 127.4, 128.0, 128.4, 128.8 (2C), 129.0, 131.1, 131.4, 134.6, 136.3, 140.3, 193.5. Anal. Calcd for C34H25NOS: C, 82.39; H, 5.08; N, 2.83%. Found C, 82.36; H, 5.04; N, 2.87%.
O), cm−1; 1H NMR (300 MHz, Acetone-d6) δH: 4.07 (s, 2H, CH2), 6.88 (td, 2H, J = 8.1, 0.9 Hz, Ar–H), 7.06 (td, 2H, J = 8.1, 0.9 Hz, Ar–H), 7.30–7.63 (m, 10H, Ar–H), 7.70–7.78 (m, 6H, Ar–H), 8.09 (d, 2H, J = 8.7 Hz, Ar–H), 10.79 (s, 1H, NH); 13C NMR (75 MHz, Acetone-d6) δC: 41.3, 104.2, 111.3, 111.6, 119.0, 119.3, 119.8, 120.4, 122.3, 122.7, 126.1, 126.6, 126.7, 127.0, 127.4, 128.0, 128.4, 128.8 (2C), 129.0, 131.1, 131.4, 134.6, 136.3, 140.3, 193.5. Anal. Calcd for C34H25NOS: C, 82.39; H, 5.08; N, 2.83%. Found C, 82.36; H, 5.04; N, 2.87%.
1-(2-Naphthyl)-2-[2-(2-naphthyl)-1H-3-indolyl]sulfanyl-1-ethanone (4f)
Isolated as colorless solid; m.p. 172–173 °C; IR (KBr): 3348 (NH), 3052 (C–H), 1659 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), cm−1; 1H NMR (300 MHz,Acetone-d6) δH: 4.05 (s, 2H, CH2), 7.11–7.26 (m, 3H, Ar–H), 7.33–7.42 (m, 3H, Ar–H), 7.47–7.53 (m, 4H, Ar–H), 7.59–7.64 (m, 4H, Ar–H), 7.67–7.83 (m, 3H, Ar–H), 7.88 (s, 1H, Ar–H), 10.69 (s, 1H, NH); 13C NMR (75 MHz, Acetone-d6) δC: 41.5, 103.0, 111.2, 111.6, 119.3, 119.7, 120.1, 120.4, 122.4, 122.9, 125.2, 125.9, 126.3, 126.4, 127.2, 127.3, 128.2, 128.6, 128.7 (2C), 129.4, 129.5, 130.4, 131.8 (2C), 132.7, 135.3, 136.7, 140.6, 192.7. Anal. Calcd for C30H21NOS: C, 81.23; H, 4.77; N, 3.16%. Found C, 81.18; H, 4.72; N, 3.21%.
O), cm−1; 1H NMR (300 MHz,Acetone-d6) δH: 4.05 (s, 2H, CH2), 7.11–7.26 (m, 3H, Ar–H), 7.33–7.42 (m, 3H, Ar–H), 7.47–7.53 (m, 4H, Ar–H), 7.59–7.64 (m, 4H, Ar–H), 7.67–7.83 (m, 3H, Ar–H), 7.88 (s, 1H, Ar–H), 10.69 (s, 1H, NH); 13C NMR (75 MHz, Acetone-d6) δC: 41.5, 103.0, 111.2, 111.6, 119.3, 119.7, 120.1, 120.4, 122.4, 122.9, 125.2, 125.9, 126.3, 126.4, 127.2, 127.3, 128.2, 128.6, 128.7 (2C), 129.4, 129.5, 130.4, 131.8 (2C), 132.7, 135.3, 136.7, 140.6, 192.7. Anal. Calcd for C30H21NOS: C, 81.23; H, 4.77; N, 3.16%. Found C, 81.18; H, 4.72; N, 3.21%.
General procedure for 1-aryl-2-(2-aryl-2-[2-phenylhydrazono]ethylsulfanyl)-1-ethanone 1-phenylhydrazone 5
A mixture of 2-[(2-oxo-2-arylethyl)sulfanyl]-1-aryl-1-ethanone 1 (1 mmol) and phenylhydrazine 2 (2.5 mmol) in ethanol (7 ml) was refluxed for 2–3 h. After completion of the reaction, monitored by TLC, the mixture was poured into ice cold water and the solid separated was purified by recrystallisation from ethanol. The spectral data for bisphenyl hydrazones are given below:1-Phenyl-2-(2-phenyl-2-[2-phenylhydrazono]ethylsulfanyl)-1-ethanone 1-phenyl hydrazone (5a)
Isolated as colorless solid; m.p. 111–112 °C [reported 112–114 °C];22IR (KBr): 3283 (NH), 3054 (C–H), 1634 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR (300 MHz,CDCl3) δH: 3.86 (s, 4H, CH2), 6.89 (t, 2H, J = 7.2 Hz, Ar–H), 7.07 (d, 4H, J = 7.5 Hz, Ar–H), 7.19–7.36 (m, 10H, Ar–H), 7.77 (d, 4H, J = 7.2 Hz, Ar–H), 8.15 (s, 2H, NH); 13C NMR (75 MHz, CDCl3) δC: 25.8, 113.5, 120.9, 125.2, 128.2, 128.6, 129.2, 137.5, 137.8, 144.5. Anal. Calcd for C28H26N4S: C, 74.63; H, 5.82; N, 12.43%. Found C, 74.60; H, 5.78; N, 12.47%.
N); 1H NMR (300 MHz,CDCl3) δH: 3.86 (s, 4H, CH2), 6.89 (t, 2H, J = 7.2 Hz, Ar–H), 7.07 (d, 4H, J = 7.5 Hz, Ar–H), 7.19–7.36 (m, 10H, Ar–H), 7.77 (d, 4H, J = 7.2 Hz, Ar–H), 8.15 (s, 2H, NH); 13C NMR (75 MHz, CDCl3) δC: 25.8, 113.5, 120.9, 125.2, 128.2, 128.6, 129.2, 137.5, 137.8, 144.5. Anal. Calcd for C28H26N4S: C, 74.63; H, 5.82; N, 12.43%. Found C, 74.60; H, 5.78; N, 12.47%.
1-(4-Methylphenyl)-2-(2-(4-methylphenyl)-2-[2-phenylhydrazono]ethylsulfanyl)-1-ethanone 1-phenylhydrazone (5b)
Isolated as colorless solid; m.p. 121–122 °C [reported 121–124 °C];22IR (KBr): 3285 (NH), 3057 (C–H), 1633 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), cm−1; 1H NMR (300 MHz,CDCl3) δH: 2.33 (s, 6H, CH3), 3.84 (s, 4H, CH2), 6.87 (t, 2H, J = 7.2 Hz, Ar–H), 7.06 (d, 4H, J = 8.1 Hz, Ar–H), 7.18–7.27 (m, 8H, Ar–H), 7.65 (d, 4H, J = 8.1 Hz, Ar–H), 8.16 (s, 2H, NH); 13C NMR (75 MHz, CDCl3) δC: 21.1, 25.9, 113.6, 120.6, 125.3, 128.3, 129.1, 129.2, 137.9, 138.0, 144.8. m/z 477.0 [M-1] calcu. 477.1 [M-1]. Anal. Calcd for C30H30N4S: C, 75.28; H, 6.32; N, 11.71%. Found C, 75.25; H, 6.28; N, 11.75%.
N), cm−1; 1H NMR (300 MHz,CDCl3) δH: 2.33 (s, 6H, CH3), 3.84 (s, 4H, CH2), 6.87 (t, 2H, J = 7.2 Hz, Ar–H), 7.06 (d, 4H, J = 8.1 Hz, Ar–H), 7.18–7.27 (m, 8H, Ar–H), 7.65 (d, 4H, J = 8.1 Hz, Ar–H), 8.16 (s, 2H, NH); 13C NMR (75 MHz, CDCl3) δC: 21.1, 25.9, 113.6, 120.6, 125.3, 128.3, 129.1, 129.2, 137.9, 138.0, 144.8. m/z 477.0 [M-1] calcu. 477.1 [M-1]. Anal. Calcd for C30H30N4S: C, 75.28; H, 6.32; N, 11.71%. Found C, 75.25; H, 6.28; N, 11.75%.
1-(4-Chlorophenyl)-2-(2-(4-chlorophenyl)-2-[2-phenylhydrazono]ethylsulfanyl)-1-ethanone 1-phenylhydrazone (5c)
Isolated as colorless solid; m.p. 135–136 °C [reported 135–137 °C];22IR (KBr): 3280 (NH), 3055 (C–H), 1632 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), cm−1; 1H NMR (300 MHz,CDCl3) δH: 3.69 (s, 4H, CH2), 6.93 (t, 2H, J = 7.2 Hz, Ar–H), 7.03 (d, 4H, J = 8.4 Hz, Ar–H), 7.20–7.32 (m, 8H, Ar–H), 7.64 (d, 4H, J = 8.4 Hz, Ar–H), 8.14 (s, 2H, NH); 13C NMR (75 MHz, CDCl3) δC: 25.5, 113.6, 121.2, 126.4, 128.5, 128.7, 129.3, 136.1, 136.2, 144.3. Anal. Calcd for C28H24Cl2N4S: C, 64.74; H, 4.66; N, 10.79%. Found C, 64.70; H, 4.63; N, 10.83%.
N), cm−1; 1H NMR (300 MHz,CDCl3) δH: 3.69 (s, 4H, CH2), 6.93 (t, 2H, J = 7.2 Hz, Ar–H), 7.03 (d, 4H, J = 8.4 Hz, Ar–H), 7.20–7.32 (m, 8H, Ar–H), 7.64 (d, 4H, J = 8.4 Hz, Ar–H), 8.14 (s, 2H, NH); 13C NMR (75 MHz, CDCl3) δC: 25.5, 113.6, 121.2, 126.4, 128.5, 128.7, 129.3, 136.1, 136.2, 144.3. Anal. Calcd for C28H24Cl2N4S: C, 64.74; H, 4.66; N, 10.79%. Found C, 64.70; H, 4.63; N, 10.83%.
1-(4-Bromophenyl)-2-(2-(4-bromophenyl)-2-[2-phenylhydrazono]ethylsulfanyl)-1-ethanone 1-phenylhydrazone (5d)
Isolated as colorless solid; m.p. 125–126 °C; IR (KBr): 3282 (NH), 3057 (C–H), 1634 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), cm−1; 1H NMR (300 MHz,CDCl3) δH: 3.79 (s, 4H, CH2), 6.92 (t, 2H, J = 7.2 Hz, Ar–H), 7.03 (d, 4H, J = 8.4 Hz, Ar–H), 7.19–7.27 (m, 6H, Ar–H), 7.43 (d, 4H, J = 8.4 Hz, Ar–H), 7.59 (d, 2H, J = 8.4 Hz, Ar–H), 8.13 (s, 2H, NH); 13C NMR (75 MHz, CDCl3) δC: 25.5, 113.6, 121.3, 126.7, 128.5, 129.3, 131.7, 136.1, 136.6, 144.2. m/z 604.8 [M-1] calcu. 605.0 [M-1]. Anal. Calcd for C28H24Br2N4S: C, 55.28; H, 3.98; N, 9.21%. Found C, 55.24; H, 3.95; N, 9.25%.
N), cm−1; 1H NMR (300 MHz,CDCl3) δH: 3.79 (s, 4H, CH2), 6.92 (t, 2H, J = 7.2 Hz, Ar–H), 7.03 (d, 4H, J = 8.4 Hz, Ar–H), 7.19–7.27 (m, 6H, Ar–H), 7.43 (d, 4H, J = 8.4 Hz, Ar–H), 7.59 (d, 2H, J = 8.4 Hz, Ar–H), 8.13 (s, 2H, NH); 13C NMR (75 MHz, CDCl3) δC: 25.5, 113.6, 121.3, 126.7, 128.5, 129.3, 131.7, 136.1, 136.6, 144.2. m/z 604.8 [M-1] calcu. 605.0 [M-1]. Anal. Calcd for C28H24Br2N4S: C, 55.28; H, 3.98; N, 9.21%. Found C, 55.24; H, 3.95; N, 9.25%.
1-(4-Methoxyphenyl)-2-(2-(4-methoxyphenyl)-2-[2-phenylhydrazono]ethylsulfanyl)-1-ethanone 1-phenylhydrazone (5e)
Isolated as colorless solid; m.p. 119–120 °C; IR (KBr): 3282 (NH), 3055 (C–H), 1634 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), cm−1; 1H NMR (300 MHz,CDCl3) δH: 3.77 (s, 4H, CH2), 3.80 (6H, OCH3), 6.84 (t, 2H, J = 6.9 Hz, Ar–H), 7.05 (d, 4H, J = 8.4 Hz, Ar–H), 7.18–7.31 (m, 8H, Ar–H), 7.69 (d, 4H, J = 8.4 Hz, Ar–H), 8.05 (s, 2H, NH); 13C NMR (75 MHz, CDCl3) δC: 25.7, 55.2, 113.5, 113.9, 120.5, 126.6, 129.1, 129.2, 137.9, 144.8, 159.6. m/z 511.2 [M + 1] calcu. 511.2 [M + 1]. Anal. Calcd for C30H30N4O2S: C, 70.56; H, 5.92; N, 10.97%. Found C, 70.52; H, 5.89; N, 11.01%.
N), cm−1; 1H NMR (300 MHz,CDCl3) δH: 3.77 (s, 4H, CH2), 3.80 (6H, OCH3), 6.84 (t, 2H, J = 6.9 Hz, Ar–H), 7.05 (d, 4H, J = 8.4 Hz, Ar–H), 7.18–7.31 (m, 8H, Ar–H), 7.69 (d, 4H, J = 8.4 Hz, Ar–H), 8.05 (s, 2H, NH); 13C NMR (75 MHz, CDCl3) δC: 25.7, 55.2, 113.5, 113.9, 120.5, 126.6, 129.1, 129.2, 137.9, 144.8, 159.6. m/z 511.2 [M + 1] calcu. 511.2 [M + 1]. Anal. Calcd for C30H30N4O2S: C, 70.56; H, 5.92; N, 10.97%. Found C, 70.52; H, 5.89; N, 11.01%.
General procedure for 1-aryl-2-(2-aryl-2-[2-phenylhydrazono]ethylsulfanyl)-1-ethanone 7
A mixture of 2-[(2-oxo-2-arylethyl)sulfanyl]-1-aryl-1-ethanone (1 mmol) and phenylhydrazine (1.5 mmol) in THF (5 ml) was refluxed for 2–3 h. After completion of the reaction, monitored by TLC, the mixture was allowed to cool and then poured into ice cold water and the solid separated was purified by recrystallisation from ethanol.1-Phenyl-2-(2-phenyl-2-[2-phenylhydrazono]ethylsulfanyl)-1-ethanone (7a)
Isolated as colorless solid; m.p. 143–144 °C; IR (KBr): 3245 (NH), 3055 (C–H), 1674 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1610 (C
O), 1610 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), cm−1; 1H NMR (300 MHz,CDCl3) δH: 3.79 (s, 2H, CH2), 3.83 (s, 2H, CH2), 6.82–6.89 (m, 1H, Ar–H), 7.21 (d, 2H, J = 7.8 Hz, Ar–H), 7.28–7.34 (m, 6H, Ar–H), 7.41 (d, 2H, J = 8.4 Hz, Ar–H), 7.74 (d, 2H, J = 7.8 Hz, Ar–H), 7.79 (d, 2H, J = 8.4 Hz, Ar–H), 9.59 (s, 1H, NH); 13C NMR (75 MHz, CDCl3) δC: 25.8, 36.8, 113.5, 120.8, 125.2, 127.7, 128.2, 128.5, 128.6, 128.8, 129.1, 133.9, 137.5, 137.7, 144.5, 195.2. m/z 359.1 [M-1] calcu. 359.1 [M-1]. Anal. Calcd for C22H20N2OS: C, 73.30; H, 5.59; N, 7.77%. Found C, 70.26; H, 5.54; N, 7.80%.
N), cm−1; 1H NMR (300 MHz,CDCl3) δH: 3.79 (s, 2H, CH2), 3.83 (s, 2H, CH2), 6.82–6.89 (m, 1H, Ar–H), 7.21 (d, 2H, J = 7.8 Hz, Ar–H), 7.28–7.34 (m, 6H, Ar–H), 7.41 (d, 2H, J = 8.4 Hz, Ar–H), 7.74 (d, 2H, J = 7.8 Hz, Ar–H), 7.79 (d, 2H, J = 8.4 Hz, Ar–H), 9.59 (s, 1H, NH); 13C NMR (75 MHz, CDCl3) δC: 25.8, 36.8, 113.5, 120.8, 125.2, 127.7, 128.2, 128.5, 128.6, 128.8, 129.1, 133.9, 137.5, 137.7, 144.5, 195.2. m/z 359.1 [M-1] calcu. 359.1 [M-1]. Anal. Calcd for C22H20N2OS: C, 73.30; H, 5.59; N, 7.77%. Found C, 70.26; H, 5.54; N, 7.80%.
1-(4-Methylphenyl)-2-(2-(4-methylphenyl)-2-[2-phenylhydrazono]ethylsulfanyl)-1-ethanone (7b)
Isolated as colorless solid; m.p. 116-117 °C; IR (KBr): 3247 (NH), 3056 (C–H), 1674 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1612 (C
O), 1612 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), cm−1; 1H NMR (300 MHz,CDCl3) δH: 2.35 (s, 3H, CH3), 2.41 (s, 3H, CH3), 3.81 (s, 2H, CH2), 3.86 (s, 2H, CH2), 6.87 (t, 1H, J = 6.9 Hz, Ar–H), 7.20 (d, 2H, J = 8.1 Hz, Ar–H), 7.26–7.37 (m, 6H, Ar–H), 7.70 (d, 2H, J = 8.1 Hz, Ar–H), 7.89 (d, 2H, J = 8.1 Hz, Ar–H), 9.53 (s, 1H, NH); 13C NMR (75 MHz, CDCl3) δC: 21.1, 21.7, 26.2, 36.7, 113.3, 120.0, 125.2, 128.8, 129.0, 129.1, 129.5, 132.6, 135.0, 137.7, 138.0, 145.0, 145.5, 194.9. Anal. Calcd for C24H24N2OS: C, 74.19; H, 6.23; N, 7.21%. Found C, 74.16; H, 6.19; N, 7.25%.
N), cm−1; 1H NMR (300 MHz,CDCl3) δH: 2.35 (s, 3H, CH3), 2.41 (s, 3H, CH3), 3.81 (s, 2H, CH2), 3.86 (s, 2H, CH2), 6.87 (t, 1H, J = 6.9 Hz, Ar–H), 7.20 (d, 2H, J = 8.1 Hz, Ar–H), 7.26–7.37 (m, 6H, Ar–H), 7.70 (d, 2H, J = 8.1 Hz, Ar–H), 7.89 (d, 2H, J = 8.1 Hz, Ar–H), 9.53 (s, 1H, NH); 13C NMR (75 MHz, CDCl3) δC: 21.1, 21.7, 26.2, 36.7, 113.3, 120.0, 125.2, 128.8, 129.0, 129.1, 129.5, 132.6, 135.0, 137.7, 138.0, 145.0, 145.5, 194.9. Anal. Calcd for C24H24N2OS: C, 74.19; H, 6.23; N, 7.21%. Found C, 74.16; H, 6.19; N, 7.25%.
1-(4-Chlorophenyl)-2-(2-(4-chlorophenyl)-2-[2-phenylhydrazono]ethylsulfanyl)-1-ethanone (7c)
Isolated as colorless solid; m.p. 151–152 °C; IR (KBr): 3245 (NH), 3055 (C–H), 1672 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1614 (C
O), 1614 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), cm−1; 1H NMR (300 MHz,CDCl3) δH: 3.80 (s, 2H, CH2), 3.87 (s, 2H, CH2), 6.88–6.93 (m, 1H, Ar–H), 7.31–7.34 (m, 6H, Ar–H), 7.48 (d, 2H, J = 8.7 Hz, Ar–H), 7.72 (d, 2H, J = 8.7 Hz, Ar–H), 7.94 (d, 2H, J = 8.7 Hz, Ar–H), 9.52 (s, 1H, NH); 13C NMR (75 MHz, CDCl3) δC: 26.0, 36.6, 113.4, 120.5, 126.5, 128.6, 129.0, 129.2, 129.3, 130.1, 133.3, 133.6, 136.2, 136.3, 145.1, 194.1. Anal. Calcd for C22H18Cl2N2OS: C, 61.54; H, 4.23; N, 6.52%. Found C, 61.50; H, 4.20; N, 6.56%.
N), cm−1; 1H NMR (300 MHz,CDCl3) δH: 3.80 (s, 2H, CH2), 3.87 (s, 2H, CH2), 6.88–6.93 (m, 1H, Ar–H), 7.31–7.34 (m, 6H, Ar–H), 7.48 (d, 2H, J = 8.7 Hz, Ar–H), 7.72 (d, 2H, J = 8.7 Hz, Ar–H), 7.94 (d, 2H, J = 8.7 Hz, Ar–H), 9.52 (s, 1H, NH); 13C NMR (75 MHz, CDCl3) δC: 26.0, 36.6, 113.4, 120.5, 126.5, 128.6, 129.0, 129.2, 129.3, 130.1, 133.3, 133.6, 136.2, 136.3, 145.1, 194.1. Anal. Calcd for C22H18Cl2N2OS: C, 61.54; H, 4.23; N, 6.52%. Found C, 61.50; H, 4.20; N, 6.56%.
1-(4-Bromophenyl)-2-(2-(4-bromophenyl)-2-[2-phenylhydrazono]ethylsulfanyl)-1-ethanone (7d)
Isolated as colorless solid; m.p. 161–162 °C; IR (KBr): 3249 (NH), 3055 (C–H), 1675 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1610 (C
O), 1610 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), cm−1; 1H NMR (300 MHz,CDCl3) δH: 3.79 (s, 2H, CH2), 3.86 (s, 2H, CH2), 6.92 (tt, 1H, J = 8.1, 2.4 Hz, Ar–H), 7.27–7.35 (m, 4H, Ar–H), 7.47 (d, 2H, J = 8.7 Hz, Ar–H), 7.64 (d, 2H, J = 8.7 Hz, Ar–H), 7.65 (d, 2H, J = 8.7 Hz, Ar–H), 7.85 (d, 2H, J = 8.7 Hz, Ar–H), 9.50 (s, 1H, NH); 13C NMR (75 MHz, CDCl3) δC: 25.9, 36.6, 113.4, 120.6, 121.9, 126.8, 129.2, 129.5, 130.2, 131.5, 132.3, 133.7, 136.3, 136.6, 145.0, 194.3. Anal. Calcd for C22H18Br2N2OS: C, 50.98; H, 3.50; N, 5.41%. Found C, 50.95; H, 3.46; N, 5.46%.
N), cm−1; 1H NMR (300 MHz,CDCl3) δH: 3.79 (s, 2H, CH2), 3.86 (s, 2H, CH2), 6.92 (tt, 1H, J = 8.1, 2.4 Hz, Ar–H), 7.27–7.35 (m, 4H, Ar–H), 7.47 (d, 2H, J = 8.7 Hz, Ar–H), 7.64 (d, 2H, J = 8.7 Hz, Ar–H), 7.65 (d, 2H, J = 8.7 Hz, Ar–H), 7.85 (d, 2H, J = 8.7 Hz, Ar–H), 9.50 (s, 1H, NH); 13C NMR (75 MHz, CDCl3) δC: 25.9, 36.6, 113.4, 120.6, 121.9, 126.8, 129.2, 129.5, 130.2, 131.5, 132.3, 133.7, 136.3, 136.6, 145.0, 194.3. Anal. Calcd for C22H18Br2N2OS: C, 50.98; H, 3.50; N, 5.41%. Found C, 50.95; H, 3.46; N, 5.46%.
1-(4-Methoxyphenyl)-2-(2-(4-methoxyphenyl)-2-[2-phenylhydrazono]ethylsulfanyl)-1-ethanone (7e)
Isolated as colorless solid; m.p. 146–147 °C; IR (KBr): 3245 (NH), 3053 (C–H), 1675 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1615 (C
O), 1615 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), cm−1; 1H NMR (300 MHz,CDCl3) δH: 3.74 (s, 2H, CH2), 3.77 (s, 2H, CH2), 3.79 (s, 6H, OCH3), 6.82–6.85 (m, 1H, Ar–H), 7.04 (d, 2H, J = 8.7 Hz, Ar–H), 7.24–7.30 (m, 4H, Ar–H), 7.67 (d, 2H, J = 9.0 Hz, Ar–H), 7.72 (d, 2H, J = 8.7 Hz, Ar–H), 7.92 (d, 2H, J = 9.0 Hz, Ar–H), 9.53 (s, 1H, NH); 13C NMR (75 MHz, CDCl3) δC: 26.0, 36.4, 55.2, 55.3, 113.4, 113.9, 114.9, 120.4, 126.6, 129.1, 129.2, 131.0, 132.4, 137.9, 144.8, 159.7, 164.1, 193.7. Anal. Calcd for C24H24N2O3S: C, 68.55; H, 5.75; N, 6.66%. Found C, 68.51; H, 5.72; N, 6.70%.
N), cm−1; 1H NMR (300 MHz,CDCl3) δH: 3.74 (s, 2H, CH2), 3.77 (s, 2H, CH2), 3.79 (s, 6H, OCH3), 6.82–6.85 (m, 1H, Ar–H), 7.04 (d, 2H, J = 8.7 Hz, Ar–H), 7.24–7.30 (m, 4H, Ar–H), 7.67 (d, 2H, J = 9.0 Hz, Ar–H), 7.72 (d, 2H, J = 8.7 Hz, Ar–H), 7.92 (d, 2H, J = 9.0 Hz, Ar–H), 9.53 (s, 1H, NH); 13C NMR (75 MHz, CDCl3) δC: 26.0, 36.4, 55.2, 55.3, 113.4, 113.9, 114.9, 120.4, 126.6, 129.1, 129.2, 131.0, 132.4, 137.9, 144.8, 159.7, 164.1, 193.7. Anal. Calcd for C24H24N2O3S: C, 68.55; H, 5.75; N, 6.66%. Found C, 68.51; H, 5.72; N, 6.70%.
General procedure for 1-(aryl)-2-[2-(aryl)-1H-3-indolyl]sulfanyl-1-ethanone 1-phenylhydrazone 6
A mixture of 1-aryl-2-[(2-aryl-1H-3-indolyl)sulfanyl]-1-ethanone 4 (1 mmol) and phenylhydrazine (1.5 mmol) in ethanol (10 ml) was refluxed for 2–3 h. After completion of the reaction, monitored by TLC, the mixture was poured into ice cold water and the solid separated was recrystallised from ethyl acetate to get pure product.1-(4-Methylphenyl)-2-[2-(4-methylphenyl)-1H-3-indolyl]sulfanyl-1-ethanone 1-phenylhydrazone (6a)
Isolated as colorless solid; m.p. 145–146 °C; IR (KBr): 3375 (NH), 3272 (NH), 3045 (C–H), 1598 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1; 1H NMR (300 MHz,CDCl3) δH: 2.31 (s, 3H, CH3), 2.32 (s, 3H, CH3), 3.84 (s, 2H, CH2), 6.58 (d, 2H, J = 8.4 Hz, Ar–H), 6.79 (t, 1H, J = 7.5 Hz, Ar–H), 7.02 (d, 2H, J = 7.8 Hz, Ar–H), 7.08–7.16 (m, 4H, Ar–H), 7.28–7.35 (m, 3H, Ar–H), 7.43 (d, 2H, J = 8.1 Hz, Ar–H), 7.50 (d, 2H, J = 7.5 Hz, Ar–H), 7.54 (s, 1H, NH), 7.93 (m, 1H, Ar–H), 8.25 (brs, 1H, NH); 13C NMR (75 MHz, CDCl3) δC: 21.2, 21.3, 30.2, 100.5, 111.5, 113.1, 119.0, 119.9, 121.3, 123.2, 125.3, 128.2, 128.4, 128.7, 128.8, 129.3, 130.9, 134.9, 135.4, 137.4, 138.6, 140.6, 142.5, 145.1. Anal. Calcd for C30H27N3S: C, 78.06; H, 5.90; N, 9.10%. Found C, 78.02; H, 5.85; N, 9.14%.
N) cm−1; 1H NMR (300 MHz,CDCl3) δH: 2.31 (s, 3H, CH3), 2.32 (s, 3H, CH3), 3.84 (s, 2H, CH2), 6.58 (d, 2H, J = 8.4 Hz, Ar–H), 6.79 (t, 1H, J = 7.5 Hz, Ar–H), 7.02 (d, 2H, J = 7.8 Hz, Ar–H), 7.08–7.16 (m, 4H, Ar–H), 7.28–7.35 (m, 3H, Ar–H), 7.43 (d, 2H, J = 8.1 Hz, Ar–H), 7.50 (d, 2H, J = 7.5 Hz, Ar–H), 7.54 (s, 1H, NH), 7.93 (m, 1H, Ar–H), 8.25 (brs, 1H, NH); 13C NMR (75 MHz, CDCl3) δC: 21.2, 21.3, 30.2, 100.5, 111.5, 113.1, 119.0, 119.9, 121.3, 123.2, 125.3, 128.2, 128.4, 128.7, 128.8, 129.3, 130.9, 134.9, 135.4, 137.4, 138.6, 140.6, 142.5, 145.1. Anal. Calcd for C30H27N3S: C, 78.06; H, 5.90; N, 9.10%. Found C, 78.02; H, 5.85; N, 9.14%.
1-(4-Chlorophenyl)-2-[2-(4-chlorophenyl)-1H-3-indolyl]sulfanyl-1-ethanone 1-phenylhydrazone (6b)
Isolated as colorless solid; m.p. 203–204 °C; IR (KBr): 3378 (NH), 3272 (NH), 3048 (C–H), 1598 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1; 1H NMR (300 MHz,CDCl3) δH: 3.75 (s, 2H, CH2), 6.46 (d, 2H, J = 8.4 Hz, Ar–H), 6.82 (t, 1H, J = 7.2 Hz, Ar–H), 7.05–7.21 (m, 7H, Ar–H), 7.28–7.34 (m, 5H, Ar–H, NH), 7.41 (d, 2H, J = 8.4 Hz, Ar–H), 7.93 (m, 1H, Ar–H), 8.18 (brs, 1H, NH); 13C NMR (75 MHz, CDCl3) δC: 28.9, 100.5, 111.8, 113.0, 120.4, 121.2, 121.6, 123.8, 126.3, 128.0, 128.6, 128.9, 129.2, 129.8, 130.8, 133.4, 134.6, 135.5, 135.7, 138.7, 141.6, 144.5. m/z 502.0 [M + 1] calcu. 502.0 [M + 1]. Anal. Calcd for C28H21Cl2N3S: C, 66.93; H, 4.21; N, 8.36%. Found C, 66.90; H, 4.17; N, 8.41%.
N) cm−1; 1H NMR (300 MHz,CDCl3) δH: 3.75 (s, 2H, CH2), 6.46 (d, 2H, J = 8.4 Hz, Ar–H), 6.82 (t, 1H, J = 7.2 Hz, Ar–H), 7.05–7.21 (m, 7H, Ar–H), 7.28–7.34 (m, 5H, Ar–H, NH), 7.41 (d, 2H, J = 8.4 Hz, Ar–H), 7.93 (m, 1H, Ar–H), 8.18 (brs, 1H, NH); 13C NMR (75 MHz, CDCl3) δC: 28.9, 100.5, 111.8, 113.0, 120.4, 121.2, 121.6, 123.8, 126.3, 128.0, 128.6, 128.9, 129.2, 129.8, 130.8, 133.4, 134.6, 135.5, 135.7, 138.7, 141.6, 144.5. m/z 502.0 [M + 1] calcu. 502.0 [M + 1]. Anal. Calcd for C28H21Cl2N3S: C, 66.93; H, 4.21; N, 8.36%. Found C, 66.90; H, 4.17; N, 8.41%.
1-(4-Bromophenyl)-2-[2-(4-bromophenyl)-1H-3-indolyl]sulfanyl-1-ethanone 1-phenylhydrazone (6c)
Isolated as colorless solid; m.p. 179–180 °C; IR (KBr): 3376 (NH), 3271 (NH), 3048 (C–H), 1597 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1;1H NMR (300 MHz,CDCl3) δH: 3.77 (s, 2H, CH2), 6.47 (d, 2H, J = 7.5 Hz, Ar–H), 6.83 (t, 1H, J = 7.5 Hz, Ar–H), 7.16 (t, 1H, J = 7.5 Hz, Ar–H), 7.21–7.44 (m, 13H, Ar–H), 7.96 (m, 1H, Ar–H), 8.24 (brs, 1H, NH); 13C NMR (75 MHz, CDCl3) δC: 28.8, 100.6, 111.8, 113.0, 119.0, 120.4, 121.6 (2C), 122.9, 123.8, 126.6, 129.0, 129.7, 130.0, 130.8, 130.9, 131.5, 135.5, 136.1, 138.6, 141.6, 144.5. m/z 589.9 [M + 1] calcu. 589.9 [M + 1]. Anal. Calcd for C28H21Br2N3S: C, 56.87; H, 3.58; N, 7.11%. Found C, 56.82; H, 3.54; N, 7.15%.
N) cm−1;1H NMR (300 MHz,CDCl3) δH: 3.77 (s, 2H, CH2), 6.47 (d, 2H, J = 7.5 Hz, Ar–H), 6.83 (t, 1H, J = 7.5 Hz, Ar–H), 7.16 (t, 1H, J = 7.5 Hz, Ar–H), 7.21–7.44 (m, 13H, Ar–H), 7.96 (m, 1H, Ar–H), 8.24 (brs, 1H, NH); 13C NMR (75 MHz, CDCl3) δC: 28.8, 100.6, 111.8, 113.0, 119.0, 120.4, 121.6 (2C), 122.9, 123.8, 126.6, 129.0, 129.7, 130.0, 130.8, 130.9, 131.5, 135.5, 136.1, 138.6, 141.6, 144.5. m/z 589.9 [M + 1] calcu. 589.9 [M + 1]. Anal. Calcd for C28H21Br2N3S: C, 56.87; H, 3.58; N, 7.11%. Found C, 56.82; H, 3.54; N, 7.15%.
Acknowledgements
The authors thank DST, New Delhi for funds under IRHPA program towards high resolution NMR spectrometer to Madurai Kamaraj University and UGC, New Delhi for providing facilities to the Department of Industrial Chemistry of Alagappa University through SAP scheme. Financial support from UGC, New Delhi to NP is gratefully acknowledged.References
- (a) R. J. Sundberg, Indoles; Ed.; Academic Press: San Diego, 1996 Search PubMed; (b) G. W. Gribble, Perkin Trans., 2000, 1, 1045 Search PubMed; (c) S. Cacchi and G. Fabrizi, Chem. Rev., 2005, 105, 2873 CrossRef CAS; (d) G. R. Humphrey and J. T. Kuethe, Chem. Rev., 2006, 106, 2875 CrossRef CAS; (e) S. P. Ovenden. and R. J. Capon, J. Nat. Prod., 1999, 62, 1246 CrossRef CAS.
- See for example, U. Misra, A. Hitkari, A. K. Saxena, S. Gurtu and K. Shanker, Eur. J. Med. Chem., 1996, 31, 629 CrossRef CAS.
- (a) P. Sharma, A. Kumar and P. Pandey, Indian J. Chem., 2006, 45B, 2077 CAS; (b) R. S. H. PanwarVerma, V. K. Srivastava and A. Kumar, Indian J. Chem., 2006, 2099 Search PubMed; (c) S. R. Bhusare, A. B. Shinde, R. P. Pawar and Y. B. Vibhute, Indian J. Pharm. Sci., 2004, 3, 228 Search PubMed.
- (a) A. Dandia, V. Sehgal and P. Singh, Indian J. Chem., 1993, 32B, 1288 CAS; (b) B. S. Holla and K. V. Udupa, J. Indian Chem. Soc., 1988, 65, 524 CAS.
- See for example, P. Archna, K. Rani, V. K. Bajaj, R. Srivastava and A. Chandra Kumar, Arzneim.-Forsch./Drug Res., 2003, 301 Search PubMed.
- (a) A. Kumar, K. K. Saxena, S. Gurtu, J. N. Sinha and K. Shanker, Indian Drugs, 1986, 24, 1 CAS; (b) N. Bru-Magniez, T. Guenger and J. M. Tenton, (Laboratories UPSA. Fr.) U.S. U 5,480,983 (Cl. 536-27, 62; C07H19/167), 2 Jan 1996, FR Appl. 92/138, 8 Jan 1992; 30 pp. Cont-in- part of U.S. 5,229,505 (Eng.) Chem. Abstr., 1996, 124(17) Search PubMed.
- J. Deng, T. Sanchez, N. Neamati and J. M. Briggs, J. Med. Chem., 2006, 49, 1684 CrossRef CAS.
- (a) For reviews on developments in the synthesis of indole and its derivatives, see: J. A. Joule, In Science of Synthesis: Methods of Molecular Transformations; E. J. Thomas, Ed.; Georg Thieme Verlag: Stuttgart, New York:, 2000; Vol. 10, pp 361–652 Search PubMed; (b) G. W. Gribble, J. Chem. Soc., Perkin Trans. 1, 2000, 1, 1045 RSC.
- (a) For reviews on mechanism and methodology Fischer synthesis, see: B. Robinson, The Fischer Indole Synthesis; Wiley & Sons: New York, 1982 Search PubMed; (b) D. L. Hughes, Org. Prep. Proced. Int., 1993, 25, 607 CrossRef CAS.
- (a) Y. Cheng and K. T. Chapman, Tetrahedron Lett., 1997, 38, 1497 CrossRef CAS; (b) G. L. Rebeiro and B. M. Khadilkar, Synthesis, 2001, 370 CrossRef CAS.
- (a) D. L. Hughes, Org. Prep. Proced. Int., 1993, 25, 607 CrossRef CAS; (b) A. H. Kelly, D. H. McLeod and J. Parrick, J. Chem. Soc., 1965, 43, 296 CAS; (c) M. M. Kidwai and V. K. Ahluwalia, Indian J. Chem., 1988, 27B, 962 CAS.
- (a) F. M. Miller and W. N. Schinske, J. Org. Chem., 1978, 43, 3384 CrossRef CAS; (b) D. L. Hughes and D. Zhao, J. Org. Chem., 1993, 58, 228 CrossRef CAS; (c) D. Zhao, D. L. Hughes, D. R. Bender, A. M. DeMarco and P. J. Reider, J. Org. Chem., 1991, 56, 3001 CrossRef CAS.
- (a) T. Ponpandian and S. Muthusubramanian, Tetrahedron Lett., 2011, 52, 1520 CrossRef CAS; (b) S. Saravanan, I. A. Azath and S. Muthusubramanian, J. Org. Chem., 2008, 73, 2323 CrossRef CAS; (c) N. Paul and S. Muthusubramanian, Tetrahedron Lett., 2011, 52, 3743 CrossRef CAS; (d) G. Ravindran, N. Paul, S. Muthusubramanian and S. Perumal, J. Sulfur Chem., 2008, 29, 575 CrossRef CAS; (e) M. Nagaraj, M. Boominathan, S. Muthusubramanian and N. Bhuvanesh, Org. Biomol. Chem., 2011, 9, 4642 RSC; (f) M. Boominathan, M. Nagaraj, S. Muthusubramanian and R. V. Krishnakumar, Tetrahedron, 2011, 67, 6057 CrossRef CAS; (g) N. Paul, S. Muthusubramanian and N. Bhuvanesh, New J. Chem., 2011, 35, 2607 RSC; (h) N. Paul, S. Kaladevi and S. Muthusubramanian, Helv. Chim. Acta DOI:10.1002/hlca.201100263; (i) S. Chitra, N. Paul, S. Muthusubramanian, P. Manisankar, P. Yogeeswari and D. Sriram, Eur. J. Med. Chem., 2011, 46, 4897 CrossRef CAS; (j) S. Chitra, N. Paul, S. Muthusubramanian and P. Manisankar, Green Chem., 2011, 13, 2777 RSC; (k) S. Chitra, N. Paul, S. Muthusubramanian, P. Manisankar, P. Yogeeswari and D. Sriram, Eur. J. Med. Chem., 2011, 46, 5465 CrossRef CAS.
- S. Hamid, S. Birgitta, B. Jan and J. Tomasz, Synlett, 2006, 2459 Search PubMed.
- V. Padmavathi, K. Mahesh, C. V. Subbaiah, Dandu Rangayapalle and A. Padmaja, Heteroat. Chem., 2008, 19, 261 CrossRef CAS.
- T. V. Moskovkina, Chem. Heterocycl. Compd., 2002, 38, 1190 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c1ra00878a/ |
| This journal is © The Royal Society of Chemistry 2012 |