Electrochemical properties of carbon nanodiscs
James Guo Sheng
Moo
and
Martin
Pumera
*
School of Physical and Mathematical Sciences, Division of Chemistry and Biological Chemistry, Nanyang Technological University, 21 Nanyang Link, Singapore. E-mail: pumera@ntu.edu.sg; Fax: (+65) 6791-1961
First published on 21st December 2011
Abstract
Carbon nanodiscs are new bottom-up prepared nanomaterials with well-defined diameter and thickness (of 1–2 μm and 20–50 nm, respectively). We investigate their electrochemical performance towards oxidation and reduction of small inorganic molecules as well as biomarkers. We have found that carbon nanodiscs exhibit faster observable heterogeneous electron transfer rates than graphite. However, this can be due to nanographitic impurities within the nanodiscs sample.
Introduction
Carbon materials have been in the center of electrochemical research for decades.1–7 The advantage of carbon materials for electrochemistry is due to the high conductivity,8 fast heterogeneous electron transfer kinetics,9,10 accessibility and cost.11Graphite, mesoporous and nanoporous carbons, fullerenes, carbon nanotubes and recently graphene have been intensively studied.12–14Graphene has been vigorously and rigorously researched since its re-introduction to the scientific community in 2004.15 Investigation of novel graphene-based materials has been consistently undertaken by scientists in the fraternity.16 Here we wish to investigate the electrochemistry of a new variation of graphite/graphene: the carbon nanodiscs.A carbon nanodisc can largely be described as a disc-shaped graphene stacked atop each other, therefore yielding into the well defined graphitic structures.17 These carbon nanodiscs with their well-defined nanostructures,18 coupled with fast heterogeneous electron transfer rate at the edges of graphene in the order of 0.01 cm s−1,9 may provide better electrochemical performance when used as an electrode material in comparison to graphite microparticles. It must also be noted that these carbon nanodiscs are not produced as a sole entity and are often present with materials such as carbon nanocones and carbon black.19Carbon nanodiscs, which could be considered to be a nanocone with a zero degree cone apex angle, occur in 70% by weight.18Carbon nanocones, with defined cone apex angles of 19.2, 38.9, 60, 84.6 and 112.9 degrees, occur in 20% by weight.18,19 The nanoarchitecture of nanocones is conferred to them due to the presence of pentagons at the apex and they have been predicted computationally to contain unique electronic states.20–21 Hence, the morphology and unique electronic states of the nanocones suggest that these nanocones may too possess unique electrochemical activity. Moreover, it is worthwhile to note that the high temperature treatment that was used to produce carbon nanodiscs,19 may possibly result in the annealing of the edges. The resulting folded edges have been shown to reduce electrochemical activity in graphitic materials.22 All these factors affect the electrochemical performance of the carbon nanodiscs. Therefore, it's essential for scientists to fully understand the structure and electrochemical properties of carbon nanodiscs. We use the electrochemical technique of cyclic voltammetry to determine the electrochemical activity. The characterization of carbon nanodiscs and their electrochemical properties in comparison to graphite are presented in the following text.
Experimental
Materials
Graphite microparticles (10–20 μm), graphite platelet nanofibres (99%),23carbon nanopowder (mesoporous) (99.95%), N,N-dimethylformamide (DMF), potassium phosphate dibasic, sodium phosphate monobasic, potassium ferrocyanide, potassium chloride, sodium chloride, ascorbic acid, dopamine, reduced nicotinamide adenine dinucleotide (NADH) were purchased from Sigma-Aldrich, Singapore. Nanodiscs (70%) was purchased from Strem Chemicals (USA). Glassy carbon electrodes with a diameter of 3 mm were obtained from CHInstruments.Apparatus
Voltammetric experiments were performed in a 5 mL electrochemical cell at room temperature by using a three-electrode configuration. A platinum electrode (Autolab) served as an auxiliary electrode, while an Ag/AgCl electrode (CHInstruments) served as a reference electrode. All electrochemical potentials in this paper are stated versusAg/AgCl reference electrode. All voltammetric experiments were performed on a μAutolab type III electrochemical analyzer (Eco Chemie, The Netherlands) connected to a personal computer and controlled by General Purpose Electrochemical Systems Version 4.9 software (Eco Chemie).Procedures
Glassy carbon electrode surfaces were renewed by polishing with 0.05 μM alumina particles on a cloth and ultrasonicated in ultrapure water for 5 min each. Immobilization of the carbon materials onto the working glassy carbon electrode was performed firstly by preparing a suspension of the desired material with a concentration of 1 mg mL−1 in DMF with a 5 min sonication, followed by a deposition of 1 μL aliquot of the appropriate suspension onto the electrode surface. The solvent was then allowed to evaporate at room temperature, leaving a randomly distributed film onto the glassy carbon electrode surface.Results and discussion
To investigate if the unique and well-defined structure of the carbon nanodiscs (ND)17 confers to them special electrochemical properties, the electrochemical instrumentation of cyclic voltammetry was utilized to establish their behavior (see Scheme 1 for drawing of the structure of nanodics and graphitic particles). Cyclic voltammetry is a useful technique for the characterization of the electrode surface and in the understanding of the heterogeneous electron transfer (HET) rate. In our study, glassy carbon (GC) was used as the electrochemical transducer and the graphite material was used as a reference material. Stacked graphene platelet nanofibres (SGNF) were used to simulate nanographitic impurities. Carbon nanopowder (mesoporous) (CNP) was included to play the role as the possible carbon black impurities which were stated to be present in the nanodiscs sample by the supplier.24 | ||
| Scheme 1 Schematic drawing of (A) carbon nanodisc and (B) graphite. | ||
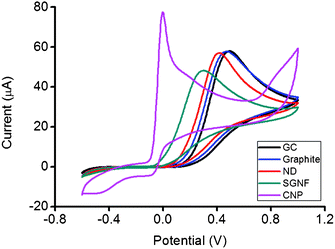
The ferro/ferricyanide redox probe was first utilized to quantify the HET rate using a technique developed by Nicolson, who associated the peak to peak separation, ΔEp with the HET rate.25 In Fig. 1, SGNF and CNP exhibited similar ΔEp values at 111 mV (k0obs = 3.616 × 10−3 cm s−1) and 93 mV (k0obs = 4.617 × 10−3 cm s−1) respectively. The reference material, graphite microparticles modified electrode has a ΔEp of 408 mV (k0obs = 6.413 × 10−5 cm s−1). ND displayed an intermediate value of ΔEp at 289 mV (k0obs = 3.226 × 10−4 cm s−1) between the values of SGNF and CNP and that of the reference graphite microparticles material. The findings appeared to suggest that the edges of nanodiscs are not annealed, as they possess similarity to open edged graphite microparticles and SGNF. To further elucidate the electrochemical performance of the ND material, we tested the ND modified electrode with other redox probes.
![Cyclic voltammograms of 5 mM [Fe(CN)6]3−/4− on GC, graphite, ND, SGNF and CNP modified electrode surfaces. Conditions: 50 mM PBS background electrolyte, pH 7.2, scan rate 100 mV s−1.](/image/article/2012/RA/c1ra00866h/c1ra00866h-f1.gif) | ||
| Fig. 1 Cyclic voltammograms of 5 mM [Fe(CN)6]3−/4− on GC, graphite, ND, SGNF and CNP modified electrode surfaces. Conditions: 50 mM PBS background electrolyte, pH 7.2, scan rate 100 mV s−1. | ||
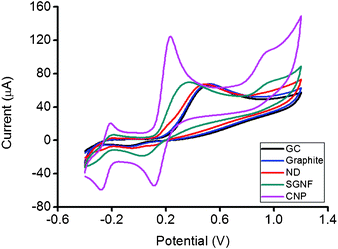
Fig. 2 shows the cyclic voltammograms of ascorbic acid using bare GC electrode and GC electrodes modified with graphite, ND, SGNF and CNP respectively. The oxidation peak of ascorbic acid for ND occurred at 416 mV, which was near but slightly lower than the potential of the oxidation peak observed for the graphite modified surface at 470 mV. For SGNF, the oxidation of ascorbic acid originated from −110 mV and reached a maximum at 300 mV. CNP showed the lowest potential for the oxidation of ascorbic acid, exhibiting the start of the oxidation from −140 mV and reaching the oxidation peak at −19 mV. Amongst all the cyclic voltammograms, the ND modified electrode with a peak of 416 mV displayed close semblance to the graphite modified surface at 470 mV, with a slight shift of the oxidation peak of ascorbic acid towards lower potential for the ND modified surface. Further clarification of the electrochemical behaviour of nanodisc was elucidated using dopamine.
 | ||
| Fig. 2 Cyclic voltammograms of 5 mM ascorbic acid on GC, graphite, ND, SGNF and CNP modified electrode surfaces. Conditions: 50 mM PBS background electrolyte, pH 7.2, scan rate 100 mV s−1. | ||
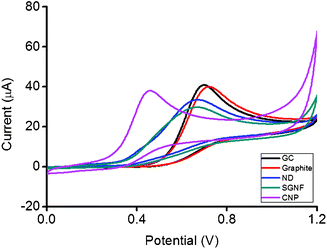
While comparing the oxidation peaks of dopamine in Fig. 3, the electrochemical behavior of ND was again shown to be close to the value of graphite, with a slight shift towards a lower potential. The oxidation peak of ND occurred at 491 mV which was near the values of graphite at 520 mV. Oxidation peak of dopamine using a SGNF modified surface occurred at 374 mV, while the reading at CNP was the lowest recorded at 234 mV. It was also helpful to note that there is a slight shoulder on dopamine at 203 mV for ND, an indication of the ND, not being the only surface present on the electrode and that there are impurities. Hence, it's worthwhile to continue to probe the surface with another analyte NADH.
 | ||
| Fig. 3 Cyclic voltammograms of 5 mM dopamine on GC, graphite, ND, SGNF and CNP modified electrode surfaces. Conditions: 50 mM PBS background electrolyte, pH 7.2, scan rate 100 mV s−1. | ||
ND and SGNF modified electrodes exhibited an almost identical potential peak during the oxidation of NADH at 669 mV in Fig. 4. It was also interesting to note that these two materials too shared similar values at the origin for oxidation of NADH, occurring at 332 mV for ND and 364 mV for SGNF. It has been proven that nanographitic impurities dominate the electrochemistry of carbon nanotubes during oxidation of NADH by our group.26 This similar phenomena that was observed in our current findings is highly probable due to the presence of these nanographitic impurities. It's also worthy to note that there is a slight shoulder peak at 416 mV in the ND modified electrode. The value is very close to that of the oxidation of NADH using a CNP modified electrode, suggesting that carbon black is present as suggested by previous findings.19 Given that only a slight shoulder is present, one may suggest that the impurities that are present in greater amount in the ND sample are those of nanographitic impurities and not of carbon black.
 | ||
| Fig. 4 Cyclic voltammograms of 5 mM NADH on GC, graphite, ND, SGNF and CNP modified electrode surfaces. Conditions: 50 mM PBS background electrolyte, pH 7.2, scan rate 100 mV s−1. | ||
We explored the structure of the nanodiscs with TEM and found that the sample was indeed a mixture of nanodiscs and nanocones, interdispersed with carbon particulate matter.19Fig. 5(A) shows area of sample with high concentration of such carbonaceous impurities.
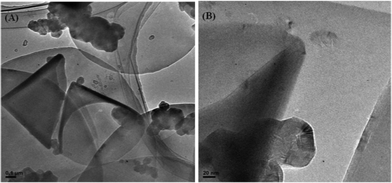 | ||
| Fig. 5 (A) TEM image of carbon nanodiscs in the presence of carbon nanocones and polyhedral particulate carbon matter. (B) TEM image of a carbon nanocone next to a polyhedral carbon particulate. | ||
On higher resolution in Fig. 5(B), the identification of these carbon particulates were that of a graphitised carbon material. Earlier reports have stated that these particulates are carbon black, a generally amorphous material.19 However, from TEM observation, these particles appear to be a crystalline and graphitized material. In an article by Krishnan et al.,27 he has described these carbon nanoparticles as bearing polyhedral or spherical shape. We believe that the carbon nanoparticles observed in the TEM imageFig. 5(B) was that of what they have observed. Similar observation has been made in case of carbon nanotubes with carbon nanoonions as polyhedral impurities by Compton and coworkers.28 Compton's group demonstrated that such nanoonions dominate electrochemistry of carbon nanotubes. These carbon nanoparticles, given its small weight to number of electrochemically active edges ratio, could be the explanation as to the phenomena observed in Fig. 4, where the electrochemical performance of SGNF, which too has a small weight to edges ratio, resembled that of ND.
Conclusion
Our findings suggest that carbon nanodiscs are open-edged and non-annealed as their electrochemical performance bore great similarity with graphite microparticles. The well-defined nanostructures of carbon nanodiscs have allowed for lower observed potentials with ferricyanide, ascorbic acid and dopamine, as in comparison with the graphite microparticles. Furthermore, the presence of impurities may have dominated the electrochemistry of carbon nanodiscs in the oxidation of NADH. These impurities appear to bear resemblance to nanographitic impurities. Carbon black also appeared to be present in carbon nanodiscs, but is shown to be of lesser amount than these carbon nanoparticle impurities. We have shown in our work that nanographitic impurities dominated the electrochemistry of NADH in carbon nanotubes.26 Hence, when considering an electrode material to be used, the impurities present must be taken account of. To maximize the unique architecture of the nanodiscs, continued efforts must be made to remove other unwanted impurities, such as carbon black and perhaps the carbon nanoparticles observed in our findings.References
- F. Winteler, Z. Elektrochem. Angew. Phys. Chem., 1900, 7, 356 CrossRef CAS.
- J. Harden, Phys. Z, 1902, 4, 552 Search PubMed.
- G. A. Roush, Ind. Eng. Chem., 1909, 1, 286 CrossRef CAS.
- U. Anik and S. Cevik, Microchim. Acta, 2011, 174, 207 CrossRef CAS.
- S. Cevik and U. Anik, Sens. Lett., 2010, 8, 667 CrossRef CAS.
- U. Anik and M. Cubukcu, Turkish J. Chem., 2008, 32, 711 CAS.
- M. Cubukcu, S. Timur and U. Anik, Talanta, 2007, 74, 434 CrossRef CAS.
- X. Du, I. Skachko, A. Barker and E. Y. Andrei, Nat. Nanotechnol., 2008, 3, 491 CrossRef CAS.
- T. J. Davies, M. E. Hyde and R. G. Compton, Angew. Chem., 2005, 117, 5251 CrossRef.
- M. Pumera, T. Sasaki and H. Iwai, Chem.–Asian J., 2008, 3, 2046 CrossRef CAS.
- S. Park and R. S. Ruoff, Nat. Nanotechnol., 2009, 4, 217 CrossRef CAS.
- L. Kavan, L. Dunsch, H. Kataura, A. Oshiyama, M. Otani and S. Okada, J. Phys. Chem. B, 2003, 107, 7666 CrossRef CAS.
- L. Kavan and L. Dunsch, ChemPhysChem, 2007, 8, 975 CrossRef.
- M. Pumera, Chem. Soc. Rev., 2010, 39, 4146 RSC.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666 CrossRef CAS.
- M. Pumera, Chem. Rec., 2009, 9, 211 CrossRef CAS.
- C. T. Lin, C. Y. Lee, H. T. Chiu and T. S. Chin, Langmuir, 2007, 23, 12806 CrossRef CAS.
- T. Garberg, S. N. Naess, G. Helgesen, K. D. Knudsen, G. Kopstad and A. Elgsaeter, Carbon, 2008, 46, 1535 CrossRef CAS.
- S. N. Naess, A. Elgaeter, G. Helgesen and K. D. Knudsen, Sci. Technol. Adv. Mater., 2009, 10, 065002 CrossRef.
- S. Berber, Y. K. Kwon and D. Tomanek, Phys. Rev. B: Condens. Matter, 2000, 62, 2291 CrossRef.
- M. Munoz-Navia, J. Dorantes-Davila, M. Terrones and H. Terrones, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 235403 CrossRef.
- A. Ambrosi, A. Bonnani and M. Pumera, Nanoscale, 2011, 3, 2256 RSC.
- A. Ambrosi, T. Sasaki and M. Pumera, Chem. Asian J., 2010, 5, 266 CrossRef CAS.
- http://www.strem.com/uploads/technical_notes/06-0725tech.pdf. .
- R. S. Nicholson, Anal. Chem., 1965, 37, 1351 CrossRef CAS.
- E. J. E. Stuart and M. Pumera, Chem. Eur. J., 2011, 17, 5544 CrossRef CAS.
- A. Krishnan, E. Dujardin, M. M. J. Treacy, J. Hugdahl, S. Lynum and T. W. Ebbesen, Nature, 1997, 388, 451 CrossRef CAS.
- M. C. Henstridge, L. Shao, G. G. Wildgoose, R. G. Compton, G. Tobias and M. L. H. Green, Electroanal., 2008, 20, 498 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
