Physicochemical properties of 1,2,3-triazolium ionic liquids†
Shilpi
Sanghi
a,
Erik
Willett
a,
Craig
Versek
b,
Mark
Tuominen
b and
E. Bryan
Coughlin
*a
aDepartment of Polymer Science and Engineering, University of Massachusetts, 120 Governors Drive, Amherst, MA 01003, USA. E-mail: coughlin@mail.pse.umass.edu; Tel: +1 413 577-1616
bDepartment of Physics, University of Massachusetts, Amherst 01003, USA. E-mail: tuominen@physics.umass.edu; Tel: +1 413-545-1944
First published on 23rd November 2011
Abstract
Ionic liquids composed of four different 1,2,3-triazolium cations with tosylate or triflate counter anions have been synthesized and characterized. Physicochemical properties of these ionic liquids including ion cluster behavior, thermal properties, electrochemical stability and ionic conductivity were determined and compared to corresponding imidazolium based ionic liquids. The impact of structure variations, in terms of substituents on the ring of the 1,2,3-triazolium cation and identity of the anion (i.e. tosylate versus triflate) is discussed. Stability of the 1,2,3-triazolium salts towards hydroxide ion at 80 °C was studied. Key features of 1,2,3-triazolium salts are their high electrochemical stability and ionic conductivity, comparable to imidazolium ionic liquids, but better chemical stability under alkaline conditions.
Introduction
Over the past decade, ionic liquids have gained overwhelming interest due to their unique properties including negligible vapor pressure, wide usable temperature range, nonflammability, excellent thermal stability and solvating properties, high ionic conductivity and wide electrochemical stability window.1,2 These properties of ionic liquids have made them useful in a broad range of chemical reactions and separation processes,3 as electrolytes in electronic devices,4,5 lubricant additives1 and biotechnology.6,7 Ionic liquids are composed of a bulky, organic cation such as quaternary ammonium,8,9 1,3-dialkylimidazolium,10–12 sulfonium,13 phosphonium ion14 and an inorganic or organic counter anion. A wide range of anions are employed in ionic liquids, ranging from simple halides, to main group anions such as tetrafluoroborate or hexafluorophosphate, and organic anions such as triflate or tosylate. The advantage of having such a wide selection of cations and anions is that it is possible to tailor the properties of ionic liquids for a specific application by suitable choice of component ions.Ionic liquids composed of 1-methyl-3-alkylimidazolium cation have dominated the field due to their wide liquidus range, high conductivities and low viscosities, necessary for a multitude of applications, especially electrochemistry. Properties of 1,3-dialkylimidazolium ionic liquids can be tuned by varying the alkyl groups. Although reported ionic liquids have been made by combining various cations and anions, notably absent from the ionic liquid literature is hydroxide as the counterion. Hydroxide ion has not been used as a counterion in ionic liquids because it is both a strong base and a nucleophile capable of degrading ammonium, phosphonium and imidazolium cations. However, if caustic ionic liquids could be developed, they would have potential applications as new reaction media for base catalyzed reactions,1 or electrolytes in alkaline fuel cells.15
The synthesis of 1,2,3-triazole is quite facile by the Huisgen 1,3-dipolar cycloaddition reaction.16 This heterocycle can be modified at nitrogens 1 and 3 to form substituted 1,2,3-triazolium cation. Although, the 1,2,3-triazolium cation has a structure analogous to imidazolium cation, research on triazolium based ionic salts17–20 is quite limited compared to imidazolium salts. This is partially due to the paucity of methyl triazole unlike 1-methylimidazole and the explosive nature of organic azides involved in the synthesis of 1,2,3-triazole. We note that a few articles on 1,2,3-triazolium ionic liquids have been published in last year,21–24 while this manuscript was being written, but this work was done independently. These recent reports have described the two-step one pot synthesis of triazolium salts using Cu catalyzed Huisgen 1,3-dipolar cycloaddition reaction,23 described their thermal properties18,22 and used them as a reaction media.17,19,21 Ryu and coworkers have recognized that 1,2,3-triazolium salts have higher chemical stability in basic medium compared to imidazolium salts, due to the facile deprotonation of C–2 hydrogen of imidazolium salts.24 However, potential use of triazolium salts in alkaline fuel cell membranes requires detailed study of their chemical stability under alkaline conditions, their ionic conductivity and electrochemical stability, which have not been reported previously to our knowledge. In our search for stable solvents to be used as an electrolyte in alkaline fuel cells, we have synthesized substituted 1,2,3-triazolium salts and studied their ion cluster behavior, thermal properties, chemical stability towards hydroxide ion and electrochemical properties, and compared them with properties of analogous imidazolium salts. We report the properties of 1,2,3-triazolium salts with two representative anions, tosylate (Tos) and triflate (OTf). Herein, we report the synthesis and characterization of ten different ionic liquids comprising five cations and two anions. Out of the five cations, three are derivatives of 1-alkyl/aryl-3-methyl-4-butyl triazolium ion, a fourth cation is 1,3-dimethyltriazolium which provides an insight on the effect of butyl group at the 4 position of triazolium, and 1,3-dimethylimidazolium was studied for comparison.
Results and discussion
Synthesis
The 1,2,3-triazolium salts were synthesized by the Huisgen 1,3-dipolar cycloaddition reaction between the corresponding azide and 1-hexyne, followed by alkylation with either methyl tosylate or methyl triflate, Fig. 1. The structures and abbreviations of the five cations and two anions comprising the ten different ionic liquids studied are shown in Fig. 2. For Tz1 salts, benzyl azide was purchased. For Tz2 salts, phenyl azide was synthesized following a literature procedure,25 and for Tz3 salts, methyl azide was generated in situ by reacting sodium azide and methyl iodide. 1-Methyl-4-butyl-1,2,3-triazole for Tz3 salts was synthesized under microwave conditions as described previously.26Caution must be taken in the synthesis of triazole as azides are explosive in nature. Alkylation was done using methyl tosylate or methyl triflate in almost 100% yields according to the procedure described by Begtrup et al.27 Tz4 salts were synthesized by deprotonation of 1,2,3-triazole by sodium hydroxide, followed by alkylation.28 Due to synthetic limitations, there is a trace of residual sodium tosylate and sodium triflate in Tz4Tos and Tz4OTf, respectively.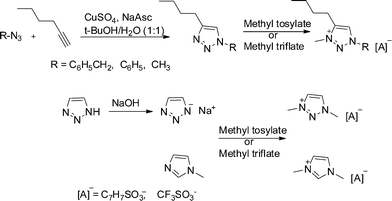 | ||
| Fig. 1 Synthesis of ionic liquids studied in this work. | ||
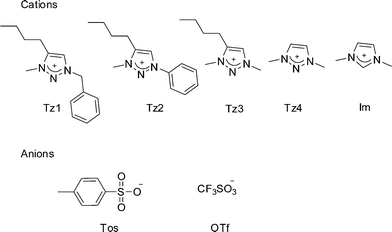 | ||
| Fig. 2 Structures and abbreviations of cations and anions studied in this work. | ||
The synthesized salts were characterized by 1H-NMR, 13C-NMR and FAB-HRMS or ESI-MS. All the characterization data are consistent with the expected structures and compositions (see the supporting information for synthesis and NMR data†). All these ionic liquids are hydrophilic in nature. To determine their moisture sensitivity, salts were dried under vacuum at 90 °C overnight and then exposed to 80% RH at 21 °C for 1 h. It was found that Tz1 and Im salts with both Tos and OTf anion absorb moisture faster (∼800 ppm in 1 h) than the other salts studied. Tz2 salts are the least hydrophilic with their water content being less than 400 ppm after 1 h of exposure. Since the properties of ionic liquids change drastically by the presence of small amounts of water, all the salts were dried overnight at 80 °C under vacuum, prior to use. All the investigated ionic liquids are miscible with solvents of high polarity: water, methanol and acetonitrile. They are immiscible with diethyl ether, chloroform and ethyl acetate. The characterization data has been classified in two series. The first series contains five salts with tosylate anion and varying cations; while the second series includes five salts with triflate anion and varying cations.
ESI-MS
Electrospray ionization mass spectrometry (ESI-MS) has been used to measure the intrinsic solvent-free strength of cation–anion interactions in ionic liquids.13,29 These interactions influence their physical and chemical properties.30 Positive ion mode ESI-MS experiments were performed using dilute methanol solutions of the ionic salts. The positive ion mode mass spectrum consisted of naked cation [C]+, dimeric clusters [C2A1]+ with a single anion [A−] and trimeric clusters [C3A2]+. Compositions higher than [C3A2]+ were not observed for any of these salts.The graphical representations of relative abundance of charged clusters of ionic liquids with tosylate anion and triflate anion are shown in Fig. 3a and 3b, respectively. No trimeric clusters were observed for any of the 1,2,3-triazolium salts. Trimeric clusters were observed for the imidazolium salts. Bini et al.30 reported the existence of multiple higher order clusters for imidazolium salts. As observed from the above comparison between triazolium salts and 1,3-dimethylimidazolium salt, the triazolium cations have lower binding strength to tosylate and triflate counter ions compared to the imidazolium cations.
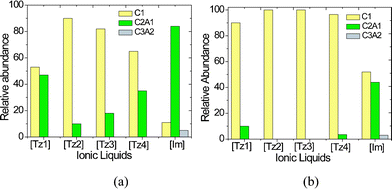 | ||
| Fig. 3 Relative abundance in ESI mass spectra of triazolium salts in methanol a) tosylate series b) triflate series. | ||
From the relative abundance data for the 1,2,3-triazolium cations studied, the qualitative order of interaction with a given anion was found to be: [Tz1]+ > [Tz4]+ > [Tz3]+ > [Tz2]+. This trend shows that the solvent-free binding strength of triazolium cations depends on the substitution at the 1 and 3 position of triazolium ring. Alkyl substitution influences the intrinsic binding strength of imidazolium cations to anions as well.30 The distribution of ESI mass spectra peaks show that the tosylate anion is much more strongly bound to the cations compared to triflate anion. This is in agreement with the report of Bini et al.30 where tosylates show stronger bonding ability than triflates with substituted imidazolium cations. These ESI-MS results explain the higher melting point and glass transition temperature observed for tosylate salts compared to triflate salts (vide infra).
Thermal stability
Thermal stability of the salts was studied by thermal gravimetric analysis (TGA) and the data are reported in Table 1, (see the supporting information for TGA traces†). Thermal stability of 1,2,3-triazolium salts are in the range of 200—300 °C, which is lower than that of 1,3-dialkylimidazolium based ionic liquids (generally, Td > 300 °C). It was observed that the thermal stability of these salts is significantly influenced by the identity of the anion, similar to that reported previously.13,24 Except for 1, 3-dimethyltriazolium salts, the triflate salts have greater thermal stability than tosylate salts. It is generally observed that the weaker the coordinating anion, the higher the thermal stability of ionic liquids.1| Entry | Salts | T g | T c | T m | T d |
|---|---|---|---|---|---|
| a Glass transition temperatures determined by DSC on second heating cycle. b Crystallization temperature determined by DSC. c Melting Point determined by DSC. d Thermal decomposition temperature corresponding to 5% weight loss, determined by TGA. NA = not applicable | |||||
| 1 | Tz1Tos | −22 | NA | NA | 240 |
| 2 | Tz1OTf | −45 | NA | NA | 245 |
| 3 | Tz2Tos | NA | 96 | 146 | 247 |
| 4 | Tz2OTf | NA | 62 | 102 | 310 |
| 5 | Tz3Tos | −33 | 73 | 106 | 238 |
| 6 | Tz3OTf | −67 | NA | NA | 228 |
| 7 | Tz4Tos | −25 | 29 | 99 | 240 |
| 8 | Tz4OTf | −77 | NA | NA | 221 |
| 9 | ImTos | −29 | 28 | 95 | 324 |
| 10 | ImOTf | NA | −37 | 37 | 417 |
Phase behavior, melting point and glass transition temperature
The phase behavior of these salts were studied by differential scanning calorimetry (DSC) and the data for glass transition temperatures (Tg), crystallization temperatures (Tc) and melting points (Tm) are summarized in Table 1. Tz1 salts are amorphous while Tz2 salts are crystalline in nature. The glass transition temperatures of all the salts studied is below 0 °C. Tz3Tos, Tz4Tos, ImTos are liquids at high temperature and become glassy upon cooling. One of the reasons for high melting point of Tz3, Tz4 and Im salts is symmetrical substitution at the 1 and 3 position of these cations. Salts with symmetrical cations exhibit higher melting point compared to salts with asymmetrical cations due to their higher lattice energy.12 For example, if the structure of symmetrical 1,3-dimethylimidazolium triflate is changed to asymmetrical 1-ethyl-3-methylimidazolium triflate, the melting point decreases from 37 °C to −9 °C.12 The phase behavior of Tz3 and Tz4 salts are very similar indicating that the thermal properties are influenced only by the substitution at the 1 and 3 position of triazolium ring and not by the butyl group at the 4 position.All the tosylate salts exhibit melting points around 100 °C, at much higher temperature compared to the triflate salts. This could be explained by the higher binding strength of cations with tosylates compared to triflates as seen by ESI-MS. The ionic salts such as 1-alkyl-3-methylbenzotriazolium tosylate, 1,2,3-triazolium tosylate, 1,3-dialkylimidazolium tosylate exhibited melting points at higher temperatures compared to salts with halides, triflate or tetrafluoroborate counter anions.18,31,32 Although the triazolium salts reported here are not room temperature ionic liquids, they could be useful for solid electrolyte applications.
Chemical stability towards hydroxide ion
The stability of ionic liquids in alkaline environment is an important property towards making caustic ionic liquids. The alkaline stability of quaternary ammonium cations has been studied quite extensively by Pivovar and coworkers for fuel cell applications.33,34 We investigated the chemical stability of 1,2,3-triazolium and 1,3-dimethylimidazolium salts under alkaline conditions. We prepared 0.05 M solutions of Tz1Tos, Tz2Tos, Tz3Tos and ImTos in 0.05 M NaOH/D2O and heated these at 80 °C in NMR tubes. The stability of each of these cations was evaluated by recording 1H-NMR spectra at regular intervals. In 1H-NMR, the integration of the area under the butyl group resonances at 4 position of triazolium cation and that of resonances of counter ion, versus that of the substituents at 1 and 3 position, yielded a quantitative measure of the cation remaining. The counterion, p-toluene sulfonate acts as an internal standard (singlet at δ 2.2 ppm and doublets at δ 7.4 and δ 7.2 ppm).Tz2 and Tz3 cations were stable for up to 10 days with no perceptible decompositions. The Tz1 cation degraded in 1 day forming 1-methyl-5-butyl triazole, and the imidazolium cation degraded within 2 h upon heating, Fig. 4.
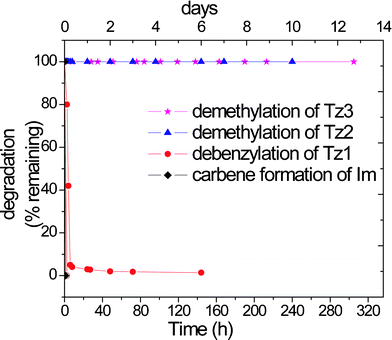 | ||
| Fig. 4 Degradation of triazolium cations and imidazolium cation at 80 °C in 0.05 M NaOH/D2O. | ||
The 1H-NMR of Tz2 and Tz3 cations, showed a gradual decrease in the intensity of resonances corresponding to protons at the 5 position of triazole, while in 13C-NMR, the singlet corresponding to the carbon at the 5 position changed to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 triplet. This indicates the 1H for 2H exchange at the 5 position of Tz2 and Tz3 cations. The rate of hydrogen for deuterium exchange was higher in Tz2 salt compared to Tz3 salt as seen in Fig. 5. Similar observations related to hydrogen for deuterium exchange rates have been reported previously.27,35
1 triplet. This indicates the 1H for 2H exchange at the 5 position of Tz2 and Tz3 cations. The rate of hydrogen for deuterium exchange was higher in Tz2 salt compared to Tz3 salt as seen in Fig. 5. Similar observations related to hydrogen for deuterium exchange rates have been reported previously.27,35
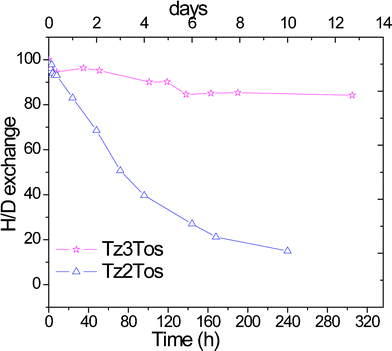 | ||
| Fig. 5 H–D exchange in solutions of Tz2Tos and Tz3Tos in 0.05 M NaOH/D2O at 80 °C. | ||
The most caustic test conditions reported to validate the stability of cations for alkaline anion exchange membrane fuel cells are 2 M NaOH at a temperature of 80 °C.33 When the concentration of NaOH was increased to 2 M in the above NMR experiments, Tz2 and Tz3 cations degraded within a day. This indicates that 1,2,3-triaolium salts have chemical stability in basic medium suitable for base catalyzed reactions such as Baylis–Hillman Reaction, but not enough for their applicability in alkaline fuel cell membranes.
Conductivity
The conductivity of ionic liquid is an important property for use as an electrolyte in batteries, fuel cells, solar cells and double layer capacitors. We measured the conductivity of eight neat ionic liquids based on Tz1, Tz2, Tz3 and Im cations with tosylate or triflate anions, over a temperature range of 25 °C to 180 °C under anhydrous conditions (see supporting information†). The measured conductivity of 1,3-dimethylimidazolium salts (Fig. 6) is in good agreement with the published data on similar imidazolium salts, thereby confirming the accuracy of our conductivity measurements.36,37 Tz2Tos, Tz2OTf and Tz3Tos show a sudden drop in conductivity due to crystallization in the conductivity cell during cooling.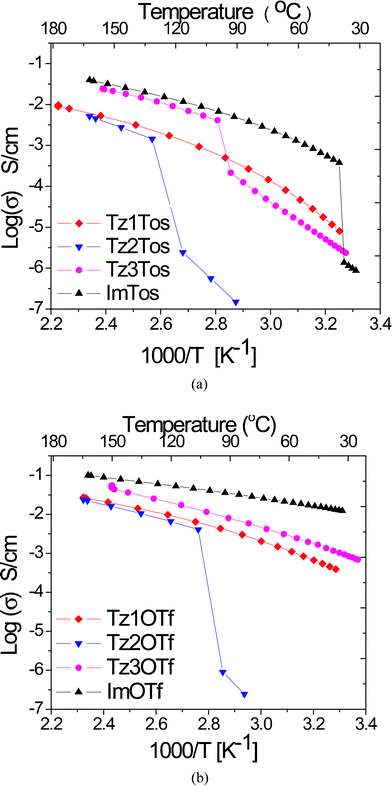 | ||
| Fig. 6 Ionic conductivity as a function of temperature a) tosylate series b) triflate series. | ||
From both the tosylate salt and triflate salt series, it can be seen that conductivity decreases in the order: Im ≈ Tz3 > Tz1 ≈ Tz2. This trend is consistent with the size of cation.36 The conductivity of Tz2 and Tz1 salts are almost identical, while that of Tz3 and Im salts are similar. Given that the substituents at N–atom of imidazolium and triazolium cations are the same, their ionic conductivity is of the same order of magnitude. This conductivity is more than that reported for quaternary ammonium and sulfonium salts.1 From Walden's rule, the conductivity of ionic liquids is mainly governed by their viscosity, with conductivity being inversely proportional to the viscosity.1 The magnitude of the Walden product depends on the ion size, mainly the cationic size. Since the conductivity of 1,3-dimethyl-1,2,3-triazolium and 1,3-dimethylimidazolium salts with analogous structures are similar, the viscosity of 1,3-dimethyl-1,2,3-triazolium salts would be as low as that of 1,3-dimethylimidazolium salts. Pairwise comparisons (see the supporting information for plots†) between any two ionic salts studied here with the same cation and different anion, e.g. Tz1Tos versus Tz1OTf or Tz2Tos versus Tz2OTf, shows that triflates have higher ionic conductivity than tosylates. This could be explained by two reasons: i) smaller size of triflate ion than tosylate ion, which imparts higher mobility to triflate ion ii) less ionic association between triflate anion and cations compared to that between tosylate anion and cations, as observed by ESI-MS, generates more diffusive species that can contribute to ionic conductivity.
Electrochemical stability
The electrochemical stability of ionic liquids is an important parameter for their application in electrochemical devices (batteries, supercapacitors), electrochemical synthesis, metal deposition etc. The electrochemical stability of the salts was evaluated by cyclic voltammetry. Measurements were carried out using 0.1 M solution of these salts in acetonitrile over a voltage range of ± 3.2 V, using a platinum working electrode, silver wire immersed in 0.01 M AgNO3 in acetonitrile reference electrode, and platinum counter electrode. The electrochemical window of ImOTf was measured as 4.31 V which is similar to that reported for ethylmethylimidazolium triflate (EMIM-OTf) by Grätzel et al. on a platinum electrode.1,12The cyclic voltammograms of both; tosylate series and triflate series are shown in Fig. 7 and the data for cathodic (Ecathodic) and anodic (Eanodic) limits and electrochemical windows (EW) are summarized in Table 2. All the triazolium salts prepared, except Tz2OTf, have an electrochemical stability window of more than 4 V. For each pair of salts with the same cation, but different anion, Ecathodic values are similar indicating that the cathodic stability of these salts with electrochemically stable anions is limited by the decomposition of the cation. The cations studied here, exhibit reductive stability in the following order: Tz3 ≈ Tz4 ≈ Im > Tz1 > Tz2. The reduction potential of salts containing Tz3, Tz4 and Im cation are similar. This indicates that electrochemical stabilities of 1,2,3-triazolium cations are influenced by the substitution pattern at the 1 and 3 positions and not the 4 position of the triazole ring. These results clearly indicate that depending upon the substituents at the nitrogen atom, the triazolium salts may have cathodic stability comparable to imidazolium salts.
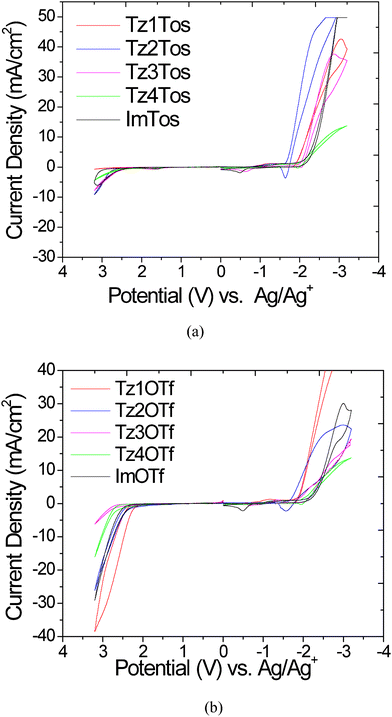 | ||
| Fig. 7 Cyclic voltammograms for a) tosylate series b) triflate series. Sweep rate: 50 mV s−1. The ferrocene/ferrocenium couple had a measured potential of 0.124 V versus Ag/Ag+ in acetonitrile. The oxidation current observed around −0.5 V for Im and Tz3 salts and around −1.6 V for Tz2 salt appears to result from formation of a surface film due to the reduction of some species at potential more negative than their respective Ecathodic limit (see the supporting information†). | ||
| Entry | Salts | E cathodic (V) | E anodic (V) | EW (V) |
|---|---|---|---|---|
| 1 | Tz1Tos | −1.79 | 2.56 | 4.35 |
| 2 | Tz1OTf | −1.86 | 2.17 | 4.03 |
| 3 | Tz2Tos | −1.6 | 2.53 | 4.13 |
| 4 | Tz2OTf | −1.67 | 2.12 | 3.79 |
| 5 | Tz3Tos | −2.1 | 2.52 | 4.62 |
| 6 | Tz3OTf | −2.1 | 2.4 | 4.5 |
| 7 | Tz4Tos | −2.08 | 2.58 | 4.66 |
| 8 | Tz4OTf | −2.08 | 2.39 | 4.47 |
| 9 | ImTos | −2.12 | 2.55 | 4.67 |
| 10 | ImOTf | −2.13 | 2.18 | 4.31 |
The oxidative stability of all tosylates is in the range of 2.5–2.6 V while for triflate containing salts is in the range of 2.15–2.4 V. The tosylate salt series exhibit higher chemical stability towards oxidation than triflates. Different Eanodic values for the salts with the same triflate anion show that the anodic decomposition of the salts is influenced by the cations as well. The anions might be more stable towards reduction and oxidation than the cations. Thus, both the cathodic and anodic decomposition potentials are influenced by the cations in case of 1,2,3-triazolium salts, which is similar to that observed for 1,3-dialkylimidazolium salts and unlike quaternary ammonium salts, where cathodic limit is governed by reduction of cation and anodic limit by oxidation of anions.8,11 Overall, 1,2,3-triazolium cations have better cathodic stability than pyridinium cations, similar to imidazolium cations, and inferior to quaternary ammonium cations.1,11,38,39
Conclusions
In summary, a series of 1,2,3-triazolium salts comprising two different anions (toslyate and triflate) were synthesized and characterized. Their key properties; thermal stability, ion aggregation behavior, alkaline stability, conductivity and electrochemical stability were evaluated and compared to those of imidazolium salts. All the 1,2,3-triazolium salts have thermal stability in the range of 200–250 °C. Although triazolium salts studied here have melting points greater than 95 °C, room temperature 1,2,3-triazolium based ionic liquids could be synthesized by appropriate choice of anion and tuning the substituents at the 1 and 3 positions of the ring. 1,2,3-Triazolium salts exhibit ionic conductivity up to 0.1 S cm−1 at 150 °C, comparable to imidazolium salts. The 1,2,3-triazolium based ionic salts exhibit a large stable electrochemical window (> 4 V), and better chemical stability towards alkaline conditions compared to imidazolium salts.Acknowledgements
This research was supported by NSF funded Center for Chemical Innovation at UMass Amherst, Grant #CHE-0739227 and the Army Research Office Grants #W911NF-08-1-0412 and #W911NF-10-1-0520. Analytical facilities were made available through the NSF-supported Materials and Research Science and Engineering Center on Polymers and DOE supported Energy Frontier Research Center at UMass Amherst. We would like to thank Dr Stephen Eyles for performing mass spectrometry experiments, Michael Thorn for helping develop ionic conductivity experimental protocol and Jimmy Lawrence for helpful suggestions on cyclic voltammetry.References
- P. Wassercheid and T. Welton, Ionic Liquids in Synthesis, Wiley-VCH, Weinheim, 2002 Search PubMed.
- S. J. Zhang, N. Sun, X. Z. He, X. M. Lu and X. P. Zhang, J. Phys. Chem. Ref. Data, 2006, 35, 1475–1517 CrossRef CAS.
- U. Domanska, Ionic Liquids in Chemical Analysis, 2009, 1, 1–72 Search PubMed.
- M. Egashira, H. Todo, N. Yoshimoto and M. Morita, J. Power Sources, 2008, 178, 729–735 CrossRef CAS.
- C. Sirisopanaporn, A. Fernicola and B. Scrosati, J. Power Sources, 2009, 186, 490–495 CrossRef CAS.
- V. Kumar and V. Malhotra Sanjay, in Ionic Liquid Applications: Pharmaceuticals, Therapeutics, and Biotechnology, ACS Symposium Series 1038; American Chemical Society, Washington, DC, 2010, pp. 1–12 Search PubMed.
- Y. Jiang, C. Guo, H. Xia and H. Liu, in Ionic Liquid Applications: Pharmaceuticals, Therapeutics, and Biotechnology, ACS Symposium Series 1038; American Chemical Society, Washington, D.C., 2010, pp. 77–89 Search PubMed.
- Z.-B. Zhou, H. Matsumoto and K. Tatsumi, Chem.–Eur. J., 2005, 11, 752–766 CrossRef CAS.
- J. Sun, M. Forsyth and D. R. MacFarlane, J. Phys. Chem. B, 1998, 102, 8858–8864 CrossRef CAS.
- H. Tokuda, K. Hayamizu, K. Ishii, M. Susan and M. Watanabe, J. Phys. Chem. B, 2005, 109, 6103–6110 CrossRef CAS.
- Z.-B. Zhou, H. Matsumoto and K. Tatsumi, Chem.–Eur. J., 2004, 10, 6581–6591 CrossRef CAS.
- P. Bonhote, A.-P. Dias, N. Papageorgiou, K. Kalyanasundaram and M. Gratzel, Inorg. Chem., 1996, 35, 1168–1178 CrossRef CAS.
- Q. Zhang, S. Liu, Z. Li, J. Li, Z. Chen, R. Wang, L. Lu and Y. Deng, Chem.–Eur. J., 2009, 15, 765–778 CrossRef CAS.
- K. J. Fraser and D. R. MacFarlane, Aust. J. Chem., 2009, 62, 309–321 CrossRef CAS.
- J. R. Varcoe and R. C. T. Slade, Fuel Cells, 2005, 5, 187–200 CrossRef CAS.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596–2599 CrossRef CAS.
- J. Liebscher, J. Shah, Z. Yacob, S. Sadiq, S. Hanelt and H. Blumenthal, Journal of Physics: Conference Series, 2009, 182 Search PubMed.
- S. S. Khan, S. Hanelt and J. Liebscher, ARKIVOC (Gainesville, FL, U. S.), 2009, 193–208 CAS.
- J. Shah, S. S. Khan, H. Blumenthal and J. Liebscher, Synthesis, 2009, 3975–3982 CAS.
- O. A. Ivashkevich, V. E. Matulis, A. S. Lyakhov, I. N. Grigorieva, P. N. Gaponik, G. T. Sukhanov, Y. V. Filippova and A. G. Sukhanova, Chem. Heterocycl. Compd., 2009, 45, 1218–1225 CrossRef CAS.
- Y.-K. Jeong, D.-Y. Kim, Y.-S. Choi and J.-S. Ryu, Org. Biomol. Chem., 2011, 9, 374–378 CAS.
- F. Q. Zhao, L. Xue, X. L. Xing, R. Z. Hu, Z. M. Zhou, H. X. Gao, J. H. Yi, S. Y. Xu and Q. Pei, Sci. China Chem., 2011, 54, 461–474 CrossRef CAS.
- J. T. Fletcher, M. E. Keeney and S. E. Walz, Synthesis, 2010, 3339–3345 CrossRef CAS.
- Y. Jeong and J.-S. Ryu, J. Org. Chem., 2010, 75, 4183–4191 CrossRef CAS.
- J. Anderson, U. Madsen, F. Bjorkling and X. Liang, Synlett, 2005, 2209–2213 Search PubMed.
- P. Appukkuttan, W. Dehaen, V. V. Fokin and E. Van der Eycken, Org. Lett., 2004, 6, 4223–4225 CrossRef CAS.
- M. Begtrup, Acta Chem. Scand., 1971, 25, 249–259 CrossRef CAS.
- M. Begtrup and P. Larsen, Acta Chem. Scand., 1990, 44, 1050–1057 CrossRef CAS.
- F. C. Gozzo, L. S. Santos, R. Augusti, C. S. Consorti, J. Dupont and M. N. Eberlin, Chem.–Eur. J., 2004, 10, 6187–6193 CrossRef CAS.
- R. Bini, O. Bortolini, C. Chiappe, D. Pieraccini and T. Siciliano, J. Phys. Chem. B, 2007, 111, 598–604 CrossRef CAS.
- S. A. Forsyth and D. R. MacFarlane, J. Mater. Chem., 2003, 13, 2451–2456 RSC.
- V. Strehmel, E. Reynaud, H. Wetzel, E. Görnitz and A. Laschewsky, Macromol. Symp., 2009, 275-276, 242–249 CrossRef CAS.
- B. R. Einsla, S. Chempath, L. R. Pratt, J. M. Boncella, J. Rau, C. Macomber and B. S. Pivovar, ECS Trans., 2007, 11, 1173–1180 CrossRef CAS.
- C. S. Macomber, J. M. Boncella, B. S. Pivovar and J. A. Rau, J. Therm. Anal. Calorim., 2008, 93, 225–229 CrossRef CAS.
- M. Begtrup, Bull. Soc. Chim. Belg., 1988, 97, 573–597 CrossRef CAS.
- J. Vila, L. M. Varela and O. Cabeza, Electrochim. Acta, 2007, 52, 7413–7417 CrossRef CAS.
- O. Zech, A. Stoppa, R. Buchner and W. Kunz, J. Chem. Eng. Data, 2010, 55, 1774–1778 CrossRef CAS.
- Z.-B. Zhou, H. Matsumoto and K. Tatsumi, Chem.–Eur. J., 2006, 12, 2196–2212 CrossRef CAS.
- A. Noda and M. Watanabe, Electrochim. Acta, 2000, 45, 1265–1270 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, TGA plots, ionic conductivity plots and cyclic voltammograms. See DOI: 10.1039/c1ra00286d |
| This journal is © The Royal Society of Chemistry 2012 |
