Multi-amine-functionalized cubic Fd![[3 with combining macron]](https://www.rsc.org/images/entities/h2_char_0033_0304.gif) m mesoporous silica by an anionic surfactant templating route
m mesoporous silica by an anionic surfactant templating route
Shao-Xin
Deng
,
Xin-Lei
Zhang
,
Jin-Gui
Wang
,
Hui-Jing
Zhou
,
Ping-Chuan
Sun
and
Tie-Hong
Chen
*
Institute of New Catalytic Materials Science, Key Laboratory of Advanced Energy Materials Chemistry (MOE), College of Chemistry, Nankai University, Tianjin, 300071, PR China. E-mail: chenth@nankai.edu.cn
First published on 24th November 2011
Abstract
Ordered multi-amine functionalized mesoporous silica with a 3D cubic Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m mesostructure was synthesized with anionic surfactant N-lauroylsarcosine sodium (Sar-Na) as a template, and [3-(2-aminoethyl)-aminopropyl]trimethoxysilane (DAPS) or 3-[2-(2-aminoethylamino)ethylamino]propyl-trimethoxysilane (TAPS) as co-structure directing agents (CSDAs). To our knowledge, this is the first time the use of DAPS and TAPS as CSDAs in an anionic surfactant templated system have been reported. Using DAPS and TAPS as the CSDA favors the formation of a 3D cubic mesocaged solid with Fd
m mesostructure was synthesized with anionic surfactant N-lauroylsarcosine sodium (Sar-Na) as a template, and [3-(2-aminoethyl)-aminopropyl]trimethoxysilane (DAPS) or 3-[2-(2-aminoethylamino)ethylamino]propyl-trimethoxysilane (TAPS) as co-structure directing agents (CSDAs). To our knowledge, this is the first time the use of DAPS and TAPS as CSDAs in an anionic surfactant templated system have been reported. Using DAPS and TAPS as the CSDA favors the formation of a 3D cubic mesocaged solid with Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m symmetry, while with 3-aminopropyltriethoxysilane (APS) as the CSDA, only the p6mm mesoporous phase was formed. The geometrical change of the CSDA resulted in a change of the surfactant packing parameter, leading to the change of the silica mesophase from 2D-hexagonal p6mm to 3D cubic Fd
m symmetry, while with 3-aminopropyltriethoxysilane (APS) as the CSDA, only the p6mm mesoporous phase was formed. The geometrical change of the CSDA resulted in a change of the surfactant packing parameter, leading to the change of the silica mesophase from 2D-hexagonal p6mm to 3D cubic Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m symmetry. After extraction of the templates, multi-amine-functionalized cubic Fd
m symmetry. After extraction of the templates, multi-amine-functionalized cubic Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m mesoporous silicas were obtained. The multi-amine functionlized mesoporous silicas exhibit excellent properties in adsorption and removal of heavy metal ions in solution as well as the support of noble metal nanoparticles.
m mesoporous silicas were obtained. The multi-amine functionlized mesoporous silicas exhibit excellent properties in adsorption and removal of heavy metal ions in solution as well as the support of noble metal nanoparticles.
Introduction
Since the discovery of mesoporous silicates in the early 1990’s,1–3 various kinds of mesoporous materials with different mesophases have been developed,4,5 such as cylindrical structures, bicontinuous Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d symmetries,6,7 body centered cubic Im
d symmetries,6,7 body centered cubic Im![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m8 and cage type structures with Pm
m8 and cage type structures with Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) n symmetry.9 Ordered 3D cubic mesoporous silicas are highly interesting and would be more advantageous than those with uni-dimensional mesopores, due to the lower resistance to pore blocking and easier diffusion of the molecules in the pore channels.
n symmetry.9 Ordered 3D cubic mesoporous silicas are highly interesting and would be more advantageous than those with uni-dimensional mesopores, due to the lower resistance to pore blocking and easier diffusion of the molecules in the pore channels.
Surface functionalization of mesoporous silica is helpful for applications in catalysis, adsorption and removal of heavy metals, separation, adsorption of CO2 and supports for metal nanoparticles.10–16 Co-condensation and post-synthesis methods are general methods17,18 to introduce functional groups into the mesoporous matrix. Materials synthesized by the co-condensation method often exhibit less ordered mesostructure and lower stability. The post-grafted functional groups normally have a limited loading amount in the mesopores and the distribution of functional groups was not uniform. In 2003, Che and Tatsumi19 firstly reported the synthesis of highly ordered anionic surfactant templated mesoporous silica materials with an anionic surfactant and co-structure directing agent (CSDA) through a new S−N+∼I− pathway, where S stands for the anionic surfactant, N for the cationic amino group of CSDA, and I for the inorganic species. In this pathway, aminosilane (e.g., 3-aminopropyltrimethoxysilane) and quaternized aminosilane (e.g., N-trimethoxylsilylpropyl-N,N,N-tributylammonium) were used as the CSDAs. Due to the interaction between the surfactant and the CSDA, an important characteristic of the mesostructured silicates, synthesized by the co-structure-directing route, is that after extraction of the surfactant, the functional groups from the CSDA have a relatively high loading amount on the pore surface and are homogeneously distributed within the mesoporous channels.
The co-structure-directing route to synthesize mesoporous silicas could be well controlled by the degree of ionization of the carboxylate surfactants and the charge density of positively charged amino groups of the CSDA.20 The effect of the ionization degree of the anionic surfactant has been widely studied.20–22 In previous report, typical CSDAs include 3-aminopropyltrimethoxylsilane (APMS), 3-aminopropyltriethoxylsilane (APS) and N-trimethoxysilylpropyl-N,N,N-trimethylammonium chloride (TMAPS). APS was also used to tune the mesophase of silica with a cationic surfactant as the template.23 However, the preparation of mesoporous silica using di-aminosilane and tri-aminosilane as CSDA has not been reported.
Herein, we report the synthesis of multi-amine-functionalized cubic Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m mesoporous silica by using anionic surfactant N-lauroylsarcosine sodium (Sar-Na) as the template, [3-(2-aminoethyl)aminopropyl]trimethoxysilane (DAPS) or 3-[2-(2-aminoethylamino)-ethylamino]propyltrimethoxysilane (TAPS) as the CSDA. To our knowledge, this is the first time the tuning of the mesophase by using DAPS and TAPS as the CSDA in the anionic surfactant templated system has been reported. After extraction of the anionic surfactant template by ethanol solution with ethanolamine, multi-amine-functionalized 3D cubic Fd
m mesoporous silica by using anionic surfactant N-lauroylsarcosine sodium (Sar-Na) as the template, [3-(2-aminoethyl)aminopropyl]trimethoxysilane (DAPS) or 3-[2-(2-aminoethylamino)-ethylamino]propyltrimethoxysilane (TAPS) as the CSDA. To our knowledge, this is the first time the tuning of the mesophase by using DAPS and TAPS as the CSDA in the anionic surfactant templated system has been reported. After extraction of the anionic surfactant template by ethanol solution with ethanolamine, multi-amine-functionalized 3D cubic Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m mesoporous silica was obtained. The amino functionalized silica has been considered as an excellent candidate for the adsorption and removal heavy metal cations in solution and as support to immobilize noble metal nanoparticles. In this paper, we used multi-amine-functionalized mesoporous silica as the support to prepare the catalyst and tested the catalytic activity by reduction of 4-nitrophenol.
m mesoporous silica was obtained. The amino functionalized silica has been considered as an excellent candidate for the adsorption and removal heavy metal cations in solution and as support to immobilize noble metal nanoparticles. In this paper, we used multi-amine-functionalized mesoporous silica as the support to prepare the catalyst and tested the catalytic activity by reduction of 4-nitrophenol.
Experimental
Chemicals and materials
N-Lauroylsarcosine sodium (Sar-Na) was purchased from Merck, [3-(2-aminoethyl)aminopropyl]trimethoxysilane (DAPS) was from Aladdin, 3-aminopropyltriethoxysilane (APS) and 3-[2-(2-aminoethylamino)ethylamino]propyl-trimethoxysilane (TAPS) were from Acros. All the chemical agents were used without further purification.Synthesis of mesoporous silica with cubic Fd![[3 with combining macron]](https://www.rsc.org/images/entities/h3_char_0033_0304.gif) m phase
m phase
In a typical synthesis, 0.58 g of Sar-Na was completely dissolved in 60.0 mL deionized water under stirring at room temperature. Then 8.0 mL of HCl (0.1 M) was added to the surfactant solution under vigorous stirring. The solution was stirring for 0.5 h at room temperature. Next, 1.319 g 20wt% DAPS/ethanol (or TAPS/ethanol) was added to the above solution under vigorous stirring. After further stirring for 10 min, 3.0 g of tetraethylsiloxane (TEOS) was added to the above solution. The solution was stirred at room temperature for another 2 h, and then was transferred to an reaction tube, which was placed in an oven at 80 °C for 24 h. The final product was filtered, washed with deionized water, and dried at 60 °C. To prepare the pure mesoporous silica without the surfactant and aminopropyl moieties, the as-synthesized samples were calcined at 550 °C for 6 h. To obtain the amino-functionalized mesoporous silicas, the templates were removed by a Soxhlet extraction in 100 mL of ethanol solution with 20 mL of ethanoamine for 12 h, and the above extraction procedure was repeated 4 times. Then the samples were washed with ethanol and dried at 60 °C. The samples synthesized with DAPS and TAPS as CSDA were denoted as D-AMS and T-AMS, respectively.
Adsorption of metal ions
Amino-functionalized mesoporous silica (AMS-ex or D-AMS-ex or T-AMS-ex) (10 mg) was added to 50 mL, 200 ppm Pb(NO3)2 solution and the solution pH value was adjusted to 8.5. Then the solution was stirred for 2 h at room temperature. Finally, the sample was obtained by centrifugation, washed with deionized water and dried at 60 °C. The adsorption amount of metal ions was investigated by atomic adsorption spectroscopy (AAS).Preparation of D-AMS-Pd catalyst and catalytical reaction
D-AMS-ex (1 g) was suspended in 9 mL (0.0485 M) H2PdCl4 solution and stirred for 2 h at room temperature. Finally, the catalyst was obtained by filtration, washed with deionized water and dried at 60 °C. The sample was reduced by a NaBH4 aqueous solution. The catalytic activity of the D-AMS-Pd was tested by catalytic reduction of 4-nitrophenol. In a typical reaction, 0.25 mL, 0.01 M 4-nitrophenol aqueous solution and 5 mL, 0.1 M NaBH4 aqueous solution were added to 40 mL deionized water in a colorimetric tube under magnetic stirring. After adding 10 mg D-AMS-Pd catalyst particles, the bright yellow solution gradually faded as the reaction proceeded. The reaction progress can be monitored by taking a small portion of the reaction mixture at a regular time interval and the kinetics of 4-nitrophenol reduction was studied by UV-vis spectroscopy.Material characterization
X-Ray diffraction (XRD) patterns were obtained on a Rigaku D/max-2500 diffractometer, with Cu Kα radiation (λ = 1.5418 Å) at 40 kV and 100 mA. The XRD patterns were collected in the range 0.6–6 degree in 2θ/θ scanning mode with a 0.02 degree step and scanning speed of 1 degree/min. Small-angle X-ray scattering (SAXS) experiments were performed on a Bruker Nanostar small-angle X-ray scattering system. TEM observations were performed on a Philips Tecnai F20 microscope, operating at 200 kV. All samples subjected to TEM measurements were dispersed in ethanol ultrasonically and were dropped on copper grids. SEM images were obtained with a Shimadzu Model SS-550 instrument operating at 15 kV. Nitrogen adsorption–desorption isotherms were measured on a BELSORP-mini II sorption analyzer at 77 K. Before measurements, the samples were dried under dry N2 flow at 350 °C for 5 h for calcined samples and at 150 °C for 5 h for extracted samples. The specific surface areas were calculated using the BET (Brunauer–Emmett–Teller) method. The total pore volumes were obtained at a relative pressure p/p0 of 0.99. The pore-size distribution was calculated from the adsorption branch using the BJH (Barett–Joyner–Halenda) method. Diffuse reflectance UV-vis spectra were measured on a Shimadzu Model 2450 spectrometer. The Fourier transform infrared (FTIR) transmission spectra were recorded using Bio-Rad FTS 6000 spectrometers. The elemental analysis was performed on an Elementar Vario-EL instrument.Results and discussion
Fig. 1 (A) shows a powder XRD pattern of the calcined mesoporous silica prepared with Sar-Na as the template and 3-aminopropyltrimethoxysilane (APS) as the CSDA. The three mesoporous diffraction peaks of the XRD pattern can be well indexed to (100), (110) and (200) reflections of the hexagonal p6mm structure. That is to say, with Sar–Na as the template, APS as the CSDA leads to the formation of a 2D-hexagonal mesophase. The result of the experiment is in accordance with our previous experimental results.24 | ||
| Fig. 1 (A) XRD pattern of calcined mesoporous silica synthesized with APS as the CSDA. (B) XRD patterns of (a) solvent extracted D-AMS, (b) calcined D-AMS, (c) solvent extracted T-AMS and (d) calcined T-AMS. | ||
Interestingly, with the replacement of APS by DAPS and TAPS, cubic Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m mesophases were obtained. As shown in Fig. 1 (B), both of the calcined and template-extracted D-AMS and T-AMS displayed two well-resolved diffraction peaks, which were indexed to the (220) and (311) characteristic diffractions of the cubic Fd
m mesophases were obtained. As shown in Fig. 1 (B), both of the calcined and template-extracted D-AMS and T-AMS displayed two well-resolved diffraction peaks, which were indexed to the (220) and (311) characteristic diffractions of the cubic Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m mesostructure (AMS-8 type). That is to say, in this experimental system, under the same conditions, the 2D-hexagonal mesophase and the cubic Fd
m mesostructure (AMS-8 type). That is to say, in this experimental system, under the same conditions, the 2D-hexagonal mesophase and the cubic Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m mesophase were obtained when APS and DAPS (or TAPS) were used as the CSDA, respectively. Based on the results, it is known that the mesostructures of the AMS materials were tuned by the type of CSDA. According to the previous report, the introduction of the CSDA makes it possible to provide adequate electrostatic interactions between the inorganic species and anionic surfactant head groups, and thus the CSDA is the vital factor for the successful synthesis of AMS mesoporous silicas.25 The effect of the CSDA in these experimental conditions was examined and explained that due to the size of DAPS or TAPS being larger than the size of APS, the steric effect would increase the effective interfacial area of the head group of the surfactant, and then the packing parameter (g) would be reduced and therefore a cage type cubic mesophase was favored.
m mesophase were obtained when APS and DAPS (or TAPS) were used as the CSDA, respectively. Based on the results, it is known that the mesostructures of the AMS materials were tuned by the type of CSDA. According to the previous report, the introduction of the CSDA makes it possible to provide adequate electrostatic interactions between the inorganic species and anionic surfactant head groups, and thus the CSDA is the vital factor for the successful synthesis of AMS mesoporous silicas.25 The effect of the CSDA in these experimental conditions was examined and explained that due to the size of DAPS or TAPS being larger than the size of APS, the steric effect would increase the effective interfacial area of the head group of the surfactant, and then the packing parameter (g) would be reduced and therefore a cage type cubic mesophase was favored.
The morphologies of the D-AMS and T-AMS were characterized by SEM. As shown in Fig. 2a and 2c, the D-AMS consisted of submicron particles as shown in the SEM image, and the morphology of T-AMS was larger irregular blocks. To further identify the mesopore arrangement, TEM observation was performed. The TEM images confirmed that the Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m mesostructure was successfully synthesized when DAPS and TAPS were used as the CSDA.
m mesostructure was successfully synthesized when DAPS and TAPS were used as the CSDA.
![SEM and TEM images of D-AMS (a, b) and T-AMS (c, d). The TEM image in (b) was taken along the [110] direction and the image in (d) was taken along the [211] direction. Insets in (b) and (d) are Fourier transform diffractograms of the central area of the projected TEM images.](/image/article/2012/RA/c1ra00363a/c1ra00363a-f2.gif) | ||
| Fig. 2 SEM and TEM images of D-AMS (a, b) and T-AMS (c, d). The TEM image in (b) was taken along the [110] direction and the image in (d) was taken along the [211] direction. Insets in (b) and (d) are Fourier transform diffractograms of the central area of the projected TEM images. | ||
Nitrogen adsorption–desorption isotherms and pore size distribution curves of the extracted and calcined D-AMS and T-AMS samples are shown in Fig. 3. As shown in Fig. 3(A), all the curves are typical type-IV isotherms with a sharp capillary condensation step at relative pressure (p/p0) between 0.4 and 0.6, which may indicate that the samples are typical mesoporous materials. The pore size distribution curves (Fig. 3(B)) of the four samples were determined from the adsorption branch of the adsorption–desorption isotherms. All the pore size distribution curves exhibited sharp peaks, indicating uniform pore channels.
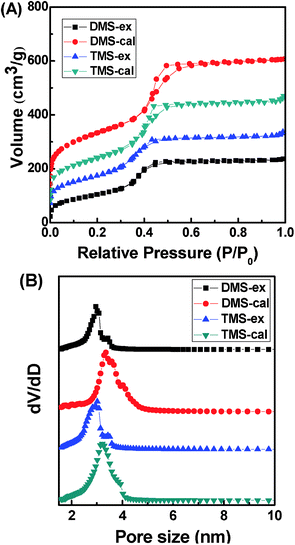 | ||
| Fig. 3 (A) Nitrogen adsorption–desorption isotherms and (B) the pore size distribution of the solvent extracted and calcined samples of D-AMS and T-AMS. | ||
The specific surface area, pore volume, pore diameters and a0 of the materials are summarized in Table 1. For the solvent-extracted samples, because of the presence of the surface functionalized amino groups in the mesochannels, the pore size and the surface areas were smaller than that of the calcined samples. Nonetheless, D-AMS-ex and T-AMS-ex possess a relatively high specific surface area, 384 and 462 m2 g−1, respectively.
| Sample | Meso-structure | a0 (nm)a | Surface area (m2 g−1)b | Pore diameter (nm)c | Pore volume (cm3 g−1)d |
|---|---|---|---|---|---|
| a The unit cell parameters (a0) were calculated from the XRD patterns, a0 = d220*(h2 + k2 +l2)1/2 for 3D cubic phase and a0 = d100*(4/3)1/2 for 2D hexagonal phase. b The specific surface areas were calculated by using the Brunauer–Emmett–Teller (BET) method. c The pore size was obtained from the pore size distribution curves calculated by the Barett–Joyner–Halenda (BJH) method using the adsorption branch of the isotherm. d The total pore volume was calculated from the amount of adsorbed N2 at P/P0 = 0.99. | |||||
| AMS-ex | p6mm | 6.4 | 321 | 2.8 | 0.45 |
| D-AMS-ex |
Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
17.0 | 384 | 3.0 | 0.40 |
| D-AMS-cal |
Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
16.1 | 838 | 3.4 | 0.79 |
| T-AMS-ex |
Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
17.0 | 462 | 3.2 | 0.49 |
| T-AMS-cal |
Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
16.1 | 645 | 3.5 | 0.62 |
In order to investigate the effect the of amount of acid to the mesostructure when DAPS and TAPS were used as the CSDA, the samples were synthesized by using different amounts of HCl. With DAPS as the CSDA, it was found that a mesophase transformation from the cage type cubic Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m to the 2D-hexagonal P6mm mesostructure occurred when the amount of acid was increased. As shown in Fig. 4(A), when the amount of HCl was 8 ml (a), there is only two peaks, one phase, which belongs to (220) and (311) of 3D cubic Fd
m to the 2D-hexagonal P6mm mesostructure occurred when the amount of acid was increased. As shown in Fig. 4(A), when the amount of HCl was 8 ml (a), there is only two peaks, one phase, which belongs to (220) and (311) of 3D cubic Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m mesostructure. With the increase of the amount of HCl to 12 ml (b), three peaks in the range 2θ = 1.3–3° started to appear at the expense of the cubic phase peaks. The three peaks of the sample were indexed to a set of cubic Fd
m mesostructure. With the increase of the amount of HCl to 12 ml (b), three peaks in the range 2θ = 1.3–3° started to appear at the expense of the cubic phase peaks. The three peaks of the sample were indexed to a set of cubic Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase peaks (220), (311), and a 2D-hexagonal phase peak (100). It might be estimated that the sample consisted of two types of mesophases: 2D-hexagonal phase and cage type cubic phase. As the amount of HCl was further increased to 16 mL (c), there is only one well resolved peak which belongs to (100) of the 2D-hexagonal mesophase. This could be indicating that the cubic Fd
m phase peaks (220), (311), and a 2D-hexagonal phase peak (100). It might be estimated that the sample consisted of two types of mesophases: 2D-hexagonal phase and cage type cubic phase. As the amount of HCl was further increased to 16 mL (c), there is only one well resolved peak which belongs to (100) of the 2D-hexagonal mesophase. This could be indicating that the cubic Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m mesophase was formed at first, and then converted to the hexagonal mesophase through increasing the amount of HCl when DAPS was used as the CSDA in the anionic surfactant templated system.
m mesophase was formed at first, and then converted to the hexagonal mesophase through increasing the amount of HCl when DAPS was used as the CSDA in the anionic surfactant templated system.
 | ||
| Fig. 4 (A) SAXS patterns of calcined D-AMS and (B) XRD patterns of calcined T-AMS by changing the amount of 0.1 M HCl: (a) 8 ml, (b) 12 ml, (c) 16 ml. | ||
However, with TAPS as the CSDA, as shown in Fig. 4(B), the Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m mesophase remained unchanged when the acid amount was increased. That is to say, with TAPS as the CSDA, the Fd
m mesophase remained unchanged when the acid amount was increased. That is to say, with TAPS as the CSDA, the Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m mesophase could be achieved within a wider range. Although the cubic Fd
m mesophase could be achieved within a wider range. Although the cubic Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m structure has been previously reported by using anionic,26 gemini cationic27 and copolymer surfactants,28 the block copolymer-templating and cationic surfactant syntheses were so complicated and many experimental parameters were noted to strongly affect the resulting mesostructures,29,30 and the cubic silica mesostructure could be synthesized only in a narrow range.
m structure has been previously reported by using anionic,26 gemini cationic27 and copolymer surfactants,28 the block copolymer-templating and cationic surfactant syntheses were so complicated and many experimental parameters were noted to strongly affect the resulting mesostructures,29,30 and the cubic silica mesostructure could be synthesized only in a narrow range.
One important characteristic of the mesostructured silicas which were synthesized by the co-structure-directing route is a uniform distribution of organic groups. To obtain the amino-functionalized mesoporous silicas, many methods for the removal of the surfactant have been attempted.31 In this paper, we adopt an ion-exchange extraction method with an ethanol/ethanolamine solution as the extractant to remove the surfactant. FTIR spectra of the as-synthesized and the extracted samples are displayed in Fig. 5. The bands at 2850 and 2928 cm−1 in FTIR spectra can be assigned to the symmetrical and asymmetrical stretching vibrations of the methylene groups of the CSDA and the surfactant. It can be seen that the intensities of the bands at 2850 and 2928 cm−1 obviously decreased after extraction, which indicated an efficient template extraction. The XRD pattern in Fig. 1(B) shows that the mesostructure of the extracted samples remained well after the extraction process. Furthermore, as shown in Table 1, D-AMS-ex and T-AMS-ex have high specific surface area, 384 and 462 m2 g−1, respectively. The elemental analysis results (Table 2) show that the content of amino groups in AMS-es, D-AMS-ex and T-AMS-ex are 1.74, 2.46 and 2.99 mmol g−1, respectively.
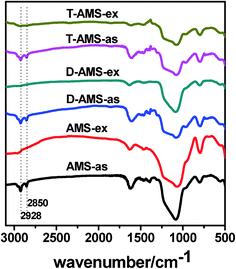 | ||
| Fig. 5 The FTIR spectra of the as-synthesized mesostructured silicas and the extracted samples. | ||
| Sample | N content (mmol g−1) | Initial Pb2+ concentration (ppm) | After adsorption (ppm) | Adsorption capacity (mg g−1) | Adsorption efficiency (%) |
|---|---|---|---|---|---|
| AMS-ex | 1.74 | 200 | 19.6 | 902 | 90 |
| D-AMS-ex | 2.46 | 200 | 10.2 | 949 | 95 |
| T-AMS-ex | 2.99 | 200 | 5.5 | 972 | 97 |
The amino functionalized mesoporous silica has potential applications in base catalysis, adsorption and removal of heavy metals, adsorption of carbon dioxide and supports for metal nanoparticles. In this paper, AMS-ex, D-AMS-ex and T-AMS-ex were used as adsorbents to absorb Pb2+ metal ions. Multi-amine functionalized materials exhibited efficient property in removing Pb2+ in solution. As shown in Table 2, the Pb2+ concentration in solution was greatly reduced after adsorption with the multi-amine functionalized D-AMS and T-AMS and the adsorption efficiency of Pb2+ reaches 95% and 97%, respectively. When AMS-ex was used as the adsorbent, by contrast, the adsorption efficiency of Pb2+ is 90%.
The multi-amine functionalized mesoporous materials also adsorb Pd2+ ion efficiently. As shown in Fig. 6, after the solution containing PdCl42− was stirred at room temperature for 2 h, the color of the solution changed from brown to pale yellow (Fig. 6b), colorless (Fig. 6c,d) when AMS-ex, D-AMS-ex and T-AMS-ex were used as adsorbents, respectively. The Pd2+ adsorption amount was 35, 45, 49 mg g−1, respectively for AMS-ex, D-AMS-ex and T-AMS-ex, and the corresponding adsorption efficiency of Pd2+ were 75%, 97% and 100%, respectively. The multi-amine functionalized 3D cubic mesoporous silicas can absorb metal ions efficiently, due to the interaction between metal ions and amino groups on the walls of channels, and the adsorption capacity was dependent on the amino group content.
 | ||
| Fig. 6 Photos of H2PdCl4 solution (a), after adsorption with AMS (b), after adsorption with D-AMS (c) and after adsorption with T-AMS (d). | ||
D-AMS-ex was used as the support to absorb Pd2+ ions to prepare catalyst D-AMS-Pd. The catalytic activity of D-AMS-Pd was tested by catalytic reduction of 4-nitrophenol. Fig. 7a shows the UV-vis spectra for the reduction of 4-nitrophenol measured at different times during the progress of the reaction. The UV-vis spectra has shown that the maximum ultraviolet absorption spectrum of 4-nitrophenol is 400 nm and the maximum ultraviolet absorption spectrum of product is 300 nm. It was found that the peak height at 400 nm decreases and the peak at 300 nm increases gradually with time after the addition of D-AMS-Pd catalyst. A successive decrease of peak intensity at 400 nm with time can be taken into consideration to obtain the rate constant. The relationship of ln(C/C0) versus time is shown in Fig. 7b, where C is the concentration at time t and C0 is the initial concentration. The ratio of C/C0 was measured from the relative intensity ratio of the respective absorbance A/A0, at 400 nm. The linear relationship of ln(C/C0) versus time was observed, indicating that the reactions followed first-order kinetics. The observed rate constant for the D-AMS-Pd catalyst was 1.2 × 10−3 S−1 calculated directly from the slope of the straight line.
 | ||
| Fig. 7 (A) Time-dependent UV-vis spectral changes of the reaction mixture catalyzed by D-AMS-Pd. (B) Plot of ln(C/C0) versus time for D-AMS –Pd catalyst. | ||
The TEM micrograph of the D-AMS-Pd catalyst (Fig. 8a) clearly shows that the catalyst has large domains of ordered 3D cubic Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m mesostructures, which indicate that the highly ordered mesostructure of the mesoporous material is unchanged after the immobilization of noble metal Pd nanoparticles. We further carried out TEM observation for the recovered D-AMS-Pd catalyst after catalytic reduction of 4-nitrophenol. As shown in Fig. 8c, the highly ordered 3D cubic mesostructure of the catalyst was not destroyed after the catalytic reaction. The result demonstrated that synthetic multi-amine functionalized mesoporous silicas have good stability. As shown in Fig. 8b, the TEM image shows that the dispersion of the Pd particles in the fresh D-AMS-Pd catalyst is quite homogeneous and the sizes of the Pd particles are very uniform. The TEM micrograph for the D-AMS-Pd catalyst after use (Fig. 8d) revealed that Pd nanoparticles were not aggregated. There was no observable leaching of Pd nanoparticles during the reaction. The interaction between metal ions and amino groups prevent the Pd nanoparticles from leaching, which further suggested that there was no leaching of the surface amino groups in the application. The above results confirmed that the mesoporous material is a good host for nanoparticles and the multi-amine functionalized mesoporous silica has good stability.
m mesostructures, which indicate that the highly ordered mesostructure of the mesoporous material is unchanged after the immobilization of noble metal Pd nanoparticles. We further carried out TEM observation for the recovered D-AMS-Pd catalyst after catalytic reduction of 4-nitrophenol. As shown in Fig. 8c, the highly ordered 3D cubic mesostructure of the catalyst was not destroyed after the catalytic reaction. The result demonstrated that synthetic multi-amine functionalized mesoporous silicas have good stability. As shown in Fig. 8b, the TEM image shows that the dispersion of the Pd particles in the fresh D-AMS-Pd catalyst is quite homogeneous and the sizes of the Pd particles are very uniform. The TEM micrograph for the D-AMS-Pd catalyst after use (Fig. 8d) revealed that Pd nanoparticles were not aggregated. There was no observable leaching of Pd nanoparticles during the reaction. The interaction between metal ions and amino groups prevent the Pd nanoparticles from leaching, which further suggested that there was no leaching of the surface amino groups in the application. The above results confirmed that the mesoporous material is a good host for nanoparticles and the multi-amine functionalized mesoporous silica has good stability.
 | ||
| Fig. 8 TEM images of the D-AMS-Pd catalyst: (a, b) the D-AMS-Pd catalyst, (c, d) the D-AMS-Pd catalyst after used in catalytic reduction of 4-nitrophenol. | ||
Conclusion
In summary, multi-amine functionalized 3D cubic Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m mesostructured silica (AMS-8) was firstly synthesized by anionic surfactant templated route with DAPS and TAPS as the CSDA. The geometrical change in the CSDA led to a change of mesophase from 2D-hexagonal P6mm to 3D cubic Fd
m mesostructured silica (AMS-8) was firstly synthesized by anionic surfactant templated route with DAPS and TAPS as the CSDA. The geometrical change in the CSDA led to a change of mesophase from 2D-hexagonal P6mm to 3D cubic Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. After extraction of the anionic surfactant, multi-amine functionalized cubic Fd
m. After extraction of the anionic surfactant, multi-amine functionalized cubic Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m mesoporous silicas were obtained which can adsorb metal ions effectively in solution. Furthermore, the D-AMS-ex was used as a support to absorb Pd2+ ions to prepared supported catalyst and the catalytic activity was tested by catalytic reduction of 4-nitrophenol. It can be expected that these cubic silicas with multi-amine functional groups within the cage-like mesopore would have promising applications in adsorption and catalysis.
m mesoporous silicas were obtained which can adsorb metal ions effectively in solution. Furthermore, the D-AMS-ex was used as a support to absorb Pd2+ ions to prepared supported catalyst and the catalytic activity was tested by catalytic reduction of 4-nitrophenol. It can be expected that these cubic silicas with multi-amine functional groups within the cage-like mesopore would have promising applications in adsorption and catalysis.
Acknowledgements
This work was supported by NSFC (20873070, 20973095), National Basic Research Program of China (2009CB623502), RFDP (200800550007), NCET of Ministry of Education (NCET-07-0448) and MOE (IRT-0927).References
- T. Yanagisawa, T. Shimizu, K. Kuroda and C. Kato, Bull. Chem. Soc. Jpn., 1990, 63, 988 CrossRef CAS.
- C. T. Kresge and M. E. Leonowicz, Nature, 1992, 359, 710 CrossRef CAS.
- J. S. Beck and J. C. Vartuli, J. Am. Chem. Soc., 1992, 114, 10834 CrossRef CAS.
- K. Moller and T. Bein, Chem. Mater., 1998, 10, 2950 CrossRef CAS.
- J. Y. Ying, C. P. Mehnert and M. S. Wong, Angew. Chem., Int. Ed., 1999, 38, 56 CrossRef CAS.
- J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. T. W. Chu, D. H. Olson and J. L. Schlenker, J. Am. Chem. Soc., 1992, 114, 10834 CrossRef CAS.
- Y. Sakamoto, T. W. Kim, R. Ryoo and O. Terasaki, Angew. Chem., Int. Ed., 2004, 43, 5231 CrossRef CAS.
- C. Yu, Y. Yua and D. Zhao, Chem. Commun., 2000, 575 RSC.
- S. Che, S. Kamiya, O. Terasaki and T. Tatsumi, J. Am. Chem. Soc., 2001, 123, 12089 CrossRef CAS.
- H. Yang, L. Zhang, L. Zhong, Q. H. Yang, C. Li and H. Angew, Angew. Chem., Int. Ed., 2007, 46, 6861 CrossRef CAS.
- H. P. Yiu, P. A. Wright and N. P. Botting, J. Mol. Catal. B: Enzym., 2001, 15, 81 CrossRef CAS.
- J. F. Diaz, K. J. Balkus, Jr., F. Bedioui, V. Kurshev and L. Keva, Chem. Mater., 1997, 9, 61 CrossRef CAS.
- M. H Lim and A. Stein, Chem. Mater., 1999, 11, 3285 CrossRef CAS.
- J. Brown, R. Richer and L. Mercier, Microporous Mesoporous Mater., 2000, 37, 41 CrossRef.
- C. E. Fowler, S. L. Burkett and S. Mann, Chem. Commun., 1997, 1769 RSC.
- D. J. Macquarrie, D. B. Jackson, S. Tailland and K. A. Utting, J. Mater. Chem., 2001, 11, 1843 RSC.
- A. Shahbazi, H. Younesi and A. Badiei, Chem. Eng. J., 2011, 168, 505 CrossRef CAS.
- A. P. Wigh tand and M. E. Davis, Chem. Rev., 2002, 102, 589 Search PubMed.
- F. Hoffmann, M. Cornelius, J. Morell and M. Froba, Angew. Chem., Int. Ed., 2006, 45, 3216 CrossRef.
- C. B. Gao, H. B. Qiu, W. Zeng, Y. S. O. Terasaki, S. Kamiya, Q. Chen and S. Che, Chem. Mater., 2006, 18, 3904 CrossRef CAS.
- Y. Sakamoto, L. Hui, S. Che and O. Terasaki, Chem. Mater., 2009, 21, 223 CrossRef CAS.
- A. E. Garcia-Bennett, N. Kupferschmidt, Y. Sakamoto, S. Che and O. Terasaki, Angew. Chem., Int. Ed., 2005, 44, 5317 CrossRef CAS.
- R. Atluri, Y. Sakamoto and A. E. Garcia-Bennett, Langmuir, 2009, 25, 3189 CrossRef CAS.
- J. G. Wang, Q. Xiao, H. J. Zhou, P. C. Sun, D. T. Ding and T. H. Chen, J. Colloid Interface Sci., 2008, 323, 332 CrossRef CAS.
- C. B. Gao, Y. Sakamoto, O. Terasaki, K. Sakamoto and S. Che, J. Mater. Chem., 2007, 17, 3591 RSC.
- S. Che, A. E. Garcia-Bennett and T. Yokoi, Nat. Mater., 2003, 2, 801 CrossRef CAS.
- T. Yokoi, H. Yoshitake, T. Yamada, Y. Kubota and T. Tatsumi, J. Mater. Chem., 2006, 16, 1125 RSC.
- A. E. Garcia-Bennett, K. Lund and O. Terasaki, Angew. Chem., Int. Ed., 2006, 45, 2434 CrossRef CAS.
- L. Han, Y. Sakamoto, O. Terasaki, Y. Li and S. Che, J. Mater. Chem., 2007, 17, 1216 RSC.
- S. A. El-Safty, F. Mizukami and T. Hanaoka, J. Mater. Chem., 2005, 15, 2590 RSC.
- H. Q. Zheng, C. B. Gao and S. Che, Microporous Mesoporous Mater., 2008, 116, 299 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
