Self-assembly of chiral hexacatenar-bisamides into a columnar structure†
G.
Shanker‡
,
D. S. Shankar
Rao
,
S. Krishna
Prasad
and
C. V.
Yelamaggad
*
Centre for Soft Matter Research, P.B. No. 1329, Jalahalli, Bangalore 560013, India. E-mail: Yelamaggad@csmr.res.in
First published on 22nd December 2011
Abstract
Several optically active hexacatenar-bisamides derived from α-amino acids such as L-/D-leucine, L-/D-valine or L-alanine have been designed, synthesized and characterized. They exhibit enantiotropic columnar liquid crystalline phase having a rectangular symmetry as evidenced by optical polarizing microscopy (OPM), differential scanning calorimetry (DSC) and X-ray diffraction studies. Circular dichroism (CD) and FTIR experiments suggest the helical organization of these self-complementing bisamide molecules within the columns are connected through inter-molecular hydrogen bonds.
1. Introduction.
Non-covalent interactions play a vital role in the development of complex molecular architectures through a process of self-assembly, wherein these supramolecular materials formed by combining several properties have greater significance in both biological and material sciences.1 Liquid crystals (LCs) represent one such area, in which liquid crystalline materials self-organize into various functional structures leading to potential applications.2 Specific molecular interaction helps in the stabilization of these complex structures by attractive molecular forces.3 Among the non-covalent interactions, hydrogen (H)-bond is one of the most important attractive force in the self-assembly of molecules because of their strength, directionality, reversibility and selectivity.4 For example, several self-complementing systems featuring both H-acceptor and H-donor sites stabilized fascinating LC phases. This is notable in nondiscoid mesogens such as amides1b,5,9–12,14,16, hydrazides,6,7bis-benzamido-ethanes,8 twin-dendritic benzamides,9 polymerizable benzamides,9 fluorinated benzamides,10 Janus-dendritic benzamides,10ureas11 and multiple amide substituted aromatics.5–12 Among these materials, amides1b,5,9–12,14,16 formed by covalently linking two lipophilic taper-shaped segments through self-complementing units have attracted a great deal of attention owing to their spontaneous self-assembly into interesting and complex columnar (Col) LC phases driven by the H-bonding among amide groups and the microphase segregation between rigid core and flexible tails. Columnar phases specially connected through H-bonds in molecules having urea and amide groups are promising candidates for ferroelectric switching11 as well as polar order and chiral super structures.13These observations inspired us to design hexacatenar bisamides possessing chiral segments. The aim of this study is two fold. Firstly, to the best of our knowledge mesomorphic chiral bisamides have not been reported. Secondly, the molecular structure could also be compared with the structures of reported homomeric dipeptides.14 In fact, the molecular design of the present study is based on the logical presumption that bisamides with one amino acid residue and two C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and NH groups should effectively show reduced melting temperature without altering the type of mesophase.14 Besides, such a design may also help in widening of the mesophase thermal range. Here, we report the synthesis and characterization of hexacatenar bisamides where two taper-shaped lipophilic segments are covalently linked through a self-complementing chiral spacer derived from naturally occurring α-amino acids such as L-alanine, L-/D-leucine and L-/D-valine. A general molecular structure of the designed and synthesized bisamides is shown in Scheme 1.
O and NH groups should effectively show reduced melting temperature without altering the type of mesophase.14 Besides, such a design may also help in widening of the mesophase thermal range. Here, we report the synthesis and characterization of hexacatenar bisamides where two taper-shaped lipophilic segments are covalently linked through a self-complementing chiral spacer derived from naturally occurring α-amino acids such as L-alanine, L-/D-leucine and L-/D-valine. A general molecular structure of the designed and synthesized bisamides is shown in Scheme 1.
 | ||
| Scheme 1 Synthesis of chiral hexacatenar-bisamides | ||
2. Experimental
Synthesis
The proposed Hexacatenar BisAmides (HBA) were synthesized using the synthetic route shown in Scheme 1. The requisite 3,4,5-trialkoxybenzoic acids (1) and primary amines (2–7) were prepared as previously reported.14The condensation of amines (2–7) with acids (1) in the presence of 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU) and N,N-diisopropylethylamine (DIEA) to accomplish target bisamides in 60–65% yields. All these molecules were subjected for molecular structural characterization using standard spectroscopic techniques (see supporting information, ESI†).
3. Results and discussion
The phase sequence, transition temperatures and enthalpies of transition for the prepared HBA series of compounds are summarized in Table 1.| Compd. | n | R 1 | Phase transitions | T cr |
|---|---|---|---|---|
| a Peak temperatures in the DSC thermograms obtained during the first heating (phase transitions) and cooling (Tcr) cycles at 5 °C min−1. b Transition enthalpies were obtained from the DSC thermograms. c The Col–Iso phase transition was observed under optical polarizing microscope and too weak [< 0.01 kJ mol−1] to get recognized in DSC. Abbreviations: Col = Columnar phase; Colr = Rectangular columnar phase; Iso = Isotropic phase; Cr and Cr1 = Crystal; Tcr/°C= Crystallization temperature. | ||||
| HBA-1 | 10 | (S)-Methyl 15 | Colr 91 [0.7] Iso | < 20 |
| HBA-2 | 10 | (S)-Isobutyl | Cr 63 [16] Col 96 [1.4] Iso | < 20 |
| HBA-3 | 10 | (R)-Isobutyl | Cr 65 [15.4] Colc 94 [< 0.01] Iso | < 20 |
| HBA-4 | 10 | (S)-Isopropyl | Cr 45 [17.2] Cr1 89 [9.8] Col 99 [2.5] Iso | < 20 |
| HBA-5 | 10 | (R)-Isopropyl | Cr 44 [9.7] Cr1 88 [11.6] Colr 99 [3.2] Iso | < 20 |
| HBA-6 | 12 | (S)-Methyl 15 | Cr 49 [85.6] Colc 89 [< 0.01] Iso | < 20 |
| HBA-7 | 12 | (S)-Isobutyl | Cr 44 [89] Colr 90 [0.9] Iso | < 20 |
| HBA-8 | 12 | (R)-Isobutyl | Cr 42 [60] Colc 96 [< 0.01] Iso | < 20 |
| HBA-9 | 12 | (S)-Isopropyl | Cr 48 [73.2] Cr1 73 [7.2] Col 84 [1.1] Iso | < 20 |
| HBA-10 | 12 | (R)-Isopropyl | Cr 48 [66] Cr1 73 [7.4] Colr 89 [1.2] Iso | < 20 |
Phase transitions and their temperatures are highly reversible over any number of heating/cooling cycles suggesting that these bisamides are thermally stable. All the compounds exhibit Col behavior, compound HBA-1 derived from L-alanine turned out to be a room temperature LC. Compound placed between a pair of glass slides, could be readily spread around the slide by mechanical shearing. The spread-over sample shows a birefringent texture, clearly indicating that it is already in the mesomorphic state. On cooling from the isotropic phase, it shows a mesophase at 85 °C with an uncharacteristic optical texture consisting of very tiny features (Fig. 1a). The optical texture remains unchanged till RT; in the DSC thermograms of first and second heating/cooling cycles, the signatures due to Col-Iso/Iso-Col transitions are seen (Fig. 2).
 | ||
| Fig. 1 Microphotographs of the optical textures obtained for the Col phase of (a) HBA-1 at 44 °C, (b) HBA-2 at 75 °C and (c) HBA-6 at 75 °C. | ||
 | ||
| Fig. 2 DSC traces obtained during the heating/cooling cycles scanned at a rate of 5 °C min−1 of bisamideHBA-1. | ||
Bisamide HBA-2 derived from L-leucine displays Col phase (Fig. 1b) over a wide thermal range up to −60 °C on cooling from the isotropic phase, as evidenced by both optical and calorimetric studies. Its enantiomer HBA-3 melts into mesophase at 65 °C and remains so till the isotropic phase (94 °C). Enantiomers HBA-4 and HBA-5 derived from L-valine and D-valine respectively stabilize enantiotropic Col phase. When the samples contained between a pair of untreated glass slides, cooled slowly from the isotropic phase, the Col phase appears with a dendritic growth pattern. On further cooling, the pattern fills the field of view and remains unaltered till room temperature. The bisamide HBA-6 comprising L-alanine residue and dodecyloxy tails displays dendritic growth pattern (Fig. 1c) which is characteristic for columnar phase on cooling from the isotropic phase. Similarly bisamides HBA-9 and HBA-10 also display thermodynamically stable columnar behavior. In general, no crystallization peak was observed down to −60 °C for all the compounds having decyloxy tails, as evidenced by DSC thermograms. However, an unidentified phase (M) or crystalline phase was observed in the compounds with dodecyloxy tails below the room temperature having enthalpy values between 7 and 27 [kJ mol−1]. Compounds HBA-6 to HBA-10 show this phase at −10 °C [−7.7], −8 °C [−21], −6 °C [−7.7], 16 °C [−19.5] and 13 °C [−27.3] respectively.
XRD experiment was carried on selected HBA compounds. The XRD patterns obtained for the columnar phase at 70 °C (Fig. 3a) and at 25 °C (Fig. 3b) were found to be qualitatively identical for HBA-1.
 | ||
| Fig. 3 1D intensity vs. 2θ profiles obtained for the Colr phase of HBA-1 (a) at 70 °C and (b) 25 °C. | ||
The results of indexing of 1D intensity vs. 2θ profile deduced from the 2D pattern are summarized in Table 2. The patterns showed sharp Bragg peaks in the low angle region besides a broad reflection at wide angles corresponding to the distances in the range of ∼4.5 Å that relates to the liquid-like order of the peripheral alkoxy tails. The sharp peaks are assigned to (11), (20) and (21) reflections from a rectangular lattice. The XRD pattern obtained for the Col phase of HBA-5 showed two sharp peaks at 19.7 Å and 17.1 Å in the low angle region; they are the best assigned to a rectangular columnar arrangement with cell parameters a = 34.2 Å and b = 24.2 Å (see Table 2). Columnar phase of HBA-7 having diffraction pattern at two different temperatures 70 °C and 25 °C were found to be almost identical (Fig. S1 and see Table 2). Similarly, a diffuse peak at ∼4.6 Å seen in the wide angles of both the patterns is diagnostic of liquid-like order of the chains within the columns. The low angle region of both high- and low-temperature diffractograms contained sharp peaks which are indexed as (11), (20) and (21) reflections of a rectangular lattice in the Col phase.
| Compound | T/°C | Phase | d obs/Å | d calcd/Å | Miller index hkl | Lattice parameters/Å |
|---|---|---|---|---|---|---|
| HBA-1 | 70 | Colr | 22.1 | 22.1 | 11 | |
| 20.9 | 20.8 | 20 | a = 41.6 | |||
| 16.3 | 16.3 | 21 | b = 26.1 | |||
| 4.6 | ||||||
| 25 | Colr | 22.1 | 22.1 | 11 | ||
| 20.4 | 20.4 | 20 | a = 40.9 | |||
| 16.4 | 16.1 | 21 | b = 26.3 | |||
| 4.5 | ||||||
| HBA-5 | 25 | Colr | 19.7 | 19.7 | 11 | a = 34.2 |
| 17.1 | 17.1 | 20 | b = 24.2 | |||
| 4.5 | ||||||
| HBA-7 | 70 | Colr | 23.2 | 23.3 | 11 | a = 43.5 |
| 21.7 | 21.8 | 20 | b = 27.6 | |||
| 17.2 | 17.1 | 21 | ||||
| 4.6 | ||||||
| 25 | Colr | 22.9 | 23.2 | 11 | ||
| 21.5 | 21.8 | 20 | a = 43.7 | |||
| 17.5 | 17.1 | 21 | b = 27.5 | |||
| 4.6 |
FTIR experiments were carried out on the two representative bisamides HBA-4 and HBA-10. The samples sandwiched between the KBr plates were heated up to the isotropic phase and spectra were recorded as a function of the temperature. The spectra obtained for these two samples clearly support the presence of H-bonding in the fluid columnar aggregates (see Fig. 4). In the spectra of compounds HBA-4 (Fig. 4) and HBA-10 (Fig. S2, ESI†), the occurrence of N–H stretching vibrations centered at 3290 cm−1 and 3282 cm−1, also of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1628 cm−1 and 1630 cm−1 respectively, indicate that all the NH groups are associated with C
O at 1628 cm−1 and 1630 cm−1 respectively, indicate that all the NH groups are associated with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups through (N–H••••O
O groups through (N–H••••O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)16hydrogen bonding given the fact that the non-bonded N–H and C
C)16hydrogen bonding given the fact that the non-bonded N–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations appear above 3427 cm−1 and 1650 cm−1 respectively.16a,b
O stretching vibrations appear above 3427 cm−1 and 1650 cm−1 respectively.16a,b
 | ||
| Fig. 4 FTIR spectra in the region of –CO–, –CH2– and –NH bands obtained for the compound HBA-4 as a function of temperature. | ||
Furthermore, the existence of bands due to asymmetric and symmetric C–H stretching at 2934 cm−1 (2926 cm−1) and 2850 cm−1 (2855 cm−1) in the spectra of HBA-4 (HBA-10, Fig S2) indicates the disordering of n-alkoxy tails in the Col phase.6,16 The peak intensity increases with a decreasing temperature from the isotropic phase. The change in strength clearly indicates the presence of inter-molecular hydrogen bonding in the Col phase.17
As can be seen, these bisamides possessing multiple chromophores, molecular chirality and H-bonds are bound to display interesting photophysical properties like those noted for dipeptides.14 It would be interesting to see whether these systems form helical structures governed by the nature (size and stereochemistry) of the amide linkage coupled with H–bond force. Circular Dichroism (CD) spectra of the helical columnar mesophase, in which the constituent chiral molecules within a column follow a helical array, show the exciton-split effect.18 The appearance of exciton-splitting is related to a through-space interaction between two or more chromophores exhibiting allowed π–π* absorption bands. Such an interaction splits the excited state into two energy levels. For the exciton-splitting to occur, interacting electronic transition moments must not be parallel since it is the result of a vectorial product.19 In other words, the CD bands originate from the intermolecular interaction between two or more electronic transition dipoles arranged in a helical manner.18,20 Thus, the selected mesomorphic bisamides were probed for chiroptical properties using CD spectroscopic technique to verify whether their molecular chirality has facilitated the molecular organization in helical fashion in the columnar phase.
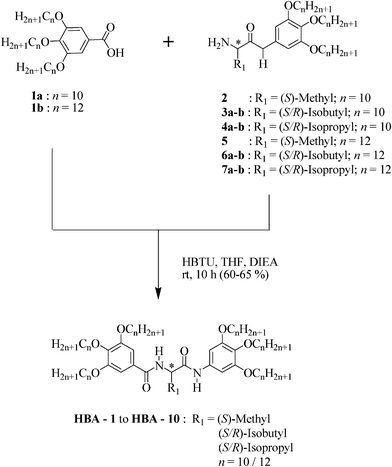
For CD experiments, thin films of neat samples (about 1 mg) held between two quartz plates were prepared and heated to their isotropic phase and cooled slowly. In order to spread the sample uniformly, the plates were mechanically sheared repeatedly while maintaining the material in the isotropic state and subsequently cooled slowly. Columnar phases of the bisamides display Cotton effect and CD spectra recorded as a function of temperature for the enantiomeric pairs HBA-4 and HBA-5 which are shown in Fig. 5a and Fig. 5b respectively. Enantiomers HBA-4 and HBA-5 exhibit mirror image cotton effect with λ = 300 nm (mdeg = −27) and 304 nm (mdeg = +33) respectively. The origin of these CD peaks in the mesophase was ascertained by recording the spectra at different temperatures by rotating the sample cells through 90°, the spectra were found to be nearly identical to those obtained in the original orientation of the samples. This clearly indicated that the signal is not arising from the possible linear dichroism (LD) of the sample.21 Furthermore, given the fact that the instrument used is capable of detecting the LD signal directly, when subjected the Col phase of HBA-4 and HBA-5 for LD measurement; no LD signals were detected. The intensity of CD signals increases with decreasing temperature, thus the above results reflects on the strength of helical aggregates. The spectra of both compounds showed absorption maxima centered around 300 nm due to π–π* transitions, CD maxima and their respective intensities derived from these spectra are tabulated in Table S1.
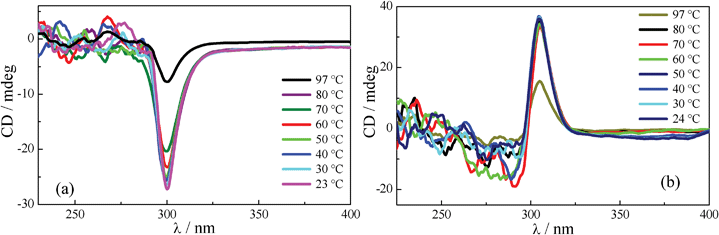 | ||
| Fig. 5 CD spectra recorded as a function of temperature in the Col phase of the enantiomers (a) HBA-4 and (b) HBA-5. | ||
Selected HBA-1 and HBA-2 compounds were investigated under low frequency AC triangular wave electric field (ITO coated cell, 6 μm) which was increased gradually. Even for high voltage up to 200 Vpp, no response was observed for the Col phase, indicating the absence of polar order in the columnar structure. Therefore, it may be assumed that columns are upright. The presence of chiral spacers makes twist from the centre of column through hydrogen bonding. IR investigation in the Col phase confirms that the N–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of adjacent molecules are interconnected and molecules twist with respect to each other by a distinct angle in a uniform sense along the column long axis.22 Furthermore, these columns organize into rectangular macroscopic columnar structure by XRD evaluation. Self-assembly of core-shell helical columns by the nanophase segregation of discrete units (H-bonding amides, π-conjugated aromatic and flexible alkoxy chains)23 makes as an attractive material for one-dimensional transportation of electrons or ions.24
O groups of adjacent molecules are interconnected and molecules twist with respect to each other by a distinct angle in a uniform sense along the column long axis.22 Furthermore, these columns organize into rectangular macroscopic columnar structure by XRD evaluation. Self-assembly of core-shell helical columns by the nanophase segregation of discrete units (H-bonding amides, π-conjugated aromatic and flexible alkoxy chains)23 makes as an attractive material for one-dimensional transportation of electrons or ions.24
As shown in our previous study,14 these kinds of bisamides have a tendency to form gels with various protic solvents through H-bond networks.25 As a preliminary investigation, representative bisamide HBA-5 was tested for its gelation ability. About 50 mg of the sample HBA-5 placed in a screw capped vial was added 1 ml of absolute ethanol and the mixture was gently warmed until the solid dissolves completely. The resulting clear solution was allowed to attain room temperature during which the gel was formed. The formation of the gel was ascertained by the test tube inversion method as illustrated in Fig. 6a. The occurrence of H-bonding in the gel was also investigated by FTIR studies and the IR band values obtained for different functional groups are shown in Table 3. The band values clearly indicate the H-bonded network in the gel. A further CD measurement was carried out at room temperature for the gel by sandwiching between the quartz plates. Fig. 6b shows the CD spectrum recorded for the gel of compound HBA-5 and the CD maxima and their respective intensities derived from this spectrum are tabulated in Table 3. The CD activity of the gel perhaps suggests the occurrence of 3D network comprising fibers having handedness.
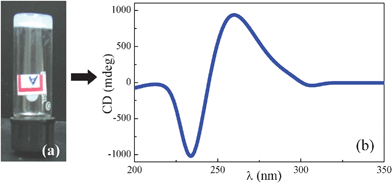 | ||
| Fig. 6 (a) Gelation test by test tube inversion method and (b) CD spectrum recorded at ambient temperature for the gel, both of HBA-5. | ||
| Compound | FTIR (cm−1) | CD | |
|---|---|---|---|
| λ max (nm) | CD (mdeg) | ||
| HBA-5 | ν N–H = 3281, νCH(as) = 2956, | 262, | +932, |
| ν CH(s) = 2854, νCO = 1631 | 234 | −1017 | |
4. Conclusions
Novel hexacatenar bisamides consisting of L-/D-leucine, L-/D-valine or L-alanine as chiral spacers incorporated between two taper-shaped lipophilic segments, have been synthesized and characterized. All the compounds display enantiotropic Col behavior. Notably, L-alanine substituted compound with decyloxy tails show the Col phase over wide thermal range through the room temperature. X-ray diffraction study carried on some representative samples revealed the rectangular symmetry. These compounds readily form stable chiral gels in ethanol. The existence of strong intermolecular hydrogen bonding was evidenced in both columnar and gel states by IR spectroscopy. The handedness of the Col phases and the gel was revealed by CD measurements. These bisamides have much lower clearing temperature when compared to the homomeric dipeptides.14 Further developments are necessary in this kind of bisamides to induce polarity in the columnar structure and also these chiral bisamides can be used as a component for room temperature Col mixtures. Super cooled helical Col phase in these materials makes promising for many applications due to its responsiveness towards external stimuli.26References
- (a) J.-M. Lehn, Supramolecular Chemistry: Concepts and Perspectives, Wiley-VCH, New York, 1995 Search PubMed; (b) H. Ringsdorf, B. Schlarb and J. Venzmer, Angew. Chem., Int. Ed. Engl., 1988, 27, 113–158 CrossRef; (c) T. Kato, Science, 2002, 295, 2414–2418 CrossRef CAS; (d) G. M. Whitesides and B. Grzybowski, Science, 2002, 295, 2418–2421 CrossRef CAS.
- (a) S. Sergeyev, W. Pisula and Y. H. Geerts, Chem. Soc. Rev., 2007, 36, 1902–1929 RSC; (b) W. Pisula, M. Zorn, J. Y. Chang, K. Müllen and R. Zentel, Macromol. Rapid Commun., 2009, 30, 1179–1202 CrossRef; (c) Z. Chen, U. Baumeister, C. Tschierske and F. Würthner, Chem.–Eur. J., 2007, 13, 450–465 CrossRef CAS; (d) W. Pisula, X. Feng and K. Müllen, Adv. Mater., 2010, 22, 3634–3649 CrossRef CAS; (e) M. Funahashi, H. Shimura, M. Yoshio and A. T. Kato, Struct. Bonding, 2008, 128, 151–179 CAS.
- (a) C. Tschierske, Annu. Rep. Prog. Chem., Sect. C, 2001, 97, 191–268 RSC; (b) J. W. Goodby, D. W. Bruce, M. Hird, C. T. Imrie and M. J. Neal, J. Mater. Chem., 2001, 11, 2631–2636 RSC; (c) I. M. Saez and J. W. Goodby, J. Mater. Chem., 2005, 15, 26–40 RSC; (d) J. W. Goodby, I. M. Saez, S. J. Cowling, V. Gortz, M. Draper, A. W. Hall, S. Sia, G. Cosquer, S.-E. Lee and E. P. Raynes, Angew. Chem., Int. Ed., 2008, 47, 2754–2787 CrossRef CAS; (e) K. Kato, N. Mizoshita and K. Kishimoto, Angew. Chem., Int. Ed., 2006, 45, 38–68 CrossRef CAS.
- (a) H. M. Keizer and R. P. Sijbesma, Chem. Soc. Rev., 2005, 34, 226–234 RSC; (b) U. Beginn, Prog. Polym. Sci., 2003, 28, 1049–1105 CrossRef CAS.
- (a) L. Brunsfeld, B. J. B. Folmer, E. W. Meijer and R. P. Sijbesma, Chem. Rev., 2001, 101, 4071–4097 CrossRef CAS; (b) L. J. Prins, D. N. Reinhoudt and P. Timmerman, Angew. Chem. Intl. Ed., 2001, 40, 2383–2402; (c) V. Percec, T. K. Bera, M. Glodde, Q. Fu, V. S. K. Balaguruswamy and P. A, Chem.–Eur. J., 2003, 9, 921–935 CrossRef CAS; (d) W. Zhou, W. Gu, Y. Xu, C. S. Pecinovsky and D. L. Gin, Langmuir, 2003, 19, 6346–6348 CrossRef CAS; (e) J. G. van, A. R. A. Palmans, B. Titulaer, J. A. J. M. Vekemans and E. W. Meijer, J. Am. Chem. Soc., 2005, 127, 5490–5494 CrossRef CAS; (f) A. R. A. Palmans, J. A. J. M. Vekemans, E. E. Havinga and E. W. Meijer, Angew. Chem., Int. Ed. Engl., 1997, 36, 2648–2651 CrossRef CAS; (g) U. Kumar, T. Kato and J. M. J. Frechet, J. Am. Chem. Soc., 1992, 114, 6630–6639 CrossRef CAS.
- S. Qu, F. Li, H. Wang, B. Bai, C. Xu, L. Zhao, B. Long and M. Li, Chem. Mater., 2007, 19, 4839–4846 CrossRef CAS.
- U. Beginn, G. Lattermann, R. Festag and J. H. Wendorff, Acta Polym., 1996, 47, 214–218 CrossRef CAS.
- G. Ungar, D. Abramic, V. Percec and J. A. Heck, Liq. Cryst., 1996, 21, 73–86 CrossRef CAS.
- V. Percec, C.-H. Ahn, T. K. Bera, G. Ungar and D. J. P. Yeardley, Chem.–Eur. J., 1999, 5, 1070–1083 CrossRef CAS.
- V. Percec, M. R. Imam, T. K. Bera, V. S. K. Balaguruswamy, M. Peterca and P. A. Heiney, Angew. Chem., Int. Ed., 2005, 44, 4739–4745 CrossRef CAS.
- K. Kishikawa, S. Nakahara, Y. Nishikawa, S. Kohmoto and M. Yamamoto, J. Am. Chem. Soc., 2005, 127, 2565–2571 CrossRef CAS.
- (a) U. Beginn and G. Lattermann, Mol. Cryst. Liq. Cryst. Sci. Technol., Sect. A, 1994, 241, 215–219 CrossRef CAS; (b) U. Stebani, G. Lattermann, M. Wittenberg and J. H. Wendorff, J. Mater. Chem., 1997, 7, 607–614 RSC.
- D. B. Amabilino, Chirality at the Nanoscale, Wiley-VCH, Weinheim 2009 Search PubMed.
- (a) C. V. Yelamaggad, G. Shanker, R. V. Ramana Rao, D. S. Shankar Rao, S. Krishna Prasad and V. V. Suresh Babu, Chem.–Eur. J., 2008, 14, 10462–10471 CrossRef CAS; (b) G. Shanker, D. S. Shanker Rao, S. Krishna Prasad and C. V. Yelamaggad, Submitted Search PubMed.
- Unable to control the racemization during the reaction of D-alanine substituted compounds, hence comparison was not possible.
- (a) H. Shen, K.-U. Jeong, M. J. Graham, S. Leng, J. X. Zheng, H. Huang, M. Guo, F. W. Harris and S. Z. D. Cheng, Soft Matter, 2006, 2, 232–242 RSC; (b) C. Xue, S. Jin, X. Weng, J. J. Ge, Z. Shen, H. Shen, J. M. Graham, K.-U. Jeong, H. Huang, D. Zhang, M. Guo, F. W. Harris and S. Z. D. Cheng, Chem. Mater., 2004, 16, 1014–1025 CrossRef CAS; (c) X.-B. Zhang and M. Li, J. Mol. Struct., 2008, 892, 490–494 CrossRef CAS.
- I. Paraschiv, M. Giesbers, B. Van Lagen, F. C. Grozema, R. D. Abello, L. D. A. Siebbeles, A. T. M. Marcelis, H. Zuilhof and E. J. R. Sudhölter, Chem. Mater., 2006, 18, 968–974 CrossRef CAS.
- (a) J. Barbera, R. Gimenez and J. L. Serrano, Chem. Mater., 2000, 12, 481–489 CrossRef CAS; (b) L. Alavarez, J. Barbera, L. Puig, P. Romero, J. L. Serrano and T. Sierra, J. Mater. Chem., 2006, 16, 3768–3773 RSC; (c) C. F. Van Nostrum, A. W. Bosman, G. H. Gelinck, P. G. Schouten, J. M. Warman, A. P. M. Kentgens, M. A. C. Devillers, A. Meijerink, S. J. Picken, U. Sohling, A.-J. Schouten and R. J. M. Nolte, Chem.–Eur. J., 1995, 1, 171–182 CAS.
- K. Nakanishi, N. Berova and R.W. Woody, Circular Dichroism: Principles and Applications, VCH Publishers, Inc, New York, NY, 1994 Search PubMed.
- (a) G. Snatzke, Angew. Chem., Int. Ed. Engl., 1979, 18, 363–377 CrossRef; (b) J. L. Serrano and T. Sierra, Chem.–Eur. J., 2000, 6, 759–766 CrossRef CAS.
- (a) J. Schellman and H. P. Jensen, Chem. Rev., 1987, 87, 1359–1399 CrossRef CAS; (b) Linear dichroism (LD) spectroscopy used to study the orientation of molecules, wherein the molecules need to have a preferential orientation. LD is the difference in absorption of light polarized parallel and perpendicular to an orientation axis using linear polarized light. Whereas, circular dichroism (CD) is the difference in absorption of left and right circular polarized light.
- (a) M. A. J. Veld, D. Haveman, A. R. A. Palmas and E. W. Meijer, Soft Matter, 2011, 7, 524–531 RSC; (b) G. Shanker, M. Prehm, C. V. Yelamaggad and C. Tschierske, J. Mater. Chem., 2011, 21, 5307–5311 RSC.
- (a) C. Tschierske, J. Mater. Chem., 1998, 8, 1485–1508 RSC; (b) C. Tschierske, J. Mater. Chem., 2001, 11, 2647–2671 RSC.
- (a) T. Kato, T. Yasuda, Y. Kamikawa and M. Yoshio, Chem. Commun., 2009, 729–739 RSC.
- (a) N. M. Sangeetha and U. Maitra, Chem. Soc. Rev., 2005, 34, 821–836 RSC; (b) N. Mizoshita, H. Monobe, M. Inoue, M. Ukon, T. Watanabe, Y. Shimizu, K. Hanabusa and T. Kato, Chem. Commun., 2002, 428–429 RSC; (c) N. Mizoshita, Y. Suzuki, K. Kishimoto, K. Hanabusa and T. Kato, J. Mater. Chem., 2002, 12, 2197–2201 RSC; (d) F. Camerel, G. Ulrich and R. Ziessel, Org. Lett., 2004, 6, 4171–4174 CrossRef CAS.
- (a) T. Ikeda, J. Mamiya and Y. Yu, Angew. Chem., Int. Ed., 2007, 46, 506–528 CrossRef CAS; (b) Y. Shimizu, A. Kurobe, H. Monobe, N. Terasawa, K. Kiyohara and K. Uchida, Chem. Commun., 2003, 1676–1677 RSC; (c) V. N. Kozhevnikov, B. Donnio and D. W. Bruce, Angew. Chem., Int. Ed., 2008, 47, 6286–6289 CrossRef CAS.
Footnotes |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c1ra00684c/ |
| ‡ Institute of Chemistry, Organic Chemistry, Martin-Luther-University Halle-Wittenberg, Kurt Mothes Str. 2, D-06120 Halle/Saale, Germany |
| This journal is © The Royal Society of Chemistry 2012 |

