A facile synthesis of a novel triptycene-containing A–B monomer: precursor to polymers of intrinsic microporosity†
Bader S.
Ghanem
*
Department of Chemistry, Faculty of Science, Taibah University, P.O. Box 30002, Almadinah Almunawarah, Saudi Arabia. E-mail: bganem@taibahu.edu.sa; bsghanem@hotmail.com
First published on 26th October 2011
Abstract
A new synthetic approach affords the first example of a triptycene-containing A–B monomer bearing both phenazine activating aromatic o-dichloride and a catecholgroup which can be used for the synthesis of a novel polymer of intrinsic microporosity (PIM) has been developed. The new polymer was characterized by GPC, IR, and 1H NMR, elemental analysis and demonstrated good porosity, thermal stability and solubility in common organic solvents such as chloroform from which a self-supporting and transparent film could be cast.
Over the past few decades, extensive research in the field of microporous materials (pore size < 2 nm) has been conducted. These materials have attracted great interest by chemists and material chemists due to their technological importance for various applications and commercial interests.1 Recently, purely organic materials with network and non-network structures, called polymers of intrinsic microporosity (PIMs)2 have been extensively studied and show high internal surface area, solubility and thermal stability. Applications of PIMs include adsorption,3 gas and liquid separation,4hydrogenstorage5 and catalysis.6 The microporosity in PIMs is intrinsic due to their highly rigid (no single bonds about which free rotation can occur) and contorted molecular structures which prohibit close packing of the polymer chains and trap sufficient internal free volume resulting in the formation of porous materials with relatively high surface area. The major advantage of PIMs over the conventional porous materials (zeolites and activated carbon) is the great ability to tailor these materials for various applications through incorporation of different functional groups in the polymerrepeat unit. In principle, PIMs can be prepared viapolycondensation reaction (a double aromatic nucleophilic substitution) using a combination of a bifunctional hydroxylated aromatic monomer and an activated fluorinated or chlorinated aromatic monomer to form rigid dibenzodioxane linking groups. For example, soluble non-network polymersPIM-1 and PIM-7 were prepared by the polymerization reaction between the commercially available 5,5′6,6′-tetrahydroxy-3,3,3′3′-tetramethyl-1,1′-spirobisindane (1) and the activated tetrafluoroterephthalonitrile monomer (2) or the specially prepared tetrachloride monomer (3) in basic medium, respectively (Scheme 1). Analysis by N2 adsorption demonstrated that these materials are microporous with surface areas of >700 m2 g−1 as calculated using the Brunauer–Emett–Teller (BET) method. The microporosity in these polymers is due to the great rigidity and the non-planar moiety resulting from the presence of the spiro-centre as a site of contortion.7
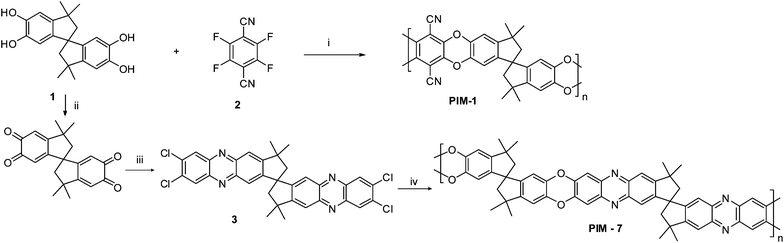 | ||
| Scheme 1 Synthesis of PIM-1 and PIM-7. Reagents and conditions: (i) K2CO3, DMF, 65 °C, 48 h; (ii) nitric acid, AcOH, C2H5OH, 0 °C, 24 h; (iii) 4,5-dichloro-1,2-diaminobenzene, AcOH, reflux, 3 h; (iv) biscatechol (A), K2CO3, 18-crown-6, DMF, 150 °C, 48 h. | ||
Recently, the synthesis of triptycene-containing insoluble network PIMs with a different alkyl chain length at the bridgehead position of the triptycene and which exhibited high apparent surface area ranging from 618 (R = octyl) to 1760 m2 g−1 (R = methyl) was reported. Interestingly, these materials showed a high uptake of hydrogen when R = methyl (about 3% by mass at 10 bar).5b
Despite the tremendous progress in the field of PIMs synthesis, there is still a need for the development of these materials that can exhibit higher free volume and which can be tailored for more applications. This can be accomplished via designing new monomers that can be utilized for their synthesis. Described herein is the facile synthesis and characterization of a novel 9,10-dibutyl triptycene–based A–B monomer 8 containing phenazine units and its use as a precursor for the synthesis of PIMs. It is noteworthy that this triptycene-containing monomer is suitable for making PIMs, since both a catecholgroup and an aromatic dichloride unit, suitably activated towards nucleophilic aromatic substitution reaction by the pyrazine ring in the phenazine structure, are present in the same molecule.7b Such an A–B monomer has a significant advantage in step-growthpolymerization over AA–BB used previously in the preparation of PIMs, due to the lack of the requirement for strict control over stoichiometric balance for attaining high molecular weight polymer. Triptycene is an attractive building block for the construction of microporous organic materials due to its high ‘internal free volume’.8 Moreover, it has been proven that triptycene derivatives can serve as ‘spacers’ in guest–host complexes,14,15 chemical and biological activities,16,17 liquid crystalline materials18,19 and molecular rotors/gyroscopes.20,21
The A–B monomer 8 was synthesised using a multi-step synthetic sequence as depicted in Scheme 2. First, 9,10-dibutyl-2,3,6,7-tetramethoxyanthracene4 was prepared according to the procedure described by Shklyaev et al. via the acid mediated reaction of veratrol (1,2-dimethoxybenzene) with the valeraldehyde.10 By adapting Zong's procedure, 9,10-dibutyl-2,3,6,7-tetramethoxytriptycene5 was prepared in good yield by the conventional Diels–Alder reaction between 9,10-dibutyl-2,3,6,7-tetramethoxyanthracene and the benzyne formed in-situ from 2-amino-4,5-dimethoxy benzoic acid.11 The o-quinone6 was prepared as a dark red solid in good yield by selective oxidation of 5 with dilute nitric acid in dichloromethane and glacial acetic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) using the procedure of Zhao et al.12 Finally, condensation reaction of the o-quinone6 with 4,5-dichloro-1,2-diaminobenzene in refluxing glacial acetic acid afforded compound 7 in good yield, which was then demethylated with BBr3 in CH2Cl2 to give the corresponding 1,2-hydroxylated triptycene 8. The structures of all new compounds were confirmed by 1H and 13C NMR, FTIR, MSspectra and elemental analysis.
1, v/v) using the procedure of Zhao et al.12 Finally, condensation reaction of the o-quinone6 with 4,5-dichloro-1,2-diaminobenzene in refluxing glacial acetic acid afforded compound 7 in good yield, which was then demethylated with BBr3 in CH2Cl2 to give the corresponding 1,2-hydroxylated triptycene 8. The structures of all new compounds were confirmed by 1H and 13C NMR, FTIR, MSspectra and elemental analysis.
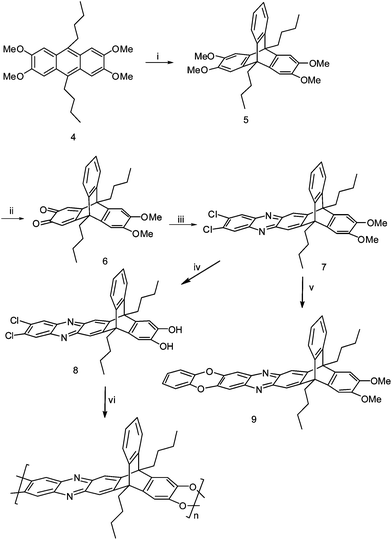 | ||
Scheme 2
Reagents and conditions: (i) 2-carboxy-benzenediazonium chloride, 1,2-dichloroethane, propylene oxide, reflux, 4 h; (ii) 0.25 M HNO3, AcOH and CH2Cl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), 5 minutes; (iii) 4,5-dichloro-1,2-diaminobenzene, AcOH, reflux, 2 h; (iv) BBr3, CH2Cl2, 2 h. K2CO3, DMF, 150 °C; (v) catechol, K2CO3, DMF, 150 °C; (vi) K2CO3, DMF, 18-crown-6, 150 °C. 1, v/v), 5 minutes; (iii) 4,5-dichloro-1,2-diaminobenzene, AcOH, reflux, 2 h; (iv) BBr3, CH2Cl2, 2 h. K2CO3, DMF, 150 °C; (v) catechol, K2CO3, DMF, 150 °C; (vi) K2CO3, DMF, 18-crown-6, 150 °C. | ||
In order to confirm the feasibility of using the pyrazine-activated dichloride monomer in the dioxane formation reaction and to optimize the reaction conditions for polymerisation, the model compound 9 was prepared from the reaction of the catecholate anion with o-dichloro compound 7 in 91% yield (Scheme 2). This model compound also aids the structural characterization of the polymer derived from monomer 8. Similarly, the A–B monomer 8 was polymerized in anhydrous DMF containing 18-crown and excess anhydrous potassium carbonate (Scheme 2). The resulting polymer was isolated and purified by repeated precipitation from CHCl3 solution into methanol to give a yellow-orange powder with spectral properties corresponding to the expected structure that match the spectroscopic data of the model compound 9.13 The elemental analysis data of the polymer are in close agreement with the theoretical values. The 1H NMR corroborates the proposed structure and shows broad signals for the aromatic and aliphatic protons, indicating a high molecular weight polymer. The aromatic signals appear as three resolved broad absorptions at δ 7.07, 7.36, and 7.81 ppm, whereas the aliphatic ones range from δ 2.90–1.14 ppm. Infrared measurements showed diagnostic absorbances at 1290 for the C–O function, and 1419–1610 for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C skeletal bands. In addition, the polymer is readily soluble in chloroform from which a self-supporting and transparent film could be cast. The good solubility was attributed to the presence of a flexible side butyl chains and the highly rigid three-dimensional structure of the triptycene unit, which prevent close packing of the polymer chains. The gel permeation chromatography (GPC) measurements using chloroform as eluent demonstrated that the polymer exhibited reasonably high average molecular masses with Mn = 30
C skeletal bands. In addition, the polymer is readily soluble in chloroform from which a self-supporting and transparent film could be cast. The good solubility was attributed to the presence of a flexible side butyl chains and the highly rigid three-dimensional structure of the triptycene unit, which prevent close packing of the polymer chains. The gel permeation chromatography (GPC) measurements using chloroform as eluent demonstrated that the polymer exhibited reasonably high average molecular masses with Mn = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Mw = 56
000, Mw = 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and a polydispersity index of 1.9 as calibrated against polystyrene standards. Standard nitrogen adsorption analysis for the powdered polymer obtained at 77 K provides an apparent BET surface area of 523 m2 g−1 and significant adsorption at low relative pressure (p/p° < 0.1), which confirm that these materials are microporous (Fig. 1). TGA analysis (nitrogen) indicated that the polymer exhibited relatively high thermal stability and initial weight loss due to thermal degradation which commences at ∼460 °C.
000 g mol−1 and a polydispersity index of 1.9 as calibrated against polystyrene standards. Standard nitrogen adsorption analysis for the powdered polymer obtained at 77 K provides an apparent BET surface area of 523 m2 g−1 and significant adsorption at low relative pressure (p/p° < 0.1), which confirm that these materials are microporous (Fig. 1). TGA analysis (nitrogen) indicated that the polymer exhibited relatively high thermal stability and initial weight loss due to thermal degradation which commences at ∼460 °C.
 | ||
| Fig. 1 Nitrogen adsorption (solid circles) and desorption (open circles) isotherms at 77 K for the polymer obtained from A–B monomer 8. | ||
In summary, we have successfully developed an efficient synthetic route for the preparation of a novel 9,10-dibutyl triptycene-based A–B monomer that contains both a pyrazine-activated dichloridegroup and a catecholgroup and used it efficiently as a precursor for the synthesis of a non-network PIMs. All synthetic procedures are simple, efficient and are suitable for scale-up. Further study on the synthesis and the use of other 9,10-dialkyl precursors for preparation of PIMs is in progress. To the best of our knowledge, this report describes the first example of a triptycene A–B monomer to be used as a precursor for the synthesis of PIMs. It is also worth noting that the presence of phenazine subunits as N-donor ligands suggests the introduction of appropriate metal ions for potential catalytic activity.9
Acknowledgements
Financial support from Taibah University is gratefully acknowledged. The author would like to thank Dr Ziad Moussa and Professor Neil McKeown for useful discussions.Notes and references
- Handbook of Porous Solids, ed. F. Schuth, K. Sing and J. Weitkman, Wiley-VCH, Berlin, 2002, vol. 1–5 Search PubMed.
- (a) N. B. McKeown and P. M. Budd, Chem. Soc. Rev., 2006, 35, 675 RSC; (b) N. B. McKeown, P. M. Budd, B. S. Ghanem and K. J. Msayib, UK Pat., appl. 0317557, 2005; International pat., appl. PCT/GB2004/003166.
- (a) P. M. Budd, B. S. Ghanem, K. J. Msayib, N. B. McKeown and C. E. Tattershall, J. Mater. Chem., 2003, 13, 2721 RSC; (b) A. V. Maffei, P. M. Budd and N. B. McKeown, Langmuir, 2006, 22, 4225 CrossRef CAS.
- (a) P. M. Budd, K. J. Msayib, C. E. Tattershal, B. S. Ghanem, K. J. Reynolds, N. B. McKeown and D. J. Fritsch, J. Membr. Sci., 2005, 251, 263 CrossRef CAS; (b) B. S. Ghanem, N. B. McKeown, P. M. Budd, J. D. Selbie and D. J. Fritsch, Adv. Mater., 2008, 20, 2766 CrossRef CAS.
- (a) N. B. Mckeown, B. S. Ghanem, K. J. Msayib, P. M. Budd, C. E. Tattershal, K. Mahmoud, D. Book and A. Walton, Angew. Chem., Int. Ed., 2006, 45, 1804 CrossRef CAS; (b) B. S. Ghanem, M. Hashem, K. Harris, K. J. Msayib, M. Xu, P. M. Budd, N. Chaukura, D. Book, S. Tedds, A. Walton and N. B. McKeown, Macromolecules, 2010, 43, 5287 CrossRef CAS.
- H. J. Mackintosh, P. M. Budd and N. B. McKeown, J. Mater. Chem., 2008, 18, 573 RSC.
- (a) P. M. Budd, B. S. Ghanem, S. Makhseed, N. B. McKeown, K. J. Msayib and C. E. Tattershall, Chem. Commun., 2004, 230 RSC; (b) B. S. Ghanem, N. B. McKeown, P. M. Budd and D. Fritsch, Macromolecules, 2008, 41, 1640 CrossRef CAS.
- (a) B. S. Ghanem, K. J. Msayib, N. B. McKeown, K. D. M. Harris, Z. Pan, P. M. Budd, A. Butler, J. Selbie, D. Book and A. Walton, Chem. Commun., 2007, 67 RSC; (b) N. T. Tsui, A. J. Paraskos, L. Torun, T. M. Swager and E. L. Thomas, Macromolecules, 2006, 39, 3350 CrossRef CAS; (c) X. Z. Zhu and C. F. Chen, J. Org. Chem., 2005, 70, 917 CrossRef CAS; (d) C. E. Godinez, G. Zepeda and M. A. Garcia-Gariby, J. Am. Chem. Soc., 2002, 124, 4701 CrossRef CAS; (e) Z. Chen and T. M. Swager, Macromolecules, 2008, 41, 6880 CrossRef CAS.
- N. B. McKeown, P. M. Budd, K. J. Msayib, B. S. Ghanem, H. J. Kingston, C. E. Tattershall, S. Makhseed, K. J. Reynolds and D. Fritsch, Chem.–Eur. J., 2005, 11, 2610 CrossRef CAS.
- Yu. V. Shklyaev and Yu. V. Nifontof, Russ. Chem. Bull., 2002, 51, 844 CrossRef CAS.
- Q. S. Zong and C.-F. Chen, Org. Lett., 2006, 8, 211 CrossRef CAS.
- J.-M. Zhao, H.-Y. Lu, J. Cao and C.-F. Chen, Tetrahedron Lett., 2009, 50, 219 CrossRef CAS.
- See ESI†.
- M. E. Rogers and B. A. Averill, J. Org. Chem., 1986, 51, 3308 CrossRef CAS.
- X. Z. Zhu and C. F. Chen, J. Am. Chem. Soc., 2005, 127, 13158 CrossRef CAS.
- X. Z. Zhu and C. F. Chen, J. Org. Chem., 2005, 70, 917 CrossRef CAS.
- N. J. Xanthopoulou, A. P. Kourounakis, S. Spyroudis and P. N. Kourounakis, Eur. J. Med. Chem., 2003, 38, 621 CrossRef CAS.
- S. J. Norvez, J. Org. Chem., 1993, 58, 2414 CrossRef CAS.
- T. M. Long and T. M. Swager, J. Mater. Chem., 2002, 12, 3407–3412 RSC.
- C. E. Godinez, G. C. Zepeda, J. Mortko, H. M. A. Dang and M. A. Garcia-Garibay, J. Org. Chem., 2004, 69, 1652–1662 CrossRef CAS.
- S. M. Hou, T. Sagara, D. C. Xu, T. R. Kelly and E. Ganz, Nanotechnology, 2003, 14, 566 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1py00423a |
| This journal is © The Royal Society of Chemistry 2012 |
