Self-repairable copolymers that change color†
Dhanya
Ramachandran
,
Fang
Liu
and
Marek W.
Urban
*
Shelby F. Thames Polymer Science Research Center, School of Polymers and High Performance Materials, The University of Southern Mississippi, Hattiesburg, Mississippi 39406, USA
First published on 10th November 2011
Abstract
Although the last decade has brought self-healing materials to the forefront of scientific interests, combining repair and sensing attributes into one material entity have not been addressed. These studies report the development of poly(methyl methacrylate/n-butylacrylate/2-[(1,3,3-trimethyl-1,3-dihydrospiro[indole-2,3′-naphtho[2,1-b][1,4]oxazin]-5-yl)amino]ethyl-2-methylacrylate) [p(MMA/nBA/SNO)] copolymer films that upon mechanical scratch undergo color changes from clear to red in the damaged area, but upon exposure to sunlight, temperature and/or acidic vapors, the damaged area is self-repaired and the initial colorless appearance is recovered. The process is reversible and driven by the ring-opening-closure of spironapthoxazine (SNO) segments to form merocyanine (MC), which are recovered back to the SNO form. Upon mechanical damage, SNO segments of the neighboring copolymer segments form inter-molecular H-bonding that stabilizes copolymer backbone, that remains in an extended conformation. External stimuli, such as light, temperature, or acidic environments cause a dissociation of the H-bonded MC pairs, which are converted back to SNO. This process is associated with the p(MMA/nBA/SNO) backbone collapse, thus pulling entangled neighboring copolymers to fill removed mass and repair a scratch. Mechanical nano-indentation analysis combined with molecular modeling and spectroscopic measurements confirm this behavior. The enclosed video clip illustrates molecular repair processes induced by visible light monitored by in situRaman imaging spectroscopy. These materials may find numerous future applications, where coupling of simultaneous color changes and reversible self-repair responses may lead to new technological paradigms.
Introduction
Although Mother Nature has the unique ability of rebuilding damage that often involves a cascade of complex biochemical events leading to physical changes, attempts at mimicking these processes are not trivial. Perhaps a simple skin cut, which triggers blood clotting and subsequent healing, represents an illustrative example of color changes that parallel self-repair. In spite of the fact that there are no synthetic materials capable of mimicking these events, simultaneous color changes induced by mechanical damages, followed by externally stimulated self-repair may be advantageous in many existing and future materials technologies. If such materials were available, designing and engineering unique devices with built-in molecular sensors capable of photochromic self-repairing responses could be possible, offering not only new application venues, but extending lifetimes of existing materials by maintaining their original beauty and integrity. These may include composite structures with built-in molecular sensors, allowing early assessments and prevention of potentially catastrophic events or molecular sensors capable of responding to an array of external or internal stimuli triggering other events. Furthermore, design and engineering of mechano-mutable devices, or even prevention of skin aging may be quite feasible.Recent studies have demonstrated that using an orchestrated choice of reactive components in polymeric matrices it is possible to achieve self-repairable networks1,2 or formulate multi-component materials with self-healing properties.3,4,5 However, photochromic sensing of damages and simultaneous self-healing have not been exploited. In this context excellent mechanochromic polymer blends6,7,8 and thermosets9,10 have been developed, and independently of these advances, self-healing concepts have been explored, ranging from covalent11 to ionic12 or coordination chemistries,13 to encapsulation of reactive components,14 or shape memory polymers.15 This study departs from these approaches and reports the development of poly(methyl methacrylate/n-butylacrylate/1, 3-dihydro-1, 3, 3-trimethylspiro[2H-indole-2, 3′-[3H]-naphth[2, 1-b][1, 4]-oxazine]-2-amino-2-methylacrylate) [p(MMA/nBA/SNO)] copolymer colloidal particles that, upon coalescence, form films capable of sensing color changes upon mechanical scratches, but upon exposure to the visible (VIS) portion of the electromagnetic radiation (∼580 nm), temperature, and/or acidic atmospheres, not only mechanical damage is repaired, but also mechanically induced red color scar vanishes. Fig. 1, A and B, illustrate the synthesis of 2-[(1,3,3-trimethyl-1,3-dihydrospiro[indole-2,3′-naphtho[2,1-b][1,4]oxazin]-5-yl)amino]ethyl 2-methylacrylate (SNO) monomer (A) and copolymerization of methyl methacrylate (MMA), n-butyl acrylate (nBA) and SNO monomers to form poly(methyl methacrylate/n-butylacrylate/2-{[1,3,3-trimethyl-2-({[(1-Z)-2-oxonaphthalen-1(2H)ylidene] amino}methylene)-2,3-dihydro-1H-indol-5-yl]amino}ethyl-2-methylacrylate) [p(MMA/nBA/MC)] random copolymer (B) in the following monomer molar ratio: 0.07/0.05/0.0013. As will be seen, ring opening-closure of spironapthoxazine (SNO) units initiates the process, which triggers backbone rearrangements of this high molecular weight copolymer.
![A. Synthesis of 2-[(1,3,3-trimethyl-1,3-dihydrospiro[indole-2,3′-naphtho[2,1-b][1,4]oxazin]-5-yl)amino]ethyl 2-methylacrylate (SNO) monomer. B. Copolymerization of methylmethacrylate (MMA), n-butyl acrylate (nBA), and 2-[(1,3,3-trimethyl-1,3-dihydrospiro[indole-2,3′-naphtho[2,1-b][1, 4]oxazin]-5-yl)amino]ethyl-2-methylacrylate (SNO) monomers in 0.07/0.05/0.0013 molar ratios resulted in pink color open ring poly(methyl methacrylate/n-butylacrylate/2-{[1,3,3-trimethyl-2-({[(1-Z)-2-oxonaphthalen-1(2-H)-ylidene]amino}methylene)-2,3-dihydro-1-H-indol-5-yl]amino}ethyl-2-methylacrylate)p(MMA/nBA/MC) copolymer, which was then heated at 95 °C for 5 min to obtain colorless films.](/image/article/2012/RA/c1ra00137j/c1ra00137j-f1.gif) | ||
| Fig. 1 A. Synthesis of 2-[(1,3,3-trimethyl-1,3-dihydrospiro[indole-2,3′-naphtho[2,1-b][1,4]oxazin]-5-yl)amino]ethyl 2-methylacrylate (SNO) monomer. B. Copolymerization of methylmethacrylate (MMA), n-butyl acrylate (nBA), and 2-[(1,3,3-trimethyl-1,3-dihydrospiro[indole-2,3′-naphtho[2,1-b][1, 4]oxazin]-5-yl)amino]ethyl-2-methylacrylate (SNO) monomers in 0.07/0.05/0.0013 molar ratios resulted in pink color open ring poly(methyl methacrylate/n-butylacrylate/2-{[1,3,3-trimethyl-2-({[(1-Z)-2-oxonaphthalen-1(2-H)-ylidene]amino}methylene)-2,3-dihydro-1-H-indol-5-yl]amino}ethyl-2-methylacrylate)p(MMA/nBA/MC) copolymer, which was then heated at 95 °C for 5 min to obtain colorless films. | ||
Results and discussion
Fig. 2, A-1, A-2 and A-3 illustrates optical images of colorless p(MMA/nBA/SNO) film (A-1) which was mechanically damaged to result in a 10 μm wide scratch (A-2), followed by exposure to VIS radiation or temperature (A-3). As seen, initially colorless‡ area a (A-1) turns reddish upon mechanical damage (A-2-a′), but when exposed to VIS radiation (∼580 nm) or temperature (T = 95 °C), turns back to its original undamaged and colorless state (A-3-a′′). The undamaged area labeled b, b′ and b′′ remains unchanged. Exposure to acidic vapors turns the entire film reddish (A-4), but the damaged area a′′′ (A-4) is being repaired, and the original colorless state (A-3) can be retrieved back upon exposure to VIS radiation (∼580 nm) or temperature (T = 95 °C).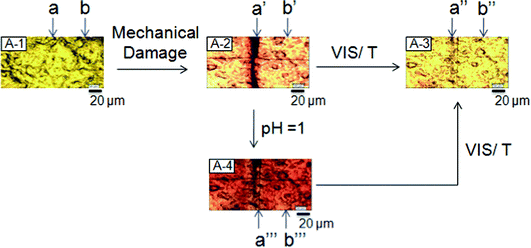 | ||
| Fig. 2 Optical images of p(MMA/nBA/SNO) films: undamaged (A-1), mechanically damaged (A-2), after exposure to VIS radiation or temperature (A-3) and after exposure to acidic vapors (A-4). Repair is achieved by either one of these conditions: visible light, temperature, or acidic pH vapors. | ||
With these macroscopic observations in mind we examined molecular processes responsible for this behavior by monitoring responses to electromagnetic radiation, temperature and acidic environments. Fig. 3, A-1 shows the optical image of p(MMA/nBA/SNO) scratched film and images A-2 and A-3 illustrate specimens after 30 (t) and 60 (t1) minutes exposure to 580 nm radiation. For reference purposes, the undamaged area is labeled b, b′ and b′′. The repair process was monitored using Raman imaging, in which we measured spatial distributions of the intensity changes of the conjugated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C band (1638 cm−1) of the ring-opened MC groups16 before (B-1-a) and after exposure to 580 nm radiation for 30 (t, B-2-a′) and 60 (t1,B-3-a′′) minutes. While navy blue areas correspond to low C
C band (1638 cm−1) of the ring-opened MC groups16 before (B-1-a) and after exposure to 580 nm radiation for 30 (t, B-2-a′) and 60 (t1,B-3-a′′) minutes. While navy blue areas correspond to low C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C intensities (ring open), red colors represent high intensities (ring closed). When damaged film (B-1-a) is exposed to 580 nm radiation, the intensity of the 1648 cm−1 band diminishes to minimum in the damaged area, as signified by the disappearance of navy blue at the expense of red color (B-3-a′′). The enclosed video clip illustrates progression of the repair as a function of time (ESI†).
C intensities (ring open), red colors represent high intensities (ring closed). When damaged film (B-1-a) is exposed to 580 nm radiation, the intensity of the 1648 cm−1 band diminishes to minimum in the damaged area, as signified by the disappearance of navy blue at the expense of red color (B-3-a′′). The enclosed video clip illustrates progression of the repair as a function of time (ESI†).
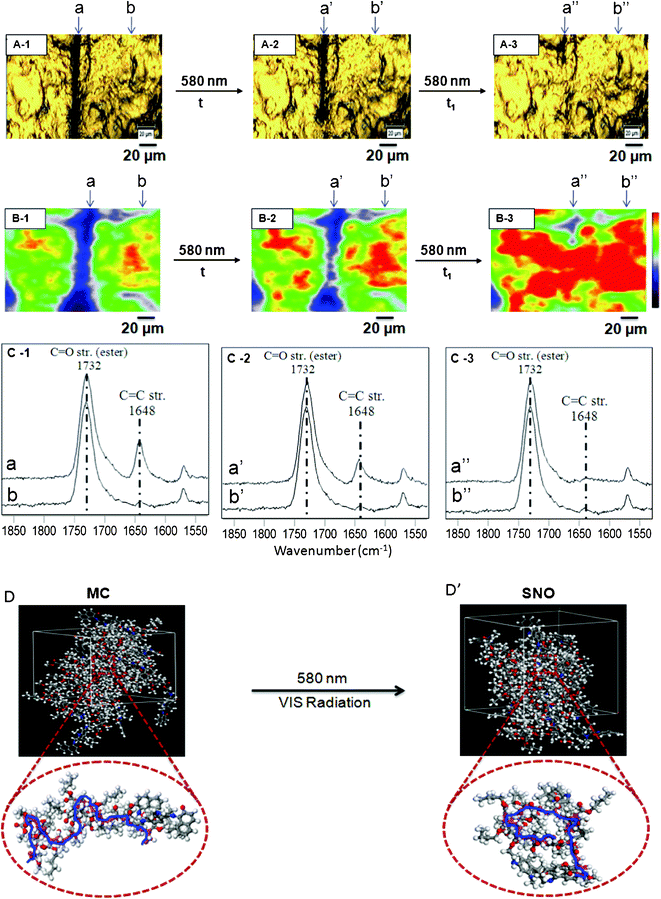 | ||
| Fig. 3 Optical images of p(MMA/nBA/SNO) films: mechanically damaged (A-1), exposure to VIS radiation (A-2, A-3). Raman images of p(MMA/nBA/SNO) film: mechanically damaged (B-1), exposure to VIS radiation (B-2, B-3). RamanSpectra of p(MMA/nBA/SNO) film: mechanically damaged (C-1), exposure to VIS radiation (C-2, C-3). Molecular modeling simulations of p(MMA/nBA/SNO) film: mechanically damaged (D) and repaired (D’) upon exposure to VIS radiation. | ||
To identify what molecular entities are responsible for simultaneous self-repair and color changes in localized areas of damage and repair we utilized spatially resolved Raman measurements and follow molecular events associated with ring-opening and closure (MC-to-SNO). Fig. 3, C-1, C-2 and C-3: traces a, a′ and a′′ illustrate Raman spectra collected from the areas labeled a, a′ and a′′ of B-1, B-2 and B-3, whereas traces b, b′ and b′′ are Raman spectra obtained from the undamaged areas labeled b, b′ and b′′ of B-1, B-2 and B-3. The band at 1732 cm−1 due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibrations of methacrylate components of p(MMA/nBA/SNO) served as a normalization band. As seen in Fig. 3, C-1, C-2, and C-3, trace b, b′ and b′′ (C-1, C-2, C-3) shows no intensity change of the 1648 cm−1 band indicating that in order for the ring to open, mechanical damage must occur. Fig. 3, trace a (C-1) shows the 1648 cm−1 band increase, indicating SNO-to-MC conversion.
O vibrations of methacrylate components of p(MMA/nBA/SNO) served as a normalization band. As seen in Fig. 3, C-1, C-2, and C-3, trace b, b′ and b′′ (C-1, C-2, C-3) shows no intensity change of the 1648 cm−1 band indicating that in order for the ring to open, mechanical damage must occur. Fig. 3, trace a (C-1) shows the 1648 cm−1 band increase, indicating SNO-to-MC conversion.
In order to convert MC to SNO, mechanically damaged films were exposed to 580 nm radiation. As seen in Fig. 3, after 30 min exposure, the intensity of the band at 1648 cm−1 decreases (trace a′, C-2), but upon further exposure for 60 min, the band disappears (trace a′′, C-3), thus manifesting MC-to-SNO conversion. To further realize molecular changes resulting from this process and its influence on the dynamics of the copolymer backbone, computer simulations were conducted. Details of the modeling experiments are outlined in the ESI.† When SNO units of p(MMA/nBA/SNO) are in the MC form, the polymer backbone (MW ∼8 × 106), which is primarily composed of MMA and nBA, exhibits a random coil-like conformation shown in Fig. 3-D. However, when the SNO form is present, the polymer backbone collapses (Fig. 3-D’). While the details of these polymer backbone changes will be further analyzed and discussed, they occur only in mechanically damaged areas, where MC-to-SNO conversions take place. With the exception of minute differences, the same observations are detected for temperature (95 °C) exposure, which are outlined and summarized in Fig. 4.
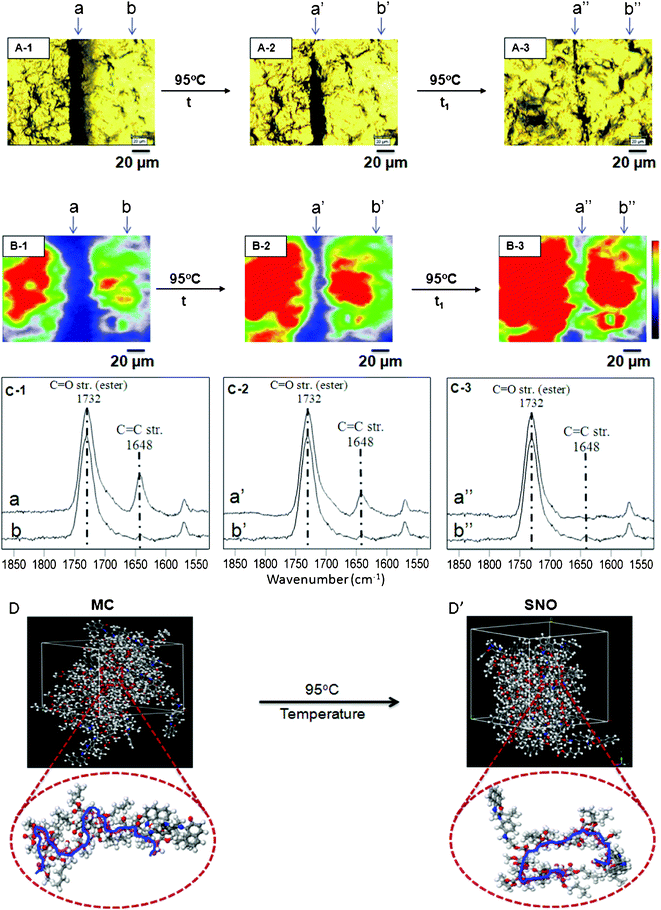 | ||
| Fig. 4 Optical images of p(MMA/nBA/SNO) films: mechanically damaged (A-1), exposure to temperature (A-2, A-3). Raman images of p(MMA/nBA/SNO) film: mechanically damaged (B-1), exposure to temperature (B-2, B-3). RamanSpectra of p(MMA/nBA/SNO) film: mechanically damaged (C-1), exposure to temperature (C-2, C-3). Molecular modeling simulations of p(MMA/nBA/SNO) film: mechanically damaged (D) and repaired (D’) upon exposure to temperature. | ||
As mentioned in the context of Fig. 2, exposure to acidic environments results in self-repair, but the entire film became reddish, which turns back to its colorless form upon exposure to VIS radiation or temperature. Fig. 5, A-1 shows optical images of p(MMA/nBA/SNO) scratched films, whereas the images A-2 and A-3 illustrate the same specimens after 30 (t) and 60 (t1) min of exposure to acidic vapors (pH = 1). The undamaged areas are labeled b, b′ and b′′′, and after 10 min exposure, the entire film turned reddish. Again, molecular events associated with this process were monitored using Raman imaging before (B-1) and after (B-2, B-3) exposure to acidic atmosphere. The Raman spectra collected from the undamaged areas b′ and b′′′ (C-2 and C-3) illustrate that the 1648 cm−1 band intensity increases, indicating SNO-to-MC hydrochloride (MC–HCl) conversion. However, no spectroscopic changes were observed for the band at 1648 cm−1 recorded from the areas a′ and a′′′ (C-2 and C-3), manifesting in the presence of the MC–HCl form after 30 and 60 min exposure. Again, knowing experimental outcomes of the MC to MC–HCL conversion and using the copolymer composition obtained in synthetic efforts, we utilized these results as an input for molecular modeling experiments. The results of modeling are illustrated in Fig. 5, D and D’ and show that, under these conditions, it is the MC–HCl form that causes the p(MMA/nBA) backbone to collapse which is also associated with repair and color changes detected in Raman spectra (ESI;† Fig. S-5, S-6, S-7). The primarily spectroscopic changes involve the following molecular components of the SNO, MC and MC–HCl units of p(MMA/nBA/SO) copolymer which are manifested by the intensity changes of the C–O–C, N–CH3, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H vibrations at 3027, 2773 and 1390 cm−1, respectively.
C–H vibrations at 3027, 2773 and 1390 cm−1, respectively.
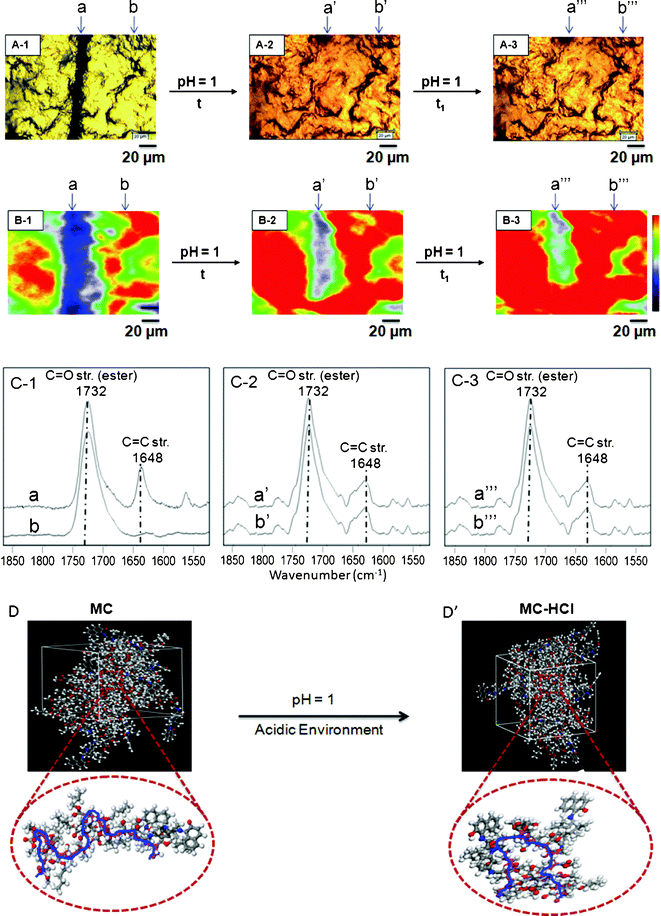 | ||
| Fig. 5 Optical images of p(MMA/nBA/SNO) films: mechanically damaged (A-1), exposure to acid environment (A-2, A-3). Raman images of p(MMA/nBA/SNO) film: mechanically damaged (B-1), exposure to acid environment (B-2, B-3). Raman spectra of p(MMA/nBA/SNO) film: mechanically damaged (C-1), exposure to acid environment (C-2, C-3). Molecular modeling simulations of p(MMA/nBA/SNO) film: mechanically damaged (D) and repaired (D’) upon exposure to acid environment. | ||
The results of the experiments described in Fig. 3–5 indicate that the MC-to-SNO transitions play a major role in color changes, but their function as a repairing entity of the entire p(MMA/nBA/SNO) copolymer film is not obvious. While the origin of macroscopic remodeling of the copolymer induced by VIS radiation, temperature and acid exposures will be further analyzed later on, let us examine mechanical responses from the areas of interest before and after self-repair by measuring loss moduli and nano hardness.17Fig. 6-I, A-1, A-2 and A-3, illustrates optical images of mechanically damaged and repaired p(MMA/nBA/SNO) films, where a, a′ and a′′ represent the areas subjected to nanoindentation before and after repair. Fig. 6-II, A, B, and C, show the results of nanoindentation measurements, where each cycle a, a′, and a′′ illustrates the force (μN) plotted as a function of displacement (nm) of undamaged (A-1), mechanically damaged (A-2) and repaired (A-3) film surfaces exposed to VIS radiation (A), temperature (B) and acid environments (C). Compared to the initial values of 28.7 (A), 28.9 (B) and 28.6 (C) MPa in the undamaged areas (a), the loss moduli in the damaged areas (a′) are diminished to 18.6 (A), 18.9 (B), and 18.5 (C) MPa, respectively. However upon repair, these values are recovered back to 27.0 (A), 27.4 (B) and 28.1 (C) MPa (a′′), respectively. The same trends are observed for nano-hardness measurements which initially were at 5.1, 5.2 and 5.1 MPa, to be reduced to 2.4, 2.35, 2.38 MPa upon scratch, and recovered to 4.1, 4.5 and 4.9 MPa. While Table S-1 of the ESI† provides further details of the nano-indentation experiments, these data indicate mechanical recovery in the damaged area.
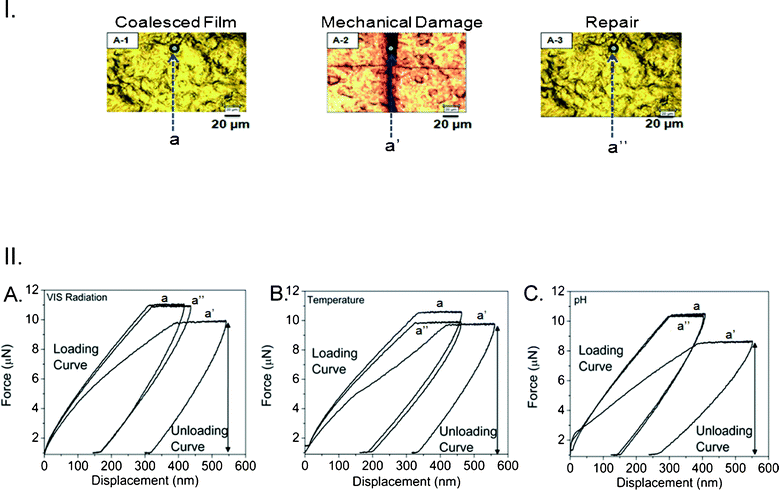 | ||
| Fig. 6 I. Optical images of p(MMA/nBA/SNO) films: coalesced (A-1), mechanically damaged (A-2) and repaired upon exposure to acid environment (A-3). II. Force (μN) plotted as a function of displacement (nm) for p(MMA/nBA/SNO) films: before damage (a); after damage (a’); after repair (a’’) upon exposure to VIS radiation (A), temperature (B) and acidic atmosphere (C). (loading conditions for cycles a, a’, and a’’: maximum force: 10, 10, and 8 μN, respectively; loading time: 15 s.) | ||
With these data in mind, let us go back to the results shown in Fig. 3–5 and realize that the MC-to-SNO conversion parallels p(MMA/nBA/SNO) backbone collapse. Considering that MW of p(MMA/nBA/SNO) is ∼8 × 106, there are significant entanglements which are reflected in simulation experiments. Furthermore, MC-to-SNO localized conversions result in going from non-bonded to bonded distances, thus enabling the access of free volume for collapse of p(MMA/nBA/SNO) backbone which, in turn, pulls entangled chains, thus filling mechanical displaced material which is amplified by a strong MC form efficacy for H-bonding. In view of these considerations Fig. 7 illustrates the summary of the simulation experiments of damaged p(MMA/nBA/MC) films, where inter-molecular H-bonding of MC groups of the neighboring chains parallels the formation of extended backbone conformations. Upon exposure to VIS radiation, temperature and acid environments, these interactions are disturbed by the ring closure, which ultimately leads to localized backbone collapse facilitated by the free volume due to MC-to-SNO localized conversion and subsequent collapse of the polymer backbone. This is illustrated in Fig. 7, B, C, and D for VIS radiation, temperature and acid exposure.
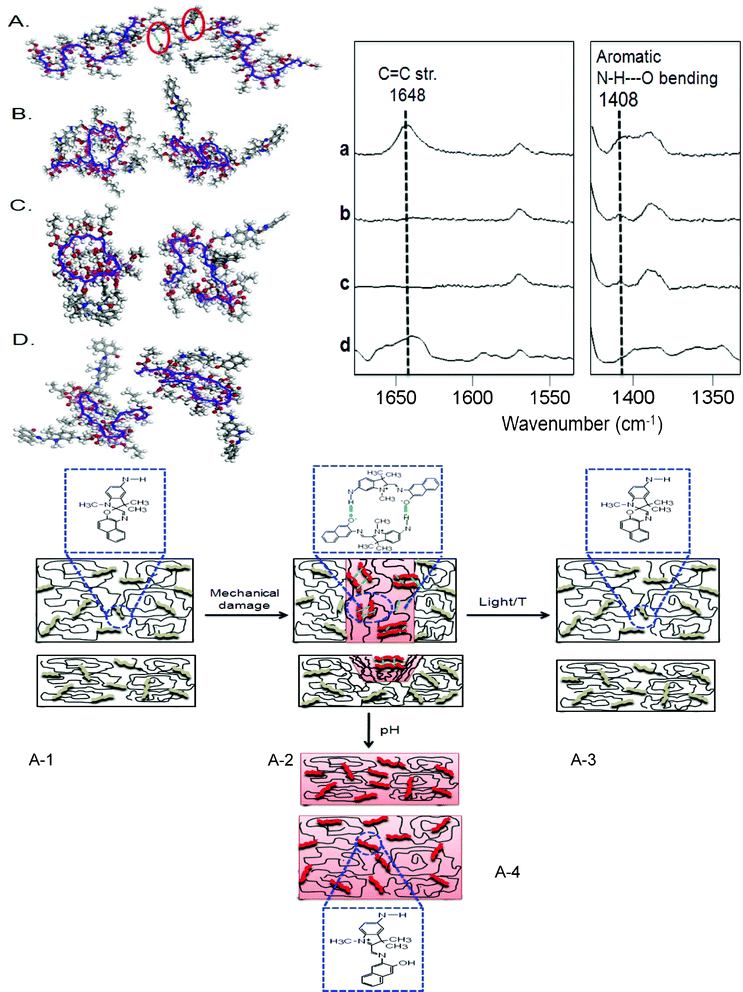 | ||
| Fig. 7 Molecular modeling simulations of p(MMA/nBA/SNO) films: mechanically damaged (A), exposure to VIS radiation (B), temperature (C) and acidic environment (D). Raman spectra of p(MMA/nBA/SNO) films: mechanically damaged (a), exposure to VIS radiation (b), temperature (c) and acidic environment (d); Schematic representation of p(MMA/nBA/SNO) films: undamaged (A-1), damaged (A-2), exposure to VIS radiation or temperature (A-3) and exposure to acidic environment (A-4). | ||
If indeed the formation of inter-molecular H-bonding parallels the extended coil copolymer backbone conformation, let us test this hypothesis experimentally. For that reason we collected Raman spectra illustrated in Fig. 7. Traces a, b, c, and d correspond to the results of the modeling experiments illustrated in Fig. 7, A, B, C, and D, and were collected before (a) damage and after repair upon exposure to VIS radiation (b), temperature (c) and acid exposures (d). As shown, the bands at 1648 and 1408 cm−1 are detected in trace a and are due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching and N–H–O bending vibrations attributed to the presence of conjugated structure of the open ring MC form and secondary amine groups, respectively. Upon repair (traces b, c and d), the conjugated MC form is converted to SNO, and the H-bonded secondary amine groups of the neighboring MC units are dissociated, as manifested by the intensity decrease of the 1408 cm−1 band. Schematic representation of these events is depicted in Fig. 7, A-1, A-2, A-3 and A-4, where the presence of closed ring SNO parallels a collapse state of the copolymer backbone, facilitating repair by pulling entanglements to fill the volume resulting from prior damage.
C stretching and N–H–O bending vibrations attributed to the presence of conjugated structure of the open ring MC form and secondary amine groups, respectively. Upon repair (traces b, c and d), the conjugated MC form is converted to SNO, and the H-bonded secondary amine groups of the neighboring MC units are dissociated, as manifested by the intensity decrease of the 1408 cm−1 band. Schematic representation of these events is depicted in Fig. 7, A-1, A-2, A-3 and A-4, where the presence of closed ring SNO parallels a collapse state of the copolymer backbone, facilitating repair by pulling entanglements to fill the volume resulting from prior damage.
In summary, these studies illustrate for the first time that simple copolymerization of film forming latex components (MMA and nBA) along with minute quantities of SNO lead to stimuli-responsive materials capable of simultaneous and controllable repairs as well as coloration/discoloration. These materials will have significant impact on many existing and emerging technologies where pairing of mechanical and photochromic responses will bring new dimensions to materials engineering.
Acknowledgements
These studies were supported by the office of Naval Research Award # N00014-07-1-1057. Partial support from the National Science Foundation MRSEC program under award no. DMR 0213883 and partially by the DMR 0215873 Major Research Instrumental (MRI) program is acknowledged. The authors are also thankful to Dr S. Morgan's lab for help in nano-indentation measurements.References
- B. Ghosh and M. W. Urban, Self-Repairing Oxetane-Substituted Chitosan Polyurethane Networks, Science, 2009, 323, 1458 CrossRef CAS.
- X. Chen, M. A. Dam, K. Ono, A. Mal, H. Shen, S. R. Nutt, K. Sheran and F. Wudl, A thermally re-mendable cross-linked polymeric material, Science, 2002, 295, 1698 CrossRef CAS.
- C. Dry, Procedures developed for self-repair of polymer matrix composite materials, Compos. Struct., 1996, 35, 263 CrossRef.
- M. R. Kessler and S. R. White, Self-activated healing of delamination damage in woven composites, Composites, Part A, 2001, 32, 683 CrossRef.
- B. Ghosh and M. W. Urban, Self-Repairing Polymeric Materials, Chap. 1 in, Handbook on Stimuli-Responsive Polymers, Ed. M. W. Urban, Wiley-VCH, 2011 Search PubMed.
- B. Crenshaw, M. Burnworth, D. Khariwala, P. A. Hiltner, P. T. Mather, R. Simha and C. Weder, Deformation-Induced Color Changes in Mechanochromic Polyethylene Blends, Macromolecules, 2007, 40, 2400 CrossRef CAS.
- J. Kunzelman, T. Chung, P. T. Mather and C. Weder, Shape Memory Polymers with built-in Threshold Tempeature Sensors, J. Mater. Chem., 2008, 18, 1082 RSC.
- A. Davis Douglas, Andrew Hamilton, Jinglei Yang, D. Cremar Lee, Dara Van Gough, L. Potisek Stephanie, T. Ong Mitchell, V. Braun Paul, J. Martínez Todd, R. White Scott, S. Moore Jeffrey and R. Sottos Nancy, Force-induced activation of covalent bonds in mechanoresponsive polymeric materials, Nature, 2009, 459, 68 CrossRef.
- K. Lee Corissa, A. Davis Douglas, R. White Scott, S. Moore Jeffrey, R. Sottos Nancy and V. Braun Paul, Force-Induced Redistribution of a Chemical Equilibrium, J. Am. Chem. Soc., 2010, 132, 16107 CrossRef.
- Greg O'Bryan, M. Wong Bryan and R. McElhanon James, Stress Sensing in Polycaprolactone Films via an Embedded Photochromic Compound, ACS Appl. Mater. Interfaces, 2010, 2, 1594 CAS.
- Yoshifumi Amamoto, Jun Kamada, Hideyuki Otsuka, Atsushi Takahara and Krzysztof Matyjaszewski, Repeatable Photoinduced Self-Healing of Covalently Cross-Linked Polymers through Reshuffling of Trithiocarbonate Units, Angew. Chem., Int. Ed., 2011, 50, 1660 CrossRef CAS.
- Philippe Cordier, François Tournilhac, Corinne Soulié-Ziakovic and Ludwik Leibler, Self-healing and thermoreversible rubber from supramolecular assembly, Nature, 2008, 451, 977 CrossRef CAS.
- Niels Holten-Andersena, J. Harrington Matthew, Henrik Birkedal, P. Leed Bruce, B. Messersmith Phillip, C. Lee Ka Yee and J. Herbert Waite, pH-induced metal–ligand cross-links inspired by mussel yield self-healing polymer networks with near-covalent elastic moduli, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 2651 CrossRef.
- S. R. White, N. R. Sottos, P. H. Geubelle, J. S. Moore, M. R. Kessler, S. R. Sriram, E. N. Brown and S. Viswanathan, Autonomic healing of polymer composites, Nature, 2000, 409, 794 CrossRef.
- M. W. Urban, Handbook of Stimuli-Responsive Materials, Wiley-VCH, 2011 Search PubMed.
- Masao Takayanagi, Munetaka Nakata, Yukihiro Ozaki, Keiji Iriyama and Mitsuo Tasumi, Intermolecular interactions of merocyanine dyes studied by visible absorption, resonance Raman and infrared spectroscopies, J. Mol. Struct., 1997, 407, 85 CrossRef CAS.
- C. C. Corten and M. W. Urban, Repairing Polymers Using Oscillating Magnetic Field, Adv. Mater., 2009, 21, 5011 CrossRef CAS.
Footnotes |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c1ra00137j/ |
| ‡ Films are actually clear, but appear yellowish due to light exposure. |
| This journal is © The Royal Society of Chemistry 2012 |
