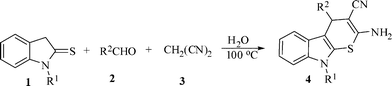Green approach to highly functionalized thiopyrano derivatives via domino multi-component reaction in water†
K. C.
Majumdar
*ab,
Sudipta
Ponra
a and
Tapas
Ghosh
a
aDepartment of Chemistry, University of Kalyani, Kalyani, 741235, W.B, India
bDepartment of Chemical Sciences, Tezpur University, Napaam, Tezpur, 784028, Assam, India. E-mail: kcm_ku@yahoo.co.in; Fax: +91 033 2582 8282; Tel: +91 033 25827521
First published on 12th December 2011
Abstract
A green, operationally simple and highly efficient one pot three-component approach for the synthesis of thiopyrano[2,3-b]indole-3-carbonitrile, thiopyrano[2,3-b]thiochromene-3-carbonitrile and dihydrothiochromeno[2,3-b]thiochromene derivatives has been developed by the domino reaction of indoline-2-thione or 4-hydroxy-2H-thiochromene-2-thione with aldehyde and malononitrile or dimedone in water at 100 °C. The significant advantages of this protocol are highlighted by short reaction time, excellent yields and formation of three new bonds and a stereocenter in one operation from easily available starting materials.
Introduction
In recent years, due to growing environmental concerns, the design of straightforward and practical chemical syntheses of drugs and fine chemicals that satisfy economic and ecological criteria is a major challenge in industry. Water is cheap and environmentally benign and is the best option for solvents due to its non-toxic, non-corrosive and non-flammable nature.1Water also possesses unique reactivity and selectivity. The green solvent water also eliminates some protection and deprotection processes for certain acidic hydrogen containing functional groups and offers an easy approach for the separation of environmentally unfriendly reagents and by-products.1a,1b The elimination of catalyst and replacement of hazardous solvents with more benign solvents is now globally accepted. Catalyst-free reactions and use of environmentally benign solvent in multi-component syntheses are particularly attractive, because they incorporate many of the green chemistry principles.Multi-component reactions (MCRs) are one-pot processes in which three or more reactants come together in a single reaction vessel to form a product containing substantial elements of all the reactants.2 Thus, design of highly efficient chemical reaction sequences that provide maximum structural complexity and diversity with a minimum number of synthetic steps to synthesize compounds with interesting properties3 is important for drug discovery and natural product like compounds. Recently, the multi-component reactions have attracted considerable attention in combinatorial and medicinal chemistry and have been designed to produce biologically active compounds.4 The multi-component reactions are advantageous compared to linear stepwise synthesis because of possible structural variations, simplicity of a one-pot procedure, atom economy, and convergent character.5
Recently a wide range of biological activities associated with the sulphur-heterocycles scaffolds have been identified.6Thiopyran and fused-thiopyran derivatives are known to exhibit anti-inflammatory,7anti-bacterials,8anti-hyperplasia,9 antipsychiatric,10analgesic, and anti-cancer11 activities and are widely present as key structural motifs in many natural products and are structurally related to plant pigments like flavonoids and anthocyanins. Thiopyran derivatives can act as modulators of the estrogen receptors12 and are found to possess a high dopamine receptor binding affinity.13Indole subunits are frequently present in many biologically active natural products.14Thiopyranoindole-annulated heterocyclic compounds are important due to their biological activity. Tetrahydrothiopyrano[2,3-b] indole are known to have analgesic activity15 and their pharmaceutically acceptable salts are useful as psychoanaleptic and nootropic drugs.16
Results and discussion
In continuation of our work on the multi-component synthesis of heterocyclic compounds17 we have designed a unique synthetic route to privileged heterocyclic scaffolds of medicinal relevance that combine synthetic efficiency of multi-component protocols with the environmental benefit of using water as a reaction medium. Herein we report the results.Thiopyrano[2,3-b]indole-3-carbonitrile derivatives (4a–m) have been synthesized in one pot by coupling various indoline-2-thione (1), aromatic aldehyde (2), malononitrile (3) in water at 100 °C in excellent yields (91–98%) without any additional reagent or catalyst (Scheme 1).
![Synthesis of thiopyrano[2,3-b]indole-3-carbonitrile derivatives.](/image/article/2012/RA/c1ra00655j/c1ra00655j-s1.gif) | ||
| Scheme 1 Synthesis of thiopyrano[2,3-b]indole-3-carbonitrile derivatives. | ||
We have carried out a series of experiments to standardize the reaction conditions for the efficient formation of bioactive heterocycles via a one-pot, three-component reaction and the results are presented in Table 1. Initial attempts were made by heating a mixture of indoline-2-thione (1a, 1 mmol), 4-methoxy benzaldehyde (2a, 1 mmol), malononitrile (3, 1 mmol) at 80 °C in ethanol in the presence of different catalysts such as InCl3, AcOH, CuI, NH4OAc (20 mole% each) separately (entries 1–4). No satisfactory result was obtained. Further attempts were made to carry out the same reaction in different solvents such as toluene, MeOH, THF, CH3CN, CH2Cl2 at different temperatures in the absence of any catalyst (entries 5–9). The results showed that the reaction can proceed successfully even without a catalyst. With a view to determine the optimized conditions of the reaction we have carried out the same experiment in water using different reaction-times and temperatures (entries 11–13 and entries 14–15). The reaction did not occur at room temperature, only cyanoolefin A, formed in situ from Knoevenagel condensation of the aldehyde 2a and malononitrile 3 was obtained (entry 10). At lower temperature the reaction gives lower yield. From the various reaction conditions in Table 1 it is clear that the reaction in water gives the best result (98% yield) and higher reaction rate when heated at 100 °C for just 30 min (Table 1, entry 13).
|
|
|||||
|---|---|---|---|---|---|
| Entry | Catalyst (mol%) | T/°C | Solvent | Time (min) | Yield (%)b |
| a Optimized reaction conditions. b Isolated yields. | |||||
| 1. | InCl3 (20) | 80 | EtOH | 30 | 42 |
| 2. | AcOH (20) | 80 | EtOH | 30 | 58 |
| 3. | CuI (20) | 80 | EtOH | 30 | 37 |
| 4. | NH4OAc (20) | 80 | EtOH | 30 | 72 |
| 5. | — | 110 | Toluene | 30 | 62 |
| 6. | — | 65 | MeOH | 30 | 59 |
| 7. | — | 65 | THF | 30 | 51, |
| 8. | — | 80 | CH3CN | 30 | 65 |
| 9. | — | 40 | CH2Cl2 | 30 | 49 |
| 10. | — | r.t. | H2O | 120 | 0 |
| 11. | — | 100 | H2O | 10 | 62 |
| 12. | — | 100 | H2O | 20 | 84 |
| 13. a | — | 100 | H2O | 30 | 98 |
| 14. | — | 80 | H2O | 30 | 71 |
| 15. | — | 90 | H2O | 30 | 87 |
To explore the scope and generality of the one-pot Knoevenagal condensation/Michael addition/cyclization reaction, a range of thiopyrano[2,3-b]indole-3-carbonitrile derivatives were synthesized from a variety of substrates under the optimized reaction conditions. Three different types of indoline-2-thione (1), and a wide range of aldehydes (aromatic and heterocyclic), 2a–k were examined. Pleasingly all of them gave excellent yields of the desired products under the optimized reaction conditions. Table 2 shows that this protocol can be applied not only to electron rich but also electron-deficient aromatic aldehydes and heterocyclic aldehydes as electronic nature of the substituents on the aromatic ring of the aldehydes did not show any influence on the yield of the products of the reaction, thus demonstrating the wide scope of this methodology. However, a complex mixture of products with very close Rf values (TLC) were obtained in the case of aliphatic aldehyde from which the desired product could not be isolated, thus, limiting the scope of this reaction to a certain extent.
|
|
|||||
|---|---|---|---|---|---|
| Entry | R1 | R2 | Time (min) | Products | Yielda(%) |
| a Isolated pure yield | |||||
| 1. | H | 4-MeOC6H4 (2a) | 30 | 4a | 98 |
| 2. | H | 4-MeC6H4 (2b) | 30 | 4b | 96 |
| 3. | H | 4-BrC6H4 (2c) | 35 | 4c | 97 |
| 4. | H | C4H4S (2d) | 40 | 4d | 95 |
| 5. | Me | C6H5 (2e) | 35 | 4e | 97 |
| 6. | Me | 4-ClC6H4 (2f) | 30 | 4f | 96 |
| 7. | Me | 3-NO2C6H4 (2g) | 40 | 4g | 93 |
| 8. | Me | benzo[d][1,3]dioxole(2h) | 35 | 4h | 95 |
| 9. | Me | 2-BrC6H4 (2i) | 35 | 4i | 91 |
| 10. | Me | 2-OMe,5-ClC6H3 (2j) | 40 | 4j | 91 |
| 11. | Me | 3,4-(OMe)2C6H3 (2k) | 30 | 4k | 94 |
| 12. | Me | 4-MeOC6H4 (2a) | 35 | 4l | 93 |
| 13. | Et | 4-MeC6H4 (2b) | 30 | 4m | 95 |
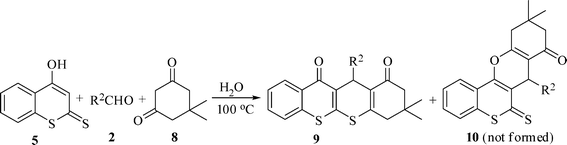
This reaction sequence is also extended to 4-hydroxy-2H-thiochromene-2-thione (5), aromatic aldehyde (2), and malononitrile (3) under the same reaction conditions as above. Although there is a possibility of the formation of benzopyran (7) and thiopyran derivatives (6), only thiopyrano[2,3-b]thiochromene-3-carbonitrile-4-one derivatives 6 were obtained in excellent yields. The electronic nature of the substituent on the aromatic ring of the aldehyde did not show any effect in this case too. The results in Table 3 show that as expected, S-alkylation is favored over O-alkylation making the protocol a highly regioselective one.
The structures of the products were characterized from their elemental analyses and spectral data. In IR-spectroscopy a peak at 2186 cm−1 region indicates the presence of a –CN group and two peaks at 3390, 3381 cm−1 indicate the presence of a –NH2group. In the 1H-NMR spectra, δH = 6.78 (s, 2H) and δH = 5.05 (s, 1H) show the presence of a NH2 group and a quaternary proton for compound 4a. For compound 6a the δC = 175.0 shows the presence of a –CO group and hence confirmed the formation of thiopyran derivatives instead of benzopyran derivatives.
A highly efficient one-pot three-component regioselective synthesis of dihydrothiochromeno[2,3-b]thiochromene derivatives have also been developed by applying the same methodology (Table 4). Here 4-hydroxy-2H-thiochromene-2-thione (5) is treated with various aromatic aldehydes (2) and dimedone (8) at 100 °C in green solvent water to give the corresponding dihydrothiochromeno[2,3-b]thiochromene derivatives (9) in high yields. (86–94%)
|
|
||||
|---|---|---|---|---|
| Entry | R2 | Time (min) | Products | Yielda (%) |
| a Isolated pure yield | ||||
| 1. | C6H5 (2e) | 45 | 9a | 94 |
| 2. | benzo[d][1,3]dioxole (2h) | 55 | 9b | 89 |
| 3. | 2-BrC6H4 (2i) | 55 | 9c | 87 |
| 4. | 2-OMe,5-ClC6H3 (2j) | 45 | 9d | 86 |
| 5. | 3,4-(OMe)2C6H3 (2k) | 60 | 9e | 93 |
| 6. | 4-FC6H4 (2l) | 60 | 9f | 91 |
The formation of products 4, 6 and 9 in this green reaction may be rationalized by the initial formation of an intermediate cyanoolefin A (isolated as a solid) from the Knoevenagel condensation of 2 and 3. Michael addition of indoline-2-thione 1 to A may generate an intermediate B (not isolable) which on cyclocondensation may give the desired product 4. Similarly Michael addition of 4-hydroxy-2H-thiochromene-2-thione 5 to cyanoolefin A may form intermediate C which may undergo intramolecular cyclization either via S-alkylation (more likely) to furnish thiochromone derivatives 6 (observed) or via O-alkylation (less likely) to give chromone derivative 7 (not obtained) (Scheme 2). Like-wise, Knoevenagel condensation of 8 and 2 gives the enone intermediate D (isolated as a solid). Michael addition of 5 to D may give intermediate E which may be followed by an intramolecular cyclization to afford the product 9. The involvement of the intermediates A and D in this green reaction was also verified by carrying out separate reactions with the isolated intermediates (Scheme 3).
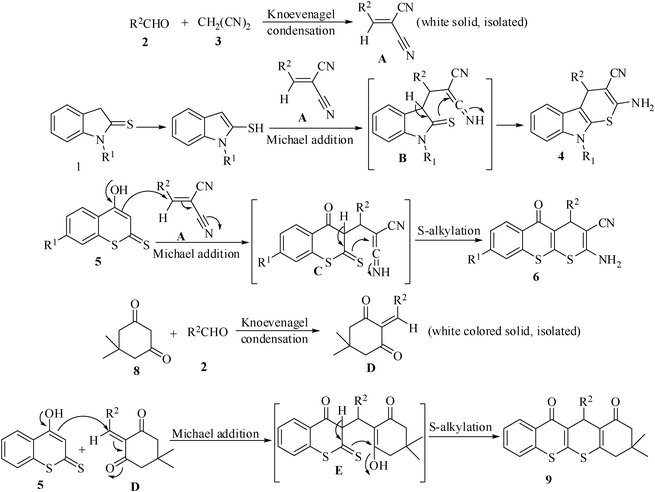 | ||
| Scheme 2 Plausible mechanism for the thiochromene derivative. | ||
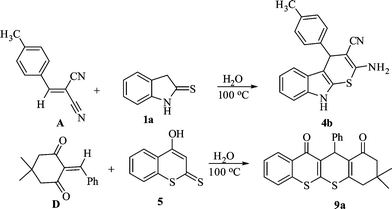 | ||
| Scheme 3 Synthesis of thiochromene derivatives from isolated intermediate A and D18. | ||
A literature survey revealed that the reports of the synthesis of thiochromones are limited.19–23 In our laboratory we have synthesized coumarin- and pyrone-annulated [6,6]-fused pyranothiopyrans using sequential Claisen rearrangements24 and tributyl tin hydride-mediated radical cyclization25 respectively. However, the above methods suffer from drawbacks such as toxic organic solvents, costly reagents, cumbersome experimental procedures, and lacking generality, expensive metal catalyst, long reaction time, multiple step and unsatisfactory yields etc. Very recently Singh et al.26 reported the synthesis of 4-aryl-3-aroyl-2-methylsulfanyl-4,6,7,8-tetrahydrothiochromen-5-ones in good to moderate yields using β-oxodithioesters with aldehydes and cyclic 1,3-diketones using P2O5 as a catalyst at 100 °C for 2–3 h. To avoid those discrepancies there has been a need to develop an efficient and convenient methodology for the synthesis of thiochromone derivatives.
Conclusion
In summary, we have developed a simple and efficient method for the diversity-oriented synthesis of a series of thiopyrano[2,3-b]indole-3-carbonitrile, thiopyrano[2,3-b]thiochromene-3-carbonitrile and dihydrothiochromeno[2,3-b]thiochromene derivatives via a one-pot multi-component reaction in water from easily available starting materials without any catalyst. To the best of our knowledge, this is the first report of thiopyrano derivatives following green chemistry principles. The significant advantages of this protocol are shorter reaction time, generally excellent yields, easily available starting materials, use of environmentally benign solvent, operational simplicity and the reaction produces three new bonds and one stereocenter in one operation. Furthermore, the methodology is suitable for diversity oriented synthesis of potentially bioactive heterocycles. We believe that this would be suitable for the generation of a library of relevant natural products synthesis.Experimental
Melting points were determined in open capillaries and are uncorrected. IR spectra were run for KBr discs on a Perkin-Elmer 120-000A apparatus (υmax in cm−1) and 1H-NMR and 13C–NMR spectra were determined for solutions in CDCl3 and DMSO-d6 with TMS as an internal standard on a Bruker DPX-400. CHN was recorded on a 2400 series II CHN analyzer Perkin Elmer. HRMS and mass spectra were recorded on a Qtof Micro instrument. Silica gel (60–120 mesh) was used for chromatographic separation. Silica gel-G [E-Mark (India)] was used for TLC. Petroleum-ether refers to the fraction between 60 °C and 80 °C.General procedure for the synthesis of thiopyrano derivatives 4
To a well-stirred suspension of the appropriate aromatic aldehyde 2 (1 equiv.) and malononitrile 3 (1 equiv.) in water (5 mL), indolin-2-thione 1 (1 equiv.) was added and the reaction mixture which was heated at 100 °C for 30–40 min. As the reaction proceeds the reaction mixture gradually becomes a deep brown color. After completion of the reaction as monitored by TLC the reaction mixture was cooled and diluted with 20 mL of water and extracted with ethyl acetate (3 × 25 mL) and dried over anhydrous Na2SO4. The solvent was distilled off. The resulting crude product was purified by filtration through a pad of silica gel (60–120 mesh) using 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether-ethyl acetate mixture as eluent to give the pure compounds (4).
1 petroleum ether-ethyl acetate mixture as eluent to give the pure compounds (4).
2-Amino-4-(4-methoxyphenyl)-4,9-dihydrothiopyrano[2,3-b]indole-3-carbonitrile (4a)
Using the general procedure starting from 100 mg (0.671 mmol) of indoline-2-thione 1a, 91.3 mg (0.671 mmol) of 4-methoxybenzaldehyde 2a, 44.3 mg (0.671 mmol) of malononitrile 3, 219 mg of the title compound 4a was isolated as a grey colored solid, mp 178–180 °C, yield = 98%, IR (KBr): 3390, 3381, 3252, 2186, 1624, 1572 cm−1. 1H NMR (400 MHz, DMSO-d6): δH = 3.69 (s, 3H), 5.05 (s, 1H), 6.78 (s, 2H), 6.84 (d, 2H, J = 8.4 Hz), 6.90 (t, 1H, J = 7.6 Hz), 7.03 (t, 1H, J = 8.0 Hz), 7.18–7.25 (m, 3H), 7.29 (t, 1H, J = 7.6 Hz), 11.45 (s, 1H). 13C NMR (100 MHz, DMSO-d6): δC = 157.9, 151.3, 137.2, 136.6, 128.3, 125.3, 121.2, 120.4, 120.0, 119.1, 117.3, 113.8, 110.7, 107.9, 74.5, 54.9. HRMS (ESI+): calcd. For C19H15N3OS: [M+Na+] 356.0834; found 356.0865.2-Amino-4-p-tolyl-4,9-dihydrothiopyrano[2,3-b]indole-3-carbonitrile (4b)
Using the general procedure starting from 100 mg (0.671 mmol) of indoline-2-thione 1a, 80.5 mg (0.671 mmol) of 4-methylbenzaldehyde 2b, 44.3 mg (0.671 mmol) of malononitrile 3, 204 mg of the title compound 4b was isolated as a grey colored solid, mp 184–186 °C, yield = 96%, IR (KBr): 3394, 3284, 3200, 2188, 1624, 1573 cm−1. 1H NMR (400 MHz, DMSO-d6): δH = 2.22 (s, 3H), 5.04 (s, 1H), 6.77 (s, 2H), 6.89 (t, 1H, J = 7.2 Hz), 7.01 (t, 1H, J = 7.6 Hz), 7.07 (d, 2H, J = 7.6 Hz), 7.18–7.21 (m, 3H), 7.28 (d, 1H, J = 8.0 Hz), 11.43 (s, 1H). 13C NMR (100 MHz, DMSO-d6): δC = 151.5, 142.1, 136.5, 135.6, 129.0, 127.2, 125.3, 121.2, 120.5, 120.0, 119.1, 117.3, 110.7, 107.8, 74.3, 40.2, 20.6. MS: m/z = 339.9 [M+Na]+. Anal. Calcd. For C19H15N3S: C, 71.90; H, 4.76; N, 13.24%. Found: C, 71.71; H, 4.90; N, 13.17%.2-Amino-4-(4-bromophenyl)-4,9-dihydrothiopyrano[2,3-b]indole-3-carbonitrile (4c)
Using the general procedure starting from 100 mg (0.671 mmol) of indoline-2-thione 1a, 124.1 mg (0.671 mmol) of 4-bromobenzaldehyde 2c, 44.3 mg (0.671 mmol) of malononitrile 3, 248 mg of the title compound 4c was isolated as a grey colored solid, mp 192–194 °C, Yield = 97%, IR (KBr): 3374, 3273, 3191, 2191, 1627, 1582 cm−1. 1H NMR (400 MHz, DMSO-d6): δH = 5.13 (s, 1H), 6.88–6.92 (m, 3H), 7.03 (t, 1H, J = 7.6 Hz), 7.21 (d, 1H, J = 7.6 Hz), 7.29 (t, 3H, J = 7.2 Hz), 7.48 (d, 2H, J = 7.6 Hz), 11.50 (s, 1H). 13C NMR (100 MHz, DMSO-d6): δC = 151.9, 144.5, 136.6, 131.4, 129.5, 125.2, 121.3, 120.7, 119.8, 119.7, 119.3, 117.2, 110.8, 107.1, 73.4. MS: m/z = 381 [M+], 383 [M++2]. Anal. Calcd. For C18H12BrN3S: C, 56.55; H, 3.16; N, 10.99%. Found: C, 56.68; H, 3.26; N, 11.04%.2-Amino-4-(thiophen-2-yl)-4,9-dihydrothiopyrano[2,3-b]indole-3-carbonitrile (4d)
Using the general procedure starting from 100 mg (0.671 mmol) of indoline-2-thione 1a, 75.2 mg (0.671 mmol) of thiophene-2-carbaldehyde 2d, 44.3 mg (0.671 mmol) of malononitrile 3, 197 mg of the title compound 4d was isolated as a grey colored solid, mp 178–180 °C, yield = 95%, IR (KBr): 3381, 3276, 3195, 2189, 1623, 1578 cm−1. 1H NMR (400 MHz, DMSO-d6): δH = 5.51 (s, 1H), 6.91 (s, 3H), 6.97 (t, 1H, J = 7.6 Hz), 7.05 (d, 2H, J = 7.6 Hz), 7.28 (d, 1H, J = 8.4 Hz), 7.32 (d, 1H, J = 8.0 Hz), 7.40 (d, 1H, J = 8.0 Hz), 11.49 (s, 1H). 13C NMR (100 MHz, DMSO-d6): δC = 152.4, 149.6, 136.5, 126.5, 125.2, 124.6, 123.7, 121.3, 120.7, 119.9, 119.3, 117.3, 110.8, 107.8, 73.9, 35.5. MS: m/z = 331.85 [M+Na]+. Anal. Calcd. For C16H11N3S2: C, 62.11; H, 3.58; N, 13.58%. Found: C, 62.33; H, 3.47; N, 13.61%.2-Amino-9-methyl-4-phenyl-4,9-dihydrothiopyrano[2,3-b]indole-3-carbonitrile (4e)
Using the general procedure starting from 100 mg (0.613 mmol) of 1-methylindoline-2-thione 1b, 65 mg (0.613 mmol) of benzaldehyde 2e, 40.5 mg (0.613 mmol) of malononitrile 3, 188.6 mg of the title compound 4e was isolated as a grey colored glassy solid, mp 196–198 °C, yield = 97%, IR (KBr): 3428, 3314, 3213, 2188, 1634, 1575 cm−1. 1H NMR (400 MHz, CDCl3): δH = 3.71 (s, 3H), 4.57 (s, 2H), 5.19 (s, 1H), 7.01 (t, 1H, J = 7.6 Hz), 7.16–7.19 (m, 2H), 7.21 (s, 1H), 7.24 (d, 1H, J = 6.0 Hz), 7.29 (d, 2H, J = 8.8 Hz), 7.33 (d, 2H, J = 7.6 Hz). 13C NMR (100 MHz, DMSO-d6): δC = 151.4, 144.9, 137.5, 128.5, 127.1, 126.6, 124.9, 123.0, 121.2, 119.9, 119.4, 117.4, 109.2, 107.5, 74.3, 40.8, 30.1. MS: m/z = 318.10 [M+H]+. Anal. Calcd. For C19H15N3S: C, 71.90; H, 4.76; N, 13.24%. Found: C, 71.89; H, 4.63; N, 13.22%.2-Amino-4-(4-chlorophenyl)-9-methyl-4,9-dihydrothiopyrano[2,3-b]indole-3-carbonitrile (4f)
Using the general procedure starting from 100 mg (0.613 mmol) of 1-methylindoline-2-thione 1b, 86 mg (0.613 mmol) of 4-cholorobenzaldehyde 2f, 40.5 mg (0.613 mmol) of malononitrile 3, 206.7 mg of the title compound 4f was isolated as a grey colored solid, mp 180–182 °C, yield = 96%, IR (KBr): 3311, 3216, 2193, 1632, 1581 cm−1. 1H NMR (400 MHz, CDCl3): δH = 3.71 (s, 3H), 4.61 (s, 2H), 5.17 (s, 1H), 7.01–7.05 (m, 1H), 7.18 (td, 2H, J = 1.2 Hz, 6.8 Hz), 7.23–7.28 (m, 5H). 13C NMR (100 MHz, DMSO-d6): δC = 151.5, 143.9, 137.6, 131.2, 129.0, 128.5, 124.8, 123.1, 121.3, 119.7, 119.5, 117.4, 109.3, 106.9, 73.7, 30.1. MS: m/z = 374.07 [M+Na]+. Anal. Calcd. For C19H14ClN3S: C, 64.86; H, 4.01; N, 11.94%. Found: C, 64.89; H, 3.97; N, 11.99%.2-Amino-9-methyl-4-(3-nitrophenyl)-4,9-dihydrothiopyrano[2,3-b]indole-3-carbonitrile (4g)
Using the general procedure starting from 100 mg (0.613 mmol) of 1-methylindoline-2-thione 1b, 92.6 mg (0.613 mmol) of 3-nitrobenzaldehyde 2g, 40.5 mg (0.613 mmol) of malononitrile 3, 206.5 mg of the title compound 4g was isolated as a light yellow fluffy solid, mp 182–184 °C, yield = 93%, IR (KBr): 3421, 3339, 3307, 2181, 1623, 1572 cm−1. 1H NMR (400 MHz, CDCl3): δH = 3.74 (s, 3H), 4.69 (s, 2H), 5.31 (s, 1H), 7.04 (d, 1H, J = 7.6 Hz), 7.19 (q, 2H, J = 8.0 Hz), 7.28 (d, 1H, J = 8.4 Hz), 7.49 (t, 1H, J = 8.0 Hz), 7.74 (d, 1H, J = 7.6 Hz), 8.08 (dd, 1H, J = 0.8 Hz, 8.0 Hz), 8.12 (d, 1H, J = 2.0 Hz). 13C NMR (100 MHz, DMSO-d6): δC = 152.2, 147.9, 147.2, 137.6, 133.9, 130.2, 124.7, 123.4, 121.9, 121.5, 119.7, 119.6, 117.3, 109.4, 106.5, 73.1, 40.1, 30.2. MS: m/z = 384.83 [M+Na]+. Anal. Calcd. For C19H14N4O2S: C, 62.97; H, 3.89; N, 15.46%. Found: C, 62.96; H, 3.89; N, 15.39%.2-Amino-4-(benzo[d][1,3]dioxol-5-yl)-9-methyl-4,9-dihydrothiopyrano[2,3-b]indole-3-carbonitrile (4h)
Using the general procedure starting from 100 mg (0.613 mmol) of 1-methylindoline-2-thione 1b, 92 mg (0.613 mmol) of benzo[d][1,3]dioxole-5-carbaldehyde 2h, 40.5 mg (0.613 mmol) of malononitrile 3, 210.4 mg of title compound 4h was isolated as light yellow fluffy solid, mp 190–192 °C, yield = 95%, IR (KBr): 3423, 3320, 3226, 2194, 1640, 1577 cm−1. 1H NMR (400 MHz, CDCl3): δH = 3.70 (s, 3H), 4.56 (s, 2H), 5.12 (s, 1H), 5.89 (dd, 2H, J = 1.2 Hz, 12.0 Hz), 6.72 (d, 2H, J = 8.4 Hz), 6.87 (dd, 1H, J = 2.0 Hz, 8.0 Hz), 7.03–7.05 (m, 1H), 7.17–7.19 (m, 1H), 7.22 (s, 1H), 7.25 (s, 1H). 13C NMR (100 MHz, DMSO-d6): δC = 151.1, 147.3, 145.9, 139.0, 137.5, 124.9, 122.9, 121.2, 120.1, 119.8, 119.4, 117.5, 109.2, 108.0, 107.5, 100.8, 79.1, 74.5, 40.4, 30.1. MS: m/z = 383.87 [M+Na]+. Anal. Calcd. For C20H15N3O2S: C, 66.46; H, 4.18; N, 11.63%. Found: C, 66.49; H, 4.17; N, 11.49%.2-Amino-4-(2-bromophenyl)-9-methyl-4,9-dihydrothiopyrano[2,3-b]indole-3-carbonitrile (4i)
Using the general procedure starting from 100 mg (0.613 mmol) of 1-methylindoline-2-thione 1b, 113.4 mg (0.613 mmol) of 2-bromobenzaldehyde 2i, 40.5 mg (0.613 mmol) of malononitrile 3, 220.5 mg of the title compound 4i was isolated as a white solid, mp 208–210 °C, yield = 91%, IR (KBr): 3424, 3314, 3219, 2185, 1636, 1571 cm−1. 1H NMR (400 MHz, CDCl3): δH = 3.71 (s, 3H), 4.58 (s, 2H), 5.87 (s, 1H), 7.00–7.06 (m, 2H), 7.14–7.17 (m, 2H), 7.23–7.28 (m, 3H), 7.57 (d, 1H, J = 8.0 Hz). 13C NMR (100 MHz, DMSO-d6): δC = 150.9, 143.8, 137.5, 132.4, 130.8, 128.9, 128.5, 124.9, 123.3, 121.6, 121.4, 119.7, 119.0, 117.0, 109.3, 106.3, 73.1, 40.1, 30.1. MS: m/z = 395 [M+], 397 [M++2]. Anal. Calcd. For C19H14BrN3S: C, 57.58; H, 3.56; N, 10.60%. Found: C, 57.51; H, 3.55; N, 10.48%.2-Amino-4-(5-chloro-2-methoxyphenyl)-9-methyl-4,9-dihydrothiopyrano[2,3-b]indole-3-carbonitrile (4j)
Using the general procedure starting from 100 mg (0.613 mmol) of 1-methylindoline-2-thione 1b, 104.5 mg (0.613 mmol) of 5-chloro-2-methoxybenzaldehyde 2j, 40.5 mg (0.613 mmol) of malononitrile 3, 212.7 mg of the title compound 4j was isolated as a grey colored solid, mp 188–190 °C, Yield = 91%, IR (KBr): 3424, 3316, 3216, 2189, 1634, 1571 cm−1. 1H NMR (400 MHz, CDCl3): δH = 3.71 (s, 3H), 3.97 (s, 3H), 4.57 (s, 2H), 5.75 (s, 1H), 6.84 (d, 1H, J = 8.8 Hz), 7.01 (t, 1H, J = 2.8 Hz), 7.04 (s, 1H), 7.09 (dd, 1H, J = 2.4 Hz, 8.8 Hz), 7.15–7.19 (m, 1H), 7.24–7.29 (m, 2H). 13C NMR (100 MHz, DMSO-d6): δC = 154.6, 152.2, 137.5, 134.8, 128.0, 127.7, 124.7, 124.4, 123.5, 121.4, 119.6, 117.1, 113.3, 109.3, 106.8, 73.2, 56.1, 33.4, 30.1. MS: m/z = 404.01 [M+Na]+. Anal. Calcd. For C20H16ClN3OS: C, 62.90; H, 4.22; N, 11.00%. Found: C, 62.79; H, 4.12; N, 11.10%.2-Amino-4-(3,4-dimethoxyphenyl)-9-methyl-4,9-dihydrothiopyrano[2,3-b]indole-3-carbonitrile (4k)
Using the general procedure starting from 100 mg (0.613 mmol) of 1-methylindoline-2-thione 1b, 101.8 mg (0.613 mmol) of 3,4-dimethoxybenzaldehyde 2k, 40.5 mg (0.613 mmol) of malononitrile 3, 217.4 mg of the title compound 4k was isolated as a colorless solid, mp 172–174 °C, yield = 94%, IR (KBr): 3416, 3356, 3258, 2192, 1621, 1588 cm−1. 1H NMR (400 MHz, DMSO-d6): δH = 3.70 (s, 6H), 3.76 (s, 3H), 5.09 (s, 1H), 6.85 (s, 2H), 6.88 (s, 2H), 6.98 (s, 2H), 7.09 (t, 1H, J = 7.6 Hz), 7.34 (d, 1H, J = 7.6 Hz), 7.42 (d, 1H, J = 8.0 Hz). 13C NMR (100 MHz, DMSO-d6): δC = 151.1, 148.5, 147.6, 137.5, 137.5, 124.9, 122.8, 121.2, 119.9, 119.4, 119.1, 117.6, 111.9, 111.2, 109.2, 107.8, 74.6, 55.4, 40.4, 30.1. MS: m/z = 416.11 [M+K]+. Anal. Calcd. For C21H19N3O2S: C, 66.82; H, 5.07; N, 11.13%. Found: C, 66.79; H, 5.06; N, 11.12%.2-Amino-4-(4-methoxyphenyl)-9-methyl-4,9-dihydrothiopyrano[2,3-b]indole-3-carbonitrile (4l)
Using the general procedure starting from 100 mg (0.613 mmol) of 1-methylindoline-2-thione 1b, 83.4 mg (0.613 mmol) of 4-methoxybenzaldehyde 2a, 40.5 mg (0.613 mmol) of malononitrile 3, 198 mg of the title compound 4l was isolated as a grey colored solid, mp 184–186 °C, yield = 93%, IR (KBr): 3392, 3386, 3252, 2181, 1622, 1573 cm−1 cm−1. 1H NMR (400 MHz, CDCl3): δH = 3.70 (s, 3H), 3.75 (s, 3H), 4.55 (s, 2H), 5.15 (s, 1H), 6.81 (d, 2H, J = 8.4 Hz), 7.01 (t, 1H, J = 7.2 Hz), 7.14–7.21 (m, 2H), 7.24 (d, 2H, J = 2.4 Hz), 7.25 (s, 1H). 13C NMR (100 MHz, DMSO-d6): δC = 158.0, 150.9, 137.5, 137.1, 128.2, 124.9, 122.8, 121.2, 119.9, 119.4, 117.5, 113.8, 109.2, 107.7, 74.7, 54.9, 30.1. MS: m/z = 347 [M+]. Anal. Calcd. For C20H17N3OS: C, 69.14; H, 4.93; N, 12.09%. Found: C, 69.11; H, 4.92; N, 12.09%.2-Amino-9-ethyl-4-p-tolyl-4,9-dihydrothiopyrano[2,3-b]indole-3-carbonitrile (4m)
Using the general procedure starting from 100 mg (0.565 mmol) of 1-ethylindoline-2-thione 1c, 67.8 mg (0.565 mmol) of 4-methylbenzaldehyde 2b, 37.3 mg (0.565 mmol) of malononitrile 3, 185.2 mg of title compound 4m was isolated as colorless solid, mp 156–158 °C, yield = 95%, IR (KBr): 3426, 3320, 3219, 2177, 1629, 1567 cm−1. 1H NMR (400 MHz, DMSO-d6): δH = 1.28 (t, 3H, J = 8.8 Hz), 2.23 (s, 3H), 4.12–4.18 (m, 2H), 5.12 (s, 1H), 6.91 (s, 2H), 6.97 (t, 1H, J = 7.6 Hz), 7.08–7.13 (m, 3H), 7.23 (d, 2H, J = 8.0 Hz), 7.29 (d, 1H, J = 7.6 Hz), 7.45 (d, 1H, J = 8.4 Hz). 13C NMR (100 MHz, DMSO-d6): δC = 150.9, 141.9, 136.5, 135.7, 129.0, 127.0, 125.2, 121.7, 121.2, 119.8, 119.4, 117.6, 109.1, 107.8, 74.6, 40.5, 30.6, 20.5, 15.0. HRMS (ESI+): calcd. For C21H19N3S: [M+Na+] 368.1198; found 368.1969.General Procedure for synthesis of thiopyrano derivatives 6
To a well-stirred suspension of the appropriate aromatic aldehyde 2 (1 equiv) and malononitrile 3 (1 equiv) in water (5 mL) appropriate 4-hydroxy-2H-thiochromene-2-thione 5 (1 equiv) was added and the reaction mixture was heated at 100 °C for 40 min. As the reaction progressed the reaction mixture becomes partly water soluble and a deep yellow oily layer appeared in the upper portion of water layer. After completion of the reaction as monitored by TLC the reaction mixture was cooled and diluted with 20 mL of water and extracted with ethyl acetate (3 × 25 mL) and dried over anhydrous Na2SO4. The solvent was distilled off. The resulting crude product was purified by filtration through a pad of silica gel (60–120 mesh) using 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether-ethyl acetate mixture as the eluent to give the pure compounds (6).
1 petroleum ether-ethyl acetate mixture as the eluent to give the pure compounds (6).
2-Amino-4-(4-methoxyphenyl)-5-oxo-4,5-dihydrothiopyrano[2,3-b]thiochromene-3-carbonitrile (6a)
Using the general procedure starting from 100 mg (0.515 mmol) of 4-hydroxy-2H-thiochromene-2-thione 5a, 70 mg (0.515 mmol) of 4-methoxybenzaldehyde 2a, 34 mg (0.515 mmol) of malononitrile 3, 173.4 mg of the title compound 6a was isolated as a grey colored solid, mp 224–226 °C, yield = 89%, IR (KBr): 3382, 3311, 3214, 2198, 1648, 1605, 1588 cm−1. 1H NMR (400 MHz, DMSO-d6): δH = 3.68 (s, 3H), 5.35 (s, 1H), 6.84 (d, 2H, J = 8.8 Hz), 7.17 (d, 2H, J = 8.4 Hz), 7.27 (s, 2H), 7.63 (t, 1H, J = 7.6 Hz), 7.75 (t, 1H, J = 7.2 Hz), 7.84 (d, 1H, J = 8.0 Hz), 8.34 (d, 1H, J = 8.0 Hz). 13C NMR (100 MHz, DMSO-d6): δC = 175.0, 158.3, 152.4, 142.1, 135.2, 133.0, 132.4, 130.5, 129.6, 128.8, 128.4, 127.7, 126.2, 119.2, 114.0, 73.0, 55.0, 40.5. MS: m/z = 400.82 [M+Na]+. Anal. Calcd. For C20H14N2O2S2: C, 63.47; H, 3.73; N, 7.40%. Found: C, 63.46; H, 3.71; N, 7.43%.2-Amino-5-oxo-4-p-tolyl-4,5-dihydrothiopyrano[2,3-b]thiochromene-3-carbonitrile (6b)
Using the general procedure starting from 100 mg (0.515 mmol) of 4-hydroxy-2H-thiochromene-2-thione 5a, 62 mg (0.515 mmol) of 4-methylbenzaldehyde 2b, 34 mg (0.515 mmol) of malononitrile 3, 164 mg of the title compound 6b was isolated as a grey colored solid, mp 258–260 °C, yield = 88%, IR (KBr): 3370, 3309, 3205, 2201, 1643, 1610, 1585 cm−1. 1H NMR (400 MHz, DMSO-d6): δH = 2.22 (s, 3H), 5.38 (s, 1H), 7.09 (d, 4H, J = 11.6 Hz), 7.27 (s, 2H), 7.65 (d, 1H, J = 7.6 Hz), 7.77 (t, 1H, J = 6.8 Hz), 7.87 (d, 1H, J = 7.6 Hz), 8.34 (d, 1H, J = 7.6 Hz). 13C NMR (100 MHz, DMSO-d6): δC = 175.0, 152.5, 142.4, 138.0, 136.3, 135.2, 132.4, 130.4, 129.6, 129.2, 128.8, 128.4, 126.5, 126.2, 119.2, 72.8, 40.9, 20.5. MS: m/z = 384.83 [M+Na]+. Anal. Calcd. For C20H14N2OS2: C, 66.27; H, 3.89; N, 7.73%. Found: C, 66.17; H, 3.91; N, 7.62%.2-Amino-4-(2-bromophenyl)-5-oxo-4,5-dihydrothiopyrano[2,3-b]thiochromene-3-carbonitrile (6c)
Using the general procedure starting from 100 mg (0.515 mmol) of 4-hydroxy-2H-thiochromene-2-thione 5a, 95.3 mg (0.515 mmol) of 2-bromobenzaldehyde 2i, 34 mg (0.515 mmol) of malononitrile 3, 182 mg of the title compound 6c was isolated as a grey colored solid, mp 226–228 °C, yield = 83%, IR (KBr): 3425, 3349, 3185, 2212, 1663, 1621, 1571 cm−1. 1H NMR (400 MHz, DMSO-d6): δH = 5.77 (s, 1H), 7.14 (t, 1H, J = 7.6 Hz), 7.19 (s, 2H), 7.24 (t, 1H, J = 7.6 Hz), 7.34 (d, 1H, J = 7.6 Hz), 7.61 (t, 2H, J = 8.0 Hz), 7.77 (t, 1H, J = 8.0 Hz), 7.90 (d, 1H, J = 8.0 Hz), 8.25 (d, 1H, J = 8.0 Hz). 13C NMR (100 MHz, DMSO-d6): δC = 160.6, 159.1, 142.0, 136.3, 133.1, 132.8, 131.4, 129.9, 129.0, 128.5, 128.1, 126.2, 123.0, 120.8, 115.7, 80.3, 72.4, 40.6. MS: m/z = 426 [M+], 428 [M++2]. Anal. Calcd. For C19H11BrN2OS2: C, 53.40; H, 2.59; N, 6.56%. Found: C, 53.39; H, 2.49; N, 6.71%.2-Amino-8-chloro-4-(4-methoxyphenyl)-5-oxo-4,5-dihydrothiopyrano[2,3-b]thiochromene-3-carbonitrile (6d)
Using the general procedure starting from 100 mg (0.437 mmol) of 7-chloro-4-hydroxy-2H-thiochromene-2-thione 5b, 59.5 mg (0.437 mmol) of 4-methoxybenzaldehyde 2a, 29 mg (0.437 mmol) of malononitrile 3, 151 mg of the title compound 6d was isolated as a grey colored solid, mp 210–212 °C, yield = 84%, IR (KBr): 3389, 3305, 3211, 2199, 1651, 1604, 1581 cm−1. 1H NMR (400 MHz, DMSO-d6): δH = 3.69 (s, 3H), 5.33 (s, 1H), 6.85 (d, 2H, J = 8.8 Hz), 7.17 (d, 2H, J = 8.4 Hz), 7.29 (s, 2H), 7.68 (dd, 1H, J = 8.8 Hz, 2.4 Hz), 8.14 (d, 1H, J = 2.0 Hz), 8.32 (d, 1H, J = 8.8 Hz). 13C NMR (100 MHz, DMSO-d6): δC = 174.4, 158.3, 152.2, 142.3, 137.5, 136.9, 132.8, 130.8, 130.6, 128.7, 128.4, 127.7, 125.5, 119.1, 114.0, 72.9, 55.0, 40.5. MS: m/z = 412 [M+]. Anal. Calcd. For C20H13ClN2O2S2: C, 58.18; H, 3.17; N, 6.78%. Found: C, 58.19; H, 3.23; N, 6.88%.General procedure for synthesis of thiopyrano derivatives 9
To a well-stirred suspension of the appropriate aromatic aldehyde 2 (1 equiv) and dimedone 8 (1 equiv) in water (5 mL) 4-hydroxy-2H-thiochromene-2-thione 5 (1 equiv) was added and the reaction mixture was heated at 100 °C for 45–60 min. As the reaction progressed the reaction mixture becomes partly water soluble and a deep yellow oily layer appeared in the upper portion of water layer. After completion of the reaction as monitored by TLC the reaction mixture was cooled and diluted with 20 mL of water and extracted with ethyl acetate (3 × 25 mL) and dried over anhydrous Na2SO4. The solvent was distilled off. The resulting crude product was purified by filtration through a pad of silica gel (60–120 mesh) using 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether-ethyl acetate mixture as the eluent to give the pure compounds (9).
1 petroleum ether-ethyl acetate mixture as the eluent to give the pure compounds (9).
3,3-Dimethyl-12-phenyl-3,4-dihydrothiochromeno[2,3-b]thiochromene-1,11(2H,12H)-dione (9a)
Using the general procedure starting from 100 mg (0.515 mmol) of 4-hydroxy-2H-thiochromene-2-thione 5a, 54.6 mg (0.515 mmol) of benzaldehyde 2e, 72.2 mg (0.515 mmol) of dimedone 8, 195.7 mg of the title compound 9a was isolated as a red colored solid, mp 134–136 °C, yield = 94%, IR (KBr): 1663, 1589 cm−1. 1H NMR (400 MHz, CDCl3): δH = 8.43 (d, 1H, J = 8.0 Hz), 7.52 (q, 1H, J = 7.2 Hz), 7.44 (t, 4H, J = 8.0 Hz), 7.19 (t, 2H, J = 7.6 Hz), 7.11 (t, 1H, J = 7.6 Hz), 6.25 (s, 1H), 2.66 (d, 1H, J = 17.6 Hz), 2.36–2.46 (m, 3H), 1.11 (s, 3H), 0.97 (s, 3H). 13C NMR (100 MHz, CDCl3): δC = 193.0, 176.1, 147.1, 142.4, 141.5, 135.5, 131.6, 131.1, 130.7, 129.9, 129.7, 128.4, 128.1, 128.0, 127.7, 126.9, 125.2, 51.5, 43.6, 37.0, 33.9, 29.5, 26.4. MS: m/z = 427.11 [M+Na]+. Anal. Calcd. For C24H20O2S2: C, 71.25; H, 4.98%. Found: C, 71.21; H, 5.05%.12-(Benzo[d][1,3]dioxol-5-yl)-3,3-dimethyl-3,4-dihydrothiochromeno[2,3-b]thiochromene-1,11(2H,12H)-dione (9b)
Using the general procedure starting from 100 mg (0.515 mmol) of 4-hydroxy-2H-thiochromene-2-thione 5a, 77.3 mg (0.515 mmol) of benzo[d][1,3]dioxole-5-carbaldehyde 2h, 72.2 mg (0.515 mmol) of dimedone 8, 205.5 mg of the title compound 9b was isolated as a red colored solid, mp 178–180 °C, yield = 89%, IR : 1662, 1625, 1615, 1589 cm−1. 1H NMR (400 MHz, CDCl3): δH = 8.44 (d, 1H, J = 7.6 Hz), 7.44–7.56 (m, 3H), 6.98 (s, 1H), 6.94 (d, 1H, J = 8.0 Hz), 6.63 (d, 1H, J = 8.0 Hz), 6.14 (s, 1H), 5.83 (d, 2H, J = 2.4 Hz), 2.65 (d, 1H, J = 17.2 Hz), 2.40 (d, 3H, J = 18.4 Hz), 1.11 (s, 3H), 0.99 (s, 3H). 13C NMR (100 MHz, CDCl3): δC = 193.0, 176.0, 147.5, 146.7, 146.4, 142.3, 135.5, 131.6, 131.1, 130.7, 129.9, 129.7, 127.7, 125.2, 122.6, 121.3, 108.8, 108.1, 100.8, 51.5, 43.5, 36.6, 33.9, 29.5, 26.5. MS: m/z = 470.97 [M+Na]+, 472.97 [M+Na+2]+ Anal. Calcd. For C25H20O4S2: C, 66.94; H, 4.49%. Found: 66.91; H, 4.54%.12-(2-Bromophenyl)-3,3-dimethyl-3,4-dihydrothiochromeno[2,3-b]thiochromene-1,11(2H,12H)-dione (9c)
Using the general procedure starting from 100 mg (0.515 mmol) of 4-hydroxy-2H-thiochromene-2-thione 5a, 95.3 mg (0.515 mmol) of 2-bromobenzaldehyde 2i, 72.2 mg (0.515 mmol) of dimedone 8, 216.2 mg of the title compound 9c was isolated as a red colored solid, mp 220–222 °C, yield = 87%, IR: 1664, 1587 cm−1. 1H NMR (400 MHz, CDCl3): δH = 8.27 (d, 1H, J = 8.0 Hz), 7.69 (d, 1H, J = 7.6 Hz), 7.49–7.58 (m, 2H), 7.39 (t, 2H, J = 6.8 Hz), 7.21 (t, 1H, J = 7.6 Hz), 6.99 (t, 1H, J = 7.2 Hz), 5.84 (s, 1H), 2.68 (d, 2H, J = 8.0 Hz), 2.27 (d, 2H, J = 6.0 Hz), 1.16 (s, 3H), 1.07 (s, 3H). 13C NMR (100 MHz, CDCl3): δC = 204.4, 195.8, 161.5, 151.8, 141.1, 138.9, 136.7, 133.8, 130.5, 128.4, 127.4, 126.3, 126.1, 123.4, 123.3, 122.5, 113.1, 50.8, 40.6, 38.0, 32.3, 29.2, 27.6. MS: m/z = 505.08 [M+Na]+. Anal. Calcd. For C24H19BrO2S2: C, 59.63; H, 3.96%. Found: C, 59.64; H, 3.89%.12-(5-Chloro-2-methoxyphenyl)-3,3-dimethyl-3,4-dihydrothiochromeno[2,3-b]thiochromene-1,11(2H,12H)-dione (9d)
Using the general procedure starting from 100 mg (0.515 mmol) of 4-hydroxy-2H-thiochromene-2-thione 5a, 87.8 mg (0.515 mmol) of 5-chloro-2-methoxybenzaldehyde 2j, 72.2 mg (0.515 mmol) of dimedone 8, 207.5 mg of the title compound 9d was isolated as a red colored solid, mp 218–220 °C, yield = 86%, IR (KBr): 1662, 1620, 1588 cm−1. 1H NMR (400 MHz, CDCl3): δH = 8.42 (d, 1H, J = 8.4 Hz), 7.44–7.56 (m, 4H), 7.06 (d, 1H, J = 8.4 Hz), 6.71 (d, 1H, J = 8.8 Hz), 6.18 (s, 1H), 3.77 (s, 3H), 2.59 (d, 1H, J = 17.2 Hz), 2.35 (d, 3H, J = 18.0 Hz), 1.10 (s, 3H), 0.95 (s, 3H). MS: m/z = 491.01 [M+Na]+. Anal. Calcd. For C25H21ClO3S2: C, 64.02; H, 4.51%. Found: C, 64.07; H, 4.50%.12-(3,4-Dimethoxyphenyl)-3,3-dimethyl-3,4-dihydrothiochromeno[2,3-b]thiochromene-1,11(2H,12H)-dione (9e)
Using the general procedure starting from 100 mg (0.515 mmol) of 4-hydroxy-2H-thiochromene-2-thione 5a, 85.5 mg (0.515 mmol) of 3,4-dimethoxybenzaldehyde 2k, 72.2 mg (0.515 mmol) of dimedone 8, 222.4 mg of the title compound 9e was isolated as a red colored solid, mp 140–142 °C, yield = 93%, IR: 1660, 1615, 1589 cm−1. 1H NMR (400 MHz, CDCl3): δH = 8.45 (d, 1H, J = 7.6 Hz), 7.44–7.56 (m, 3H), 7.12 (s, 1H), 6.93 (d, 1H, J = 8.0 Hz), 6.67 (d, 1H, J = 8.0 Hz), 6.19 (s, 1H), 3.83 (s, 3H), 3.77 (s, 3H), 2.67 (d, 1H, J = 17.6 Hz), 2.39 (d, 3H, J = 16.4 Hz), 1.12 (s, 3H), 0.99 (s, 3H). 13C NMR (100 MHz, CDCl3): δC = 193.1, 176.1, 148.7, 147.9, 146.8, 142.1, 135.5, 134.1, 131.6, 131.2, 130.8, 130.1, 129.7, 127.7, 125.2, 119.6, 111.8, 110.8, 55.8, 55.7, 51.5, 43.6, 36.5, 33.8, 29.6, 26.3. MS: m/z = 503.04 [M+K]+. Anal. Calcd. For C26H24O4S2: C, 67.21; H, 5.21%. Found: C, 67.11; H, 5.22%.12-(4-Fluorophenyl)-3,3-dimethyl-3,4-dihydrothiochromeno[2,3-b]thiochromene-1,11(2H,12H)-dione (9f)
Using the general procedure starting from 100 mg (0.515 mmol) of 4-hydroxy-2H-thiochromene-2-thione 5a, 63.9 mg (0.515 mmol) of 4-fluorobenzaldehyde 2l, 72.2 mg (0.515 mmol) of dimedone 8, 198 mg of the title compound 9f was isolated as a red colored solid, mp 182–184 °C, yield = 91%, IR: 1662, 1587 cm−1. 1H NMR (400 MHz, CDCl3): δH = 8.44 (d, 1H, J = 7.6 Hz), 7.44–7.57 (m, 5H), 6.87 (t, 2H, J = 8.0 Hz), 6.19 (s, 1H), 2.67 (d, 1H, J = 17.6 Hz), 2.40 (d, 3H, J = 17.6 Hz), 1.12 (s, 3H), 0.96 (s, 3H). 13C NMR (100 MHz, CDCl3): δC = 193.0, 176.0, 163.0, 160.6, 146.9, 142.5, 137.5, 135.5, 131.6, 131.0, 130.7, 129.8, 129.7, 129.6, 127.8, 125.2, 115.2, 115.0, 51.4, 43.6, 36.4, 33.9, 29.5, 26.3. MS: m/z = 461.03 [M+K]+. Anal. Calcd. For C24H19FO2S2: C, 68.22; H, 4.53%. Found: C, 68.26; H, 4.50%.Acknowledgements
We thank CSIR (New Delhi) and DST (New Delhi) for financial assistance. Two of us (S.P. & T.G.) are grateful to CSIR (New Delhi) for their Research Fellowships. We also thank the DST (New Delhi) for providing Bruker NMR spectrometer (400 MHz) and Perkin–Elmer CHN analyzer, UV-VIS spectrometer and Perkin-Elmer FT -IR under its FIST programme.References
- (a) U. M. Lindstrom, Organic Reactions in Water, Blackwell, Oxford, 2007 Search PubMed; (b) C. J. Li, Chem. Rev., 1993, 93, 2023 CrossRef CAS; (c) C. J. Li, Chem. Rev., 2005, 105, 3095 CrossRef CAS; (d) A. Chanda and V. V. Fokin, Chem. Rev., 2009, 109, 725 CrossRef CAS; (e) H. C. Hailes, Org. Process Res. Dev., 2007, 11, 114 CrossRef CAS.
- (a) A. Domling, Chem. Rev., 2006, 106, 17 CrossRef; (b) R. V. A. Orru and M. Degreef, Synthesis, 2003, 1471 CrossRef CAS; (c) C. Hulme and V. Gore, Curr. Med. Chem., 2003, 10, 51 CrossRef CAS; (d) C. Montagne, J. J. Shiers and M. Shipman, Tetrahedron Lett., 2006, 47, 9207 CrossRef CAS.
- (a) S. L. Schreiber, Science, 2000, 287, 1964 CrossRef CAS; (b) J.-P. Zhu, H. Bienayme, Multicomponent Reactions; Wiley-VCH: Weinheim, 2005, 1499 Search PubMed; (c) B. M. Trost, Angew. Chem., Int. Ed. Engl., 1995, 34, 259 CrossRef CAS; (d) L. F. Tietze, Chem. Rev., 1996, 96, 115 CrossRef CAS.
- (a) R. V. A. Orru and M. de Greef, Synthesis, 2003, 1471 CrossRef CAS; (b) G. Balme, E. Bossharth and N. Monteiro, Eur. J. Org. Chem., 2003, 4101 CrossRef CAS; (c) S. Bräse, C. Gil and K. Knepper, Bioorg. Med. Chem., 2002, 10, 2415 CrossRef; (d) A. Dömling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168 CrossRef.
- (a) A. Dömling, Chem. Rev., 2006, 106, 17 CrossRef; (b) D. J. Ramón and M. Yus, Angew. Chem., Int. Ed., 2005, 44, 1602 CrossRef; (c) L. F. Tietze, G. Brasche, K. M. Gericke, Domino Reaction in Organic Synthesis; Wiley-VCH: Weinheim, 2006, 542 Search PubMed; (d) J. D. Sunderhaus, C. Dockendorff and S. F. Martin, Org. Lett., 2007, 9, 4223 CrossRef CAS.
- (a) A. H. Ingall, In Comprehensive Heterocyclic Chemistry II; Boulton, A. S., McKkillop, A., ed.; Pergamon Press: Oxford, 1996, 5, 501 Search PubMed; (b) S. W. Schneller, Adv. Heterocycl. Chem., 1975, 18, 59 CrossRef CAS; (c) A. R. Katrizky and A. Bonlton, J. Adv. Heterocycl. Chem., 1975, 18, 76 Search PubMed.
- D. J. Jr. Rogier, J. S. Carter and J. J. Talley, WO 2001049675, 2001 Search PubMed.
- M. J. Brown, P. S. Carter, A. E. Fenwick, A. P. Fosberry, D. W. Hamprecht, M. J. Hibbs, R. L. Jarvest, L. Mensah, P. H. Milner, P. J. O'Hanlon, A. J. Pope, C. M. Richardson, A. West and D. R. Witty, Bioorg. Med. Chem. Lett., 2002, 12, 3171 CrossRef CAS.
- W. Quaglia, M. Pigini, A. Piergentili, M. Giannella, F. Gentili, G. Marucci, A. Carrieri, A. Carotti, E. Poggesi, A. Leonardi and C. Melchiorre, J. Med. Chem., 2002, 45, 1633 CrossRef CAS.
- L. A. Vanvliet, N. Rodenhuis, D. Dijkstra, H. Wikstrom, T. A. Pugsley, K. A. Serpa, L. T. Meltzer, T. G. Heffner, L. D. Wise, M. E. Lajiness, R. M. Huff, K. Svensson, S. Sundell and M. Lundmark, J. Med. Chem., 2000, 43, 2871 CrossRef CAS.
- (a) K. D. Berlin, D. M. Benbrook, E. C. Nelson, U.S. Patent, 2003, 6586460 Search PubMed; (b) Y. Sugita, H. Hosoya, K. Terasawa, I. Yokoe, S. Fujisawa and H. Sakagami, Anticancer Res., 2001, 21, 2629 CAS.
- (a) R. F. Kaltenbach, S. P. Robinson, G. L. Trainor, U.S. Patent Search PubMed2005/0267183 A1, December 1, 2005; (b) X. Zhang, Z. Sui, U.S. Patent Search PubMed2006/0020018 A1, January 26, 2006.
- A. Attila Sipos, M. Toth, F. K. U. Mueller, J. Lehmann and S. Berenyi, Monatsh. Chem., 2009, 140, 473 CrossRef.
- (a) J. E. Saxton, Indoles; Wiley-Interscience: New York, 1983 Search PubMed; (b) R. J. Sundberg, The Chemistry of Indoles; Academic Press: New York, 1970 Search PubMed; (c) J. S. Carle and C. Christophersen, J. Org. Chem., 1980, 45, 1586 CrossRef CAS; (d) N. Sakabe, Y. Sendo, I. Iijima and Y. Ban, Tetrahedron Lett., 1969, 10, 2527 CrossRef; (e) T. Ohmoto and K. Koike, Chem. Pharm. Bull., 1984, 32, 170 CrossRef CAS; (f) M. Shimizu, M. Ishikawa, Y. Komoda, T. Nkajima, K. Yamaguchi and N. Yoneda, Chem. Pharm. Bull., 1984, 32, 463 CrossRef CAS; (g) E. Yamanaka, M. One, S. Kasamatsu, N. Aimi and S. Sakai, Chem. Pharm. Bull., 1984, 32, 818 CrossRef CAS; (h) M. Taniguchi, T. Anjiki, M. Nakagawa and T. Hino, Chem. Pharm. Bull., 1984, 32, 2544 CrossRef CAS; (i) Y. Hashimoto, K. Shudo and T. Okamoto, Chem. Pharm. Bull., 1984, 32, 4300 CrossRef CAS; (j) Y. Nakashima, Y. Kawashima, F. Amanuma, K. Sota, A. Tanaka and T. Kameyama, Chem. Pharm. Bull., 1984, 32, 4271 CrossRef CAS; (k) S. Kamijo and Y. Yamamoto, J. Org. Chem., 2003, 68, 4764 CrossRef CAS.
- (a) S. Takada and Y. Makisumi, Chem. Pharm. Bull., 1984, 32, 872 CrossRef CAS; (b) S. Takada, N. Ishizuka, T. Sasatani, Y. Makisumi, H. Jyoyama, H. Hatakeyama, F. Asanuma and K. Hirose, Chem. Pharm. Bull., 1984, 32, 877 CrossRef CAS.
- Y. Makisumi, T. Sasatani, United States Patent Search PubMedNo. 4910318, March 20, 1990.
- (a) K. C. Majumdar, S. Ponra, D. Ghosh and A. Taher, Synlett, 2011, 104 CrossRef CAS; (b) K. C. Majumdar, S. Ponra and D. Ghosh, Synthesis, 2011, 1132 CrossRef CAS.
- A. Kumar and R. A. Maurya, Tetrahedron, 2007, 63, 1946 CrossRef CAS.
- (a) T. Katoaka, A. Tomoto, H. Shimizu and M. Hori, J. Chem. Soc., Perkin Trans. 1, 1983, 2913 RSC; (b) C. Banzatti, P. Mellini and P. Salvadori, Gazz. Chim. Ital., 1987, 117, 259 CAS; (c) P. T. Kaye, S. Afr. J. Sci., 2004, 100, 545 CAS.
- (a) E. Rasolofonjatovo, B. Treguier, O. Provot, A. Hamze, E. Morvan, J. –D. Brion and M. Alami, Tetrahedron Lett., 2011, 52, 1036 CrossRef CAS; (b) R. S. Kenny, U. C. Mashelkar, D. M. Rane and D. K. Bezawada, Tetrahedron, 2006, 62, 9280 CrossRef CAS.
- W. Wang, H. Li, J. Wang and L. Zu, J. Am. Chem. Soc., 2006, 128, 10354 CrossRef CAS.
- (a) B. I. Usachev, M. A. Shafeev and V. Y. Sosnovskikh, Russ. Chem. Bull., 2006, 55, 504 CrossRef; (b) B. I. Usachev, V. Y. Sosnovskikh, M. A. Shafeev and G. -V. Röschenthaler, Phosphorus, Sulfur Silicon Relat. Elem., 2005, 180, 1315 CrossRef CAS.
- R. Rios, H. Sunden, I. Ibrahem, G. -L. Zhao, L. Eriksson and A. Cordova, Tetrahedron Lett., 2006, 47, 8547 CrossRef CAS.
- K. C. Majumdar, U. K. Kundu and S. K. Ghosh, Org. Lett., 2002, 4, 2629 CrossRef CAS.
- K. C. Majumdar and S. Muhuri, Synthesis, 2006, 2725 CrossRef CAS.
- S. Chowdhury, G. C. Nandi, S. Samai and M. S. Singh, Org. Lett., 2011, 13, 3762 CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c1ra00655j/ |
| This journal is © The Royal Society of Chemistry 2012 |