Calix[4]pyrogallolarenes as novel high temperature inhibitors of oxidative degradation of polymers†
Przemyslaw
Ziaja
a,
Katarzyna
Jodko-Piorecka
a,
Rafal
Kuzmicz
b and
Grzegorz
Litwinienko
*a
aFaculty of Chemistry, University of Warsaw, Pasteura 1, 02-093, Warsaw, Poland. E-mail: litwin@chem.uw.edu.pl; Fax: +48 22 8222380; Tel: +48 22 8220211 ext. 333
bDepartment of Inorganic and Analytical Chemistry, Faculty of Pharmacy, Medical University of Warsaw, Banacha 1, 02-097, Warsaw, Poland
First published on 8th November 2011
Abstract
Oxidative stability of high density polyethylene (HDPE) containing C-alkylcalix[4]pyrogallolarenes was compared with the oxidative stability of HDPE containing 1,2,3-trihydroxybenzene (pyrogallol) and 2,6-di-tert-butyl-4-methylphenol (BHT). The Arrhenius kinetic parameters (Ea, Z, k) of non-isothermal thermo-oxidative decompositions indicate that C-alkylcalix[4]pyrogallolarenes are effective antioxidants acting at temperatures above 150 °C.
Oxidative decomposition of polymers is a relatively well understood process1 and this undesirable phenomenon has a crucial importance for quality of polymer products. Several classes of inhibitors are applied to improve thermal-oxidative stability of polymers,2 including phenolic antioxidants, hindered amines and organic–inorganic functional nanomaterials. Efficiency of these stabilizers is limited to moderate temperatures,3 thus, new, thermally stable and active antioxidants are needed in order to inhibit the oxidative degradation of polymers.2 In a few reports,4–6 the calix[n]arenes (where n = 4, 6 and 8) were introduced as stabilizing agents during thermal oxidation of polyethylene and polypropylene, however, there was no attempt to apply calix[n]pyrogallolarenes as antioxidants. This finding is surprising because calix[n]pyrogallolarenes are potential chain-breaking antioxidants due to a presence of 3nphenolic OHgroups.
Our preliminary measurements showed that calix[4]pyrogallolarenes are thermally stable and not volatile. ‡ Herein, we report the first experimental study in which the kinetic parameters of thermo-oxidative decomposition of high density polyethylene (HDPE) containing C-R-calix[4]pyrogallolarenes (compound A in Chart 1) were determined and compared with the activities of 1,2,3-trihydroxybenzene (pyrogallol, compound B in Chart 1) and 2,6-di-tert-butyl-4-methylphenol (BHT, compound C in Chart 1).
![Structures of: calix[4]pyrogallolarene (A), pyrogallol (B) and 2,6-di-tert-butyl-4-methylphenol (C). Symbols A1, A2, A3 in the text denote compound A with R = methyl, ethyl, undecyl, respectively.](/image/article/2012/PY/c1py00494h/c1py00494h-c1.gif) | ||
| Chart 1 Structures of: calix[4]pyrogallolarene (A), pyrogallol (B) and 2,6-di-tert-butyl-4-methylphenol (C). Symbols A1, A2, A3 in the text denote compound A with R = methyl, ethyl, undecyl, respectively. | ||
To demonstrate the antioxidant activity of the calix[4]pyrogallolarenes we determined the oxidative stability of polyethylene (HDPE) as polymer matrix. This commonly applied polyolefin is fully recyclable, the products made of HDPE can be thermally sterilized, but, unfortunately, this polymer is easily permeated by oxygen. Taking into account its high thermal stability, HDPE is an optimal model system for testing the antioxidative activity of additives at temperatures above 150 °C.
HDPE oxidation was monitored by Differential Scanning Calorimetry (DSC), a thermoanalytical technique that has been successfully applied by us7 and by other researchers8 for studies on the kinetics of non-isothermal oxidations of lipids and hydrocarbons. The first exothermal effect on the DSC curve corresponds to the primary autoxidation (i.e., production of hydroperoxides). The Arrhenius kinetic parameters of oxidation: activation energy (Ea) and pre-exponential factor (Z), were determined on the basis of the isoconversional Ozawa–Flynn–Wall (OFW) method9 not dependent on the kinetic model of thermal reaction.10
Temperatures of the extrapolated start of oxidation (Te, see Fig. 1), determined for several samples heated with different heating rates (β in K min−1), form a straight line dependence:
| log β = a/Te + b | (1) |
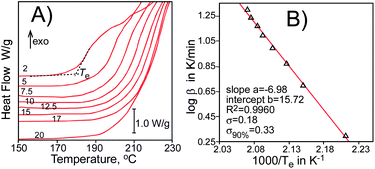 | ||
| Fig. 1 (A) DSC curves (shifted vertically for clarity) of thermooxidative degradation of pure HDPE (density 0.96 g cm−3, melting point 132–134 °C, powder). Heating rates, in K min−1, expressed as numbers. (B) Plot of log β versus 1000/Te with statistical parameters. σ90% is a standard deviation calculated at the level of confidentiality 90%. | ||
Application of eqn (1) for oxidation of HDPE (Fig. 1) gives Ea = 127 ± 6 kJ mol−1 and Z = 7.15 × 1013 s−1 (see Table S1 in the ESI†), that is in the middle of literature values (from 109 kJ mol−1 (ref. 3a) to 140 kJ mol−1 (ref. 11)) and in agreement with values for oxidation of saturated long chain fatty acid esters (115–130 kJ mol−1)7b,c and a bit higher than 108.8 kJ mol−1 reported for oxidation of n-octane.12 Korcek et al.13 determined value (Ep − 1/2Et) = 66 ± 2 kJ mol−1 (Ep and Et are the activation energies of propagation and termination, respectively) for autoxidation of n-hexadecane in the presence of tert-butyl hydroperoxide at 40–70 °C. The overall activation energy of autoxidation is Ea = Ep + ½(Ei − Et)12 and taking the activation energy of thermal initiation by decomposition of hydroperoxides, Ei = 146 kJ mol−1,14 the overall Ea for polyethylene autoxidation will be 139 kJ mol−1, also being in reasonable agreement with our results.
DSC thermograms of non-isothermal oxidation of composites § presented in Fig. 2A clearly demonstrate that compound B (pyrogallol) does not improve the oxidative stability of HDPE (i.e., Te does not increase). In contrast, addition of compound A results in a significant increase of Te. An increase of the concentration of compound B does not improve the oxidative stability, see values of log k for various concentrations, up to 0.21% (w/w) for compound B (see Fig. 3 and ESI†). Much better oxidative stability of HDPE/A systems in comparison to the HDPE/B system cannot be explained by difference in the amount of phenolic hydroxylgroups present in the same volume of polymer, because the HDPE sample containing 0.5% w/w of C-undecyl tetramer (sample 4, Fig. 2A) has the same concentration of hydroxylgroups as a sample containing 0.2% w/w of pyrogallol (see sample 2 in Fig. 2A).¶
![Panel (A): thermo-oxidative degradation of HDPE (1), HDPE containing 0.21% (w/w) of 1,2,3-trihydroxybenzene (2), 0.50% of BHT (3) and 0.50 ± 0.02% (w/w) of C-R-calix[4]pyrogallolarenes: R = undecyl (4), R = ethyl (5) and R = methyl (6). Heating rate β = 5 K min−1. Panels (B–D): thermo-oxidative degradation of HDPE containing 1,2,3-trihydroxybenzene (0.10% w/w, panel B), and C-R-calix[4]pyrogallolarenes: R = methyl (0.51%, panel C), R = undecyl (0.98%, panel D), monitored by DSC at heating rates a–h: 2, 5, 7.5, 10, 12.5, 15, 17.5 and 20 K min−1, respectively. DSC scans are shifted vertically for clarity. Other DSC curves of oxidation of HDPE containing various concentrations of antioxidants are presented in the ESI.](/image/article/2012/PY/c1py00494h/c1py00494h-f2.gif) | ||
| Fig. 2 Panel (A): thermo-oxidative degradation of HDPE (1), HDPE containing 0.21% (w/w) of 1,2,3-trihydroxybenzene (2), 0.50% of BHT (3) and 0.50 ± 0.02% (w/w) of C-R-calix[4]pyrogallolarenes: R = undecyl (4), R = ethyl (5) and R = methyl (6). Heating rate β = 5 K min−1. Panels (B–D): thermo-oxidative degradation of HDPE containing 1,2,3-trihydroxybenzene (0.10% w/w, panel B), and C-R-calix[4]pyrogallolarenes: R = methyl (0.51%, panel C), R = undecyl (0.98%, panel D), monitored by DSC at heating rates a–h: 2, 5, 7.5, 10, 12.5, 15, 17.5 and 20 K min−1, respectively. DSC scans are shifted vertically for clarity. Other DSC curves of oxidation of HDPE containing various concentrations of antioxidants are presented in the ESI†. | ||
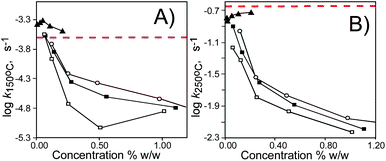 | ||
| Fig. 3 Plots of log k for HDPEoxidation at 150 °C (panel A) and at 250 °C (panel B) versus concentration of pyrogallol (▲), and C-methyl- (□), C-ethyl- (■), and C-undecyl-(○) pyrogallolarenes. Red dashed line indicates the value of log k for non-inhibited oxidation of HDPE. | ||
Fig. 2B–D illustrate the general rule: the higher the heating rate, the higher the Te values and, based on eqn (1), we calculated kinetic parameters for oxidation of HDPE samples containing five concentrations of each additive (Table 1). The Ea's listed in Table 1 are usually smaller than the value 127 ± 6 kJ mol−1 determined for non-inhibited oxidation of HDPE. However, temperatures Te (see Fig. 2 and ESI†) noticeably indicate that HDPE samples containing pyrogallolarenes are more resistant toward high temperature oxidation than the neat HDPE. This discrepancy can be explained by high temperature of the process and the compensation phenomena: at lower temperatures reaction rates are limited by enthalpy while at higher temperatures (above the isokinetic temperature) a reaction rate is limited by the entropy barrier. Both parameters, Ea and Z, were used for calculation of the rate constants and comparison of k's listed in Table 1 (and Fig. 3) evidently confirms that samples containing cyclic polyphenolsA1, A2 and A3 are more resistant toward oxidation than neat HDPE and than HDPE with additives B and C. Our data show pro-oxidative properties of B at 150 °C (see Fig. 3A). Indeed, polyphenols with two or three OHgroups in 1,2- or 1,2,3-positions sometimes can act as pro-oxidants,15 because H atom abstraction from Ar(OH)2 produces semiquinones, Ar(OH)O˙, able to react with molecular oxygen to form quinones and peroxyl radicals: Ar(OH)O˙ + O2 → Ar(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)2 + HOO˙. Surprisingly, we do not observe a similar pro-oxidative effect for calix[4]pyrogallolarenes. This intriguing feature of cyclic tetramers of pyrogallol makes them promising antioxidants and will be further studied.
O)2 + HOO˙. Surprisingly, we do not observe a similar pro-oxidative effect for calix[4]pyrogallolarenes. This intriguing feature of cyclic tetramers of pyrogallol makes them promising antioxidants and will be further studied.
| Concentrationb | E a | log Z | log k | ||
|---|---|---|---|---|---|
| 150 °C | 200 °C | 250 °C | |||
| a Full data including the parameters of eqn (1) together with statistic and kinetic parameters (also for composites with BHT) are given in the ESI.† b Concentration in % w/w. c The errors of the activation energy Ea calculated from the standard deviations of the slopes of eqn (1), see the ESI.† | |||||
| Pyrogallol | |||||
| 0.013 | 110 ± 3 | 11.92 | −3.38 | −1.95 | −0.79 |
| 0.026 | 106 ± 2 | 11.52 | −3.35 | −1.97 | −0.85 |
| 0.052 | 109 ± 2 | 11.85 | −3.32 | −1.90 | −0.76 |
| 0.104 | 113 ± 3 | 12.33 | −3.41 | −1.93 | −0.74 |
| 0.208 | 117 ± 4 | 12.77 | −3.50 | −1.96 | −0.73 |
| C-Methylcalix[4]pyrogallolarene | |||||
| 0.063 | 101 ± 5 | 10.68 | −3.54 | −2.23 | −1.16 |
| 0.127 | 112 ± 3 | 11.65 | −3.97 | −2.51 | −1.32 |
| 0.254 | 125 ± 5 | 12.44 | −4.73 | −3.10 | −1.79 |
| 0.508 | 135 ± 5 | 13.26 | −5.14 | −3.38 | −1.96 |
| 1.02 | 111 ± 9 | 10.67 | −4.85 | −3.40 | −2.22 |
| C-Ethylcalix[4]pyrogallolarene | |||||
| 0.069 | 113 ± 4 | 12.21 | −3.58 | −2.10 | −0.90 |
| 0.138 | 111 ± 4 | 11.70 | −3.84 | −2.39 | −1.21 |
| 0.276 | 117 ± 5 | 11.87 | −4.36 | −2.83 | −1.60 |
| 0.552 | 115 ± 3 | 11.41 | −4.61 | −3.11 | −1.89 |
| 1.10 | 110 ± 2 | 10.59 | −4.79 | −3.36 | −2.19 |
| C-Undecylcalix[4]pyrogallolarene | |||||
| 0.122 | 117 ± 8 | 12.53 | −3.71 | −2.19 | −0.95 |
| 0.244 | 113 ± 5 | 11.56 | −4.22 | −2.74 | −1.54 |
| 0.488 | 111 ± 7 | 11.08 | −4.37 | −2.92 | −1.75 |
| 0.976 | 110 ± 10 | 10.71 | −4.65 | −3.21 | −2.05 |
| 1.95 | 127 ± 7 | 12.19 | −5.26 | −3.60 | −2.26 |
In summary, non-isothermal DSC proved to give valuable information on the kinetics of high temperature oxidative decomposition of polyethylene. The start of oxidation detected as the first exothermal effect on the DSC curve can be used for calculation of the overall kinetic parameters: Ea, Z and k(T) of oxidation. Our results show that C-alkylcalix[4]pyrogallolarenes are effective agents increasing the oxidative stability of HDPE and efficiently inhibit oxidation of polymers that occurs at relatively high temperatures (even above 150 °C). Calix[4]pyrogallolarenes are more suitable for applications than conventional antioxidants because they are thermally stable and not volatile.
The authors gratefully acknowledge the financial support of the project (grant no N204 029936) by the Ministry of Science and Higher Education of Poland. PZ and KJP thank for the Mazowian Doctoral Scholarship. KJP thanks Foundation for Polish Science (International PhD Studies Program).
Notes and references
- (a) S. H. Hamid, Handbook of Polymer Degradation, Marcel Dekker, 2000 Search PubMed; (b) J. D. Peterson, S. Vyazovkin and C. A. Wight, Macromol. Chem. Phys., 2001, 202, 775–784 CrossRef CAS; (c) E. M. Hoàng, N. S. Allen, C. M. Liauw, E. Fontán and P. Lafuente, Polym. Degrad. Stab., 2006, 91, 1356–1362 CrossRef CAS; (d) F. Gugumus, Polym. Degrad. Stab., 2002, 76, 329–340 CrossRef CAS; (e) P. Gijsman, e-Polym., 2008, 065 Search PubMed; (f) E. T. Denisov and I. B. Afanasev, Oxidation and Antioxidants in Organic Chemistry and Biology, Taylor and Francis, 2005 Search PubMed.
- (a) G. Pritchard, Plastics Additives: an A–Z Reference, Chapman and Hall, 1998 Search PubMed; (b) H. Zweifel, R. D. Maier and M. Schiller, Plastics Additives Handbook, Hanser, 2009 Search PubMed; (c) F. Gugumus, Polym. Degrad. Stab., 1998, 60, 85–97 CrossRef CAS; (d) N. S. Allen, E. B. Zeynalov, K. Taylor and P. Birkett, Polym. Degrad. Stab., 2009, 94, 1932–1940 CrossRef CAS; (e) M. Lucarini and G. F. Pedulli, Chem. Soc. Rev., 2010, 39, 2106–2119 RSC.
- (a) Y. Bolbukh, P. Kuzema, V. Tertykh and I. Laguta, J. Therm. Anal. Calorim., 2008, 94, 727–736 CrossRef CAS; (b) R. Gensler, C. J. G. Plummer, H.-H. Kausch, E. Kramer, J.-R. Pauquet and H. Zweifel, Polym. Degrad. Stab., 2000, 67, 195–208 CrossRef CAS.
- S. Jipa, T. Zaharescu, R. Setnescu, T. Setnescu, M. Dumitru, L. M. Gorghiu, I. Mihalcea and M. Bumbac, Polym. Degrad. Stab., 2003, 80, 203–208 CrossRef CAS.
- S. Yordanova and S. Miloshev, J. Univ. Chem. Technol. Metall., 2006, 41, 397–402 Search PubMed.
- K. Chennakesavulu, M. Raviathul Basariya, P. Sreedevi, G. Bhaskar Raju, S. Prabhakar and S. Subba Rao, Thermochim. Acta, 2011, 515, 24–31 CrossRef CAS.
- (a) G. Litwinienko and T. Kasprzycka-Guttman, Thermochim. Acta., 1998, 319, 185–191 CrossRef CAS; (b) G. Litwinienko, Analysis of Lipid Oxidation by Differential Scanning Calorimetry, in Analysis of Lipid Oxidation, AOCS Publishing, 2005 Search PubMed; (c) G. Litwinienko, A. Daniluk and T. Kasprzycka-Guttman, Ind. Eng. Chem. Res., 2000, 39, 7–12 CrossRef CAS; (d) G. Litwinienko and T. Kasprzycka-Guttman, Ind. Eng. Chem. Res., 2000, 39, 13–17 CrossRef CAS; (e) M. Ulkowski, M. Musialik and G. Litwinienko, J. Agric. Food Chem., 2005, 53, 9073–9077 CrossRef CAS.
- (a) R. O. Dunn, Fuel Process. Technol., 2005, 86, 1071–1085 CrossRef CAS; (b) R. O. Dunn, Biofuels, Bioprod. Biorefin., 2008, 2, 304–318 Search PubMed; (c) B. R. Moser, J. Am. Oil Chem. Soc., 2009, 86, 699–706 CrossRef CAS; (d) G. B. Bantchev, G. Biresaw, A. Mohamed and J. Moser, Thermochim. Acta, 2011, 513, 94–99 CrossRef CAS; (e) S. I. Martínez-Monteagudo, M. D. A. Saldaña and J. J. Kennelly, J. Therm. Anal. Calorim., 2011 DOI:10.1007/s10973-011-1649-8.
- (a) J. H. Flynn and L. A. Wall, Polym. Lett., 1966, 4, 323–328 CrossRef CAS; (b) T. Ozawa, J. Therm. Anal., 1970, 2, 301–324 CrossRef CAS.
- J. Opfermann and E. Kaisersberger, Thermochim. Acta, 1992, 203, 167–175 CrossRef CAS.
- M. Iring, F. Tüdos, Zs. Fodor and T. Kelen, Polym. Degrad. Stab., 1980, 2, 143–153 CrossRef CAS.
- D. E. Van Sickle, T. Mill, F. R. Mayo, H. Richardson and C. W. Gould, J. Org. Chem., 1973, 38, 4435–4440 CrossRef.
- S. Korcek, J. H. B. Chenier, J. A. Howard and K. U. Ingold, Can. J. Chem., 1972, 50, 2285–2297 CAS.
- F. Gugumus, Polym. Degrad. Stab., 2000, 68, 337–352 CrossRef CAS.
- (a) B. Kalyanaraman, W. Korytowski, B. Pilas, T. Sarna, E. J. Land and T. G. Truscott, Arch. Biochem. Biophys., 1988, 266, 277–284 CrossRef CAS; (b) J. L. Bolton, M. A. Trush, T. M. Penning, G. Dryhurst and T. J. Monks, Chem. Res. Toxicol., 2000, 13, 135 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, DSC, TG curves, NMR spectra, derivation of the Ozawa–Flynn–Wall method and detailed analysis of kinetic data, as indicated in the text. See DOI: 10.1039/c1py00494h |
| ‡ Thermogravimetric analysis of these compounds showed that pyrogallolarenes are not volatile and are thermally stable—their decomposition starts above 260 °C, in contrast to BHT and pyrogallol (both evaporate at 80–160 °C, see TG curves presented in the ESI†). |
| § Polymeric composites were prepared in the same manner as in ref. 4–6: HDPE powder (0.50 ± 0.01 g) was ground with an appropriate volume of acetone solution of a phenol to obtain homogeneous concentration of additive in the polymer, then, the powder was left for 1 hour to dry. TG measurements showed that samples prepared in that way were acetone free and no additional drying or heating was required. |
| ¶ 0.2% w/w, i.e., 16 mM pyrogallol (Mw = 126 g mol−1) is a molar equivalent of ca. 2% w/w of C-undecylcalix[4]pyrogallolarene (Mw = 1168 g mol−1), however, correcting the number of OHgroups in monomer and tetramer, 0.2% w/w pyrogallol is equivalent of 0.5% w/w of C-undecylcalix[4]pyrogallolarene. In our comparative studies the maximal concentrations were: 0.2% for pyrogallol, 1.1% for C-methyl and C-ethyl pyrogallolarenes and 2% w/w for C-undecyl-pyrogallolarene. |
| This journal is © The Royal Society of Chemistry 2012 |
