Gender differences in UV-induced inflammation and immunosuppression in mice reveal male unresponsiveness to UVA radiation†
Vivienne E.
Reeve
*,
Munif
Allanson
,
Diane
Domanski
and
Nicole
Painter
Faculty of Veterinary Science, University of Sydney, NSW 2006, Australia. E-mail: vreeve@usyd.edu.au; Fax: +612 9351 7348
First published on 4th October 2011
Abstract
Immunosuppression attributed mainly to the UVB (290–320 nm) waveband is a prerequisite for skin cancer development in mice and humans. The contribution of UVA (320–400 nm) is controversial, but in mice UVA irradiation has been found to antagonise immunosuppression by UVB. In other studies of photoimmune regulation, protection mediated viaoestrogen receptor-β signalling was identified as a normal endogenous defence in mice, and was shown to depend on UVA irradiation. A gender bias in photoimmune responsiveness was thus suggested, and is tested in this study by comparing the UV-induced inflammatory and immune responses in male and female hairless mice. We report that male mice, which show greater skin thickness than females, developed a less intense but slower resolving sunburn inflammatory oedema, correlated with reduced epidermal expression of pro-inflammatory IL-6 than females following solar simulated UV (SSUV, 290–400 nm) exposure. On the other hand, the contact hypersensitivity reaction (CHS) was more severely suppressed by SSUV in males, correlated with increased epidermal expression of immunosuppressive IL-10. Exposure to the UVB waveband alone, or to cis-urocanic acid, suppressed CHS equally in males and females. However, whereas UVA irradiation induced immunoprotection against either UVB or cis-urocanic acid in females, this protection was significantly reduced or abrogated in males. The results indicate that males are compromised by a relative unresponsiveness to the photoimmune protective effects of UVA, alone or as a component of SSUV. This could explain the known gender bias in skin cancer development in both mice and humans.
Introduction
It is recognised that immune suppression by chronic UV irradiation is an important contributing factor for the development of experimental photocarcinogenesis in mice, and sunlight-induced skin cancer in humans.1,2 Multiple immune-modulating pathways activated by UV radiation that lead to the photoimmune suppressed state have been identified mainly from murine experiments, however, the relative importance of these pathways, their possible interactions, and their human equivalent responses, remain unclear.3,4 Studies in mice with topically applied phytoestrogenic isoflavones, such as equol, and later with the natural hormone 17β-oestradiol, demonstrated their capacity to protect against the suppression of contact hypersensitivity (CHS) induced by either solar simulated radiation (SSUV, 290–400 nm) or its cutaneous immunosuppressive photoproduct cis-urocanic acid.5 The immune protection could be abrogated by treatment with the oestrogen receptor (Er) antagonist ICI 182,780, identifying a role for Er signalling in this regulatory pathway.6 Later it was revealed that the non-classical receptor, Er-β was involved rather than the better characterised Er-α. Mice with a null mutation for Er-β (Er-β−/−) were found to have increased sensitivity to UVB (290–320 nm)-induced suppression of CHS and to have significantly greater UVB-induction of the major immunosuppressive cytokine, IL-10 in the epidermis.7In other studies, photoimmune regulating interactions between different regions of the solar UV spectrum have been reported in murine models. These indicate a protective role for the UVA waveband (320–400 nm) against both UVB radiation-induced epidermal IL-10 expression and the suppression of CHS.8,9 The UVA component of SSUV appears to act in this mode, and experimental exposures of mice to UV sources of increased UVA/UVB ratios were found to attenuate the suppression of CHS in a UVA dose-dependent manner.10Signalling by Er-β was identified as the protective mediator of this effect, and in Er-β−/− mice, the photoimmune protective effect of UVA exposure was found to be abrogated.7 Thus there is a normal endogenous protective mechanism against photoimmune suppression in the mouse that is activated by UVA exposure, and is mediated via the non-classical oestrogenic pathways.
A natural model of a relative deficiency in Er signalling is the male gender, when compared with the female, and it has been of interest to determine whether a gender bias might be detected in the ability of the skin to respond to the damaging effects of UV exposure. This study compares the UV responsiveness of male and female Skh:hr-1 hairless mice, assessed by those pathologies that are understood to be relevant to the risk of development of skin cancer. The sunburn reaction was measured as the SSUV-induced inflammatory oedema, indicated by an increase in the irradiated skinfold thickness and the level of expression of the pro-inflammatory cytokine interleukin (IL)-6 in the epidermis.11,12 Immune function was assessed by the CHS reaction, its suppression by SSUV was related to the level of expression of the immunosuppressive cytokine IL-10 in the epidermis, and the modulation of the immune response by the UVA and UVB wavebands was examined.
Methods
Mice
Age-matched groups of inbred male and female Skh:hr-1 hairless albino mice were obtained from the Veterinary Science breeding colony at 9-12 weeks of age. They were housed in groups of 3–5 mice in conventional wire-topped plastic boxes on compressed paper bedding (Fibrecycle Pty. Ltd., Mudgeeraba, Qld., Australia) at an ambient temperature of 25 °C under gold lighting (F40GO tubes, General Electric Co., Hobart, Tas., Australia) that does not emit UV radiation, and fed stock rodent pellets (Gordons Specialty Stockfeeds, Yanderra, N.S.W., Australia) and tap waterad libitum. Because of their propensity to fight and bite, male mice remained housed with their original weaned littermates throughout the experiments, and were not included if there were signs of skin damage. Occasionally male mice displayed scratch or bite marks on the dorsum post-irradiation, therefore duplicate treatment groups were initiated, and the skin-damaged mice were excluded from the study.All procedures were approved by the University of Sydney Animal Ethics Committee and complied with the current New South Wales Animal Welfare Act.
UV irradiation
The UV radiation was produced by fluorescent tube sources. SSUV was obtained from a planar bank of 6 UVA tubes (Hitachi 40 W F40T 10/BL, Tokyo, Japan) flanking a single UVB tube (Philips TL40W 12/RS, Eindhoven, The Netherlands), and was filtered through a sheet of 0.125 mm cellulose acetate (Grafix Plastics, Cleveland, OH) to remove wavelengths below 290 nm. The UVA and UVB wavebands were isolated from this source by switching off the relevant tubes, retaining the cellulose acetate filter for UVB, and substituting this with a 6 mm window glass filter that was opaque to wavelengths below 320 nm for UVA. The SSUV source could also be manipulated to produce increasing UVA/UVB ratios, by reducing the voltage to the UVB tube and increasing the exposure time to maintain a constant UVB dose, as previously described.10,13 Irradiance was measured with an International Light IL1500 radiometer (Newburyport, MA), using UVA and UVB detectors calibrated to the spectral output of the sources. The minimum oedematous dose (MEdD) of SSUV for female Skh:hr-1 mice has been previously established as containing 1.33 kJ m−2 UVB and 23.7 kJ m−2 of UVA.10Groups of mice were exposed on the dorsum, unrestrained in their boxes with the wire tops removed, either to a single dose of 2 or 3 MEdD, or 3 repeated daily doses of fractions of the MEdD of SSUV (when establishing a SSUV-dose-dependence), or the UVA or UVB component alone of the SSUV source. The UVA alone dose for optimal photoprotection against UVB has been previously established14 as approximately 400 kJ m−2, and was administered as a single exposure. This dose is equivalent to the UVA component of approximately 6–8 × minimal erythemal dose (MED) of sunlight in humans. If radiation from both UVA and UVB was given in sequence, or UVA irradiation was combined with cis-urocanic acid application, the UVA always preceded the UVB exposure or cis-urocanic acid treatment. If the UVA component of 3 MEdD SSUV was enriched, enabling increased ratios of UVA/UVB to be administered concurrently in a single exposure, the mice received a constant dose of UVB (3.99 kJ m−2) together with 71.0, 115.7 or 177.2 kJ m−2UVA.13
The inflammatory oedema response
The oedema was measured in groups of 5 mice as the increase in the mid-dorsal skinfold thickness using a spring micrometre (Interrapid, Zurich, Switzerland), before and at 24-hourly intervals up to 7 days after a single irradiation with 3 MEdD (female) of SSUV. Normal adult male mice were found to have a significantly thicker mid-dorsal skinfold measurement (average 104.0 ± 7.1 mm × 0.01) compared with age-matched female mice (81.0 ± 3.6 mm × 0.01).Treatment with cis-urocanic acid
In some experiments, the mice were treated with topical application of the immunosuppressive mediator, cis-urocanic acid, prepared in a lotion vehicle from trans-urocanic (Sigma Aldrich Corp., Castle Hill, NSW, Australia) acid as previously described.11 Aliquots of 0.1 mL containing 0.2 mg photoisomerised urocanic acids were applied to the mouse dorsum 3 times within a 24 h period, to simulate one daily UV irradiation. Control mice received the same volume of the vehicle lotion only.Induction of contact hypersensitivity (CHS)
CHS was induced in groups of 5 mice on days 8 and 9 following either single UV exposures, cis-urocanic acid applications, or the first of 3 consecutive daily UV exposures, by sensitising the unirradiated abdominal skin with 0.1 mL 2% oxazolone (Sigma) in ethanol, in order to reveal systemic changes in immune responsiveness, as previously described.15 The response was elicited on day 15 by oxazolone challenge on the ears, and the ear thicknesses were measured before and repeatedly between 16–24 h post-challenge to identify the time of the peak ear swelling in the unirradiated control mice. The group average ear swelling was obtained at this time point for each treatment group, and the percent immunosuppression was calculated from the differences between the average ear swelling measurements of the matching unirradiated and UV-irradiated treatments.Immunohistochemical detection of IL-6 and IL-10
Lateral mid-dorsal skin samples were obtained from groups of 3 male or female mice before and at 72 h following SSUV irradiation, the time point previously shown to display maximum cytokine expression.16 The exposure dose was reduced to 2 MEdD to increase the likelihood of revealing differences in cytokine expression between males and females, as the 72 h responses of IL-6 and IL-10 to 3 MEdD SSUV previously reported in female mice were very strong.16 They were fixed for 6 h in HistoChoice (Amresco, Solon, OH), processed in an automated ethanol–formalin system, wax embedded, and sections of 4 μm were cut for immunohistochemical staining, as previously described.7 The cytokines were identified using the primary antibodies goat anti-mouse IL-6 and IL-10 (R & D Systems, Minneapolis, MN), omitting this antibody for the negative control section, and the secondary antibody HRP-labelled anti-goat IgG (H+L) (Vector Laboratories Inc., Burlingame, CA). Digital images of the staining under light microscopy were obtained using a Sony HyperHAD video camera (Sony, Melbourne, Australia) and the positive brown stain intensity was semi-quantitated for selected time points using the Leica image processing and analysis system (Leica QWin version V01.00, Cambridge, UK). This was done in a blinded manner, averaged for 15 sequential fields across the skin section from each of 3 mice per treatment group.Statistical analysis
Statistical significance of the differences between treatment groups was obtained by two-way ANOVA, Student's t-test and the Bonferroni post-test.Results
Gender and SSUV-induced inflammatory sunburn oedema
The development of the inflammatory oedema in the irradiated dorsal skin, measured as the increase in the mid-dorsal skinfold thickness, was found to differ between male and female mice (Fig. 1). In females the skinfold thickness increased more acutely to a maximum at 3 days post-irradiation that was approximately twice the normal skinfold thickness, then decreased quite rapidly and was restored to almost normal by 7 days. However, in males the skinfold thickness increased more slowly, reached a maximum approximately 50% greater than normal skinfold thickness at 4 days post-irradiation, significantly (P < 0.05) less than the earlier peak in females, and decreased more gradually so that at 7 days, skinfold thickness remained elevated, similarly to the increased skinfold thickness at 1 day post-irradiation. Thus male mice displayed a more prolonged, but less intense increase in this inflammatory response than female mice. | ||
| Fig. 1 Progressive development of the inflammatory sunburn oedema presented as the average increase in mid-dorsal skinfold thickness ± SEM in groups of 5 male or female mice before and following irradiation with a single dose of 3 MEdD SSUV. Asterisks indicate significantly differing values (P < 0.05). | ||
Gender and SSUV-induced suppression of contact hypersensitivity (CHS)
The sensitivity to the suppression of CHS to oxazolone was compared between male and female mice, seeking dose-dependence at increasing SSUV doses, each administered daily for 3 consecutive days. The normal CHS response did not differ between males and females (Fig. 2a). Exposure to 3 × 0.5 MEdD was not immunosuppressive in females, but suppressed CHS by 17% in male mice (Fig. 2b). Exposure to 3 × 0.75 MEdD became slightly immunosuppressive (13% suppression) in females, whereas this dose strongly suppressed males by 45% (P < 0.001), demonstrating a significantly exacerbated response compared with females (Fig. 2b; P < 0.01). A further increase in the SSUV dose to 3 × 1 MEdD resulted in a further increase in the immunosuppression in females (30% suppression; P < 0.01), but no further increase in immunosuppression was evident in the males (Fig. 2a; 49% suppression). Thus both male and female mice displayed a SSUV-dose dependence for suppression of CHS, but male mice were significantly more sensitive than female mice and appeared to be maximally immunosuppressed at a lower SSUV dose.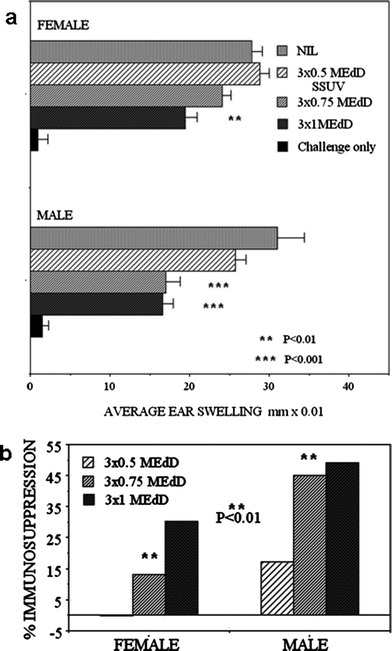 | ||
| Fig. 2 (a) The CHS response to oxazolone in groups of 5 male or female mice following 3 daily exposures to increasing doses of SSUV, presented as the average ear swelling ± SEM. Asterisks indicate values differing statistically significantly from control (Nil). Challenge only (no sensitisation) indicates a small statistically non significant irritant effect of oxazolone. (b) shows the % immunosuppression in the irradiated mice. Asterisks indicate significantly differing values. | ||
Gender and epidermal cytokine expression
There was very little expression of IL-6 or IL-10 in the unirradiated skin. The cytokines were induced by SSUV at 72 h and appeared to be restricted to, and diffuse throughout the epidermis (Fig. 3 and 4), consistent with our previous observations.9,16 Although early reports of IL-10 induction in human skin describe infiltrating macrophages in both dermis and epidermis as the source of IL-10, the radiation source differed, and the cytokine was assayed as mRNAin situ, perhaps contributing to the difference from the mouse skin response, which appeared to be strongly epidermal.17 | ||
| Fig. 3 Immunohistochemical staining of IL-6 (brown) in typical mid-dorsal skin sections before (Nil) and 72 h following irradiation with a single dose of 3 MEdD SSUV. (a) Graph shows semi-quantitation by image analysis of the relative intensity of immunopositive staining for IL-6 averaged for 15 sequential mid-dorsal fields each of 3 male and female mice (n = 45 sequential fields) before and 72 h following irradiation, expressed as arbitrary units ± SEM. Symbols indicate values differing statistically significantly. | ||
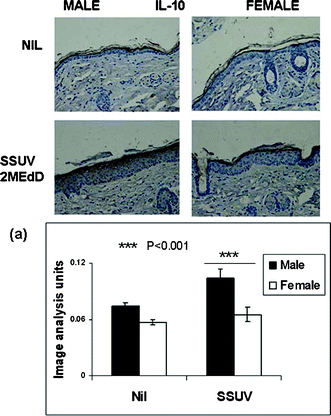 | ||
| Fig. 4 Immunohistochemical staining of IL-10 (brown) in typical mid-dorsal skin sections before (Nil) and 72 h following irradiation with a single dose of 3 MEdD SSUV. (a) Graph shows semi-quantitation by image analysis of the relative intensity of immunopositive staining for IL-10 averaged for 15 sequential mid-dorsal fields each of 3 male and female mice (n = 45 sequential fields) before and 72 h following irradiation, expressed as arbitrary units ± SEM. Symbols indicate values differing statistically significantly. | ||
Immunopositive IL-6, a hallmark cytokine indicating inflammation, was upregulated in the epidermis following SSUV irradiation. In Fig. 3, the development of IL-6 expression can be seen as brown staining in the epidermis, in typical sections taken from the mid-dorsum of male or female mice, before and at 72 h following irradiation with 2 MEdD SSUV. Both male and female unirradiated skin expressed a low level of IL-6. After SSUV exposure, the epidermal hyperplasia was apparent in both males and females, but IL-6 staining in male mice remained much less intense than in females. Thus IL-6 expression correlated with the stronger inflammatory oedema development in female mice compared with male mice. The staining intensity is semi-quantitated at 72 h by image analysis (Fig. 3a).
Immunopositive IL-10, the hallmark epidermal cytokine indicative of photoimmune suppression, developed more intensely in the male mice, and was substantially upregulated at 72 h post-SSUV, whereas the IL-10 expression remained very slight at this time point in female epidermis (Fig. 4). These levels of IL-10 expression parallel the greater immunosuppression demonstrated by the CHS response in SSUV-irradiated males compared with females. Semi-quantitation (Fig. 4a) shows a clear disparity between the two genders.
Gender and effect of UVA/UVB interaction on CHS
No differences were detected in the CHS reactions of male and female mice after a single UVA exposure (Fig. 5). However irradiation with 3 MEdD of UVB alone strongly suppressed CHS, equally in both males and females (52% and 50% suppression respectively; P < 0.001). Interestingly, if this UVB dose was preceded by the UVA irradiation (UVA + UVB), a gender bias became apparent. The UVB suppression in females was reduced from 50% to 22% (P < 0.01), but the reduction in males was not statistically significant (52% to 44%), and these mice remained highly significantly more immunosuppressed than the females (P < 0.001). This experiment revealed that males and females are equally immunosuppressed by UVB, but that males are less responsive to the immunoprotective effect of UVA radiation.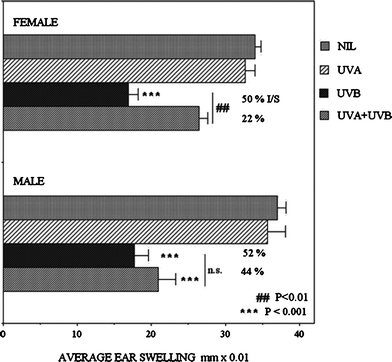 | ||
| Fig. 5 CHS responses to oxazolone presented as average ear swelling ± SEM in groups of 5 male or female mice, following irradiation with a single sub-erythemogenic dose of 400 kJ m−2UVA, 3 MEdD of UVB, or these doses of UVA followed immediately by UVB. Asterisks indicate values differing statistically significantly from control (Nil). # indicate significantly differing treatments. | ||
The equal suppression of CHS in males and females by UVB was supported by the replacement of the UVB irradiation with topical cis-urocanic acid (Fig. 6), a putative epidermal mediator of UVB-immunosuppression, resulting in 25% and 28% suppression respectively (P < 0.001). However when the cis-urocanic acid treatment was preceded by the single UVA irradiation, female mice were again protected from suppression of CHS (28% suppression reduced to 11% suppression, P < 0.01), whereas male mice remained unaffected by the UVA (25% suppression). This observation was consistent with a failure of male mice to respond to UVA radiation that is immunoprotective in females.
 | ||
| Fig. 6 CHS responses to oxazolone presented as average ear swelling ± SEM in groups of 5 male or female mice, following irradiation with a single dose of 400 kJ m−2UVA, with or without subsequent treatment with topical cis-urocanic acid lotion (c-UCA) or vehicle base lotion (Nil). Asterisks indicate values differing statistically significantly from control (Nil). # indicate significantly differing treatments. | ||
To strengthen this evidence, mice were irradiated with UVA and UVB simultaneously, as in environmental solar irradiation, with the UVB component of the radiation kept constant (equal to UVB of 3 MEdD SSUV) but the UVA content increased 1.6- and 2.5-fold. These radiation sources therefore provided UVA/UVB ratios of 17.8 (SSUV), 29.0 and 44.4 (Fig. 7). In female mice, increasing the UVA/UVB ratio to 29.0 reduced the SSUV suppression of CHS (28% to 17%), while further increasing the UVA/UVB ratio to 44.4 provided further immune protection, and these female mice produced a CHS reaction not significantly different from the unirradiated controls (3% suppression). In contrast, male mice were suppressed by 37% by SSUV, and remained 34% suppressed, not significantly different, when UVA/UVB was increased to 29.0. It required an increase in UVA/UVB to 44.4 to produce significant immunoprotection in males (22% suppression), but this slightly protected response remained significantly reduced (P < 0.01) compared with similarly irradiated female mice.
 | ||
| Fig. 7 CHS responses to oxazolone presented as average ear swelling ± SEM in groups of 5 male or female mice, following irradiation with a single dose of SSUV (3 MEdD) or of increasingly UVA-enriched SSUV providing the same UVB content as the SSUV. The increasing UVA/UVB ratios are shown as ‘A/B’. Asterisks indicate values differing statistically significantly from control (Nil). # indicate significantly differing treatments. | ||
These experiments strongly suggested that the male gender is less amenable to the photoimmune protective effects of the UVA waveband than the female.
Discussion
We have revealed a marked gender difference in the development of the inflammatory sunburn oedema reaction to SSUV. The induced male oedema was similar to the female response at 1 day post-SSUV, but then developed much less actively and remained weaker and was more prolonged than the more acute female response. We have noted that unirradiated adult male mouse skin is significantly thicker than female mouse skin, potentially providing a greater barrier to penetration, particularly of the shorter wavelength UVB rather than the UVA waveband. Histologically, it did not appear that male epidermis was thicker than female, suggesting that the dermis and sub-dermal muscle tissue may account for the normal increased male skinfold thickness. The persisting male oedema at 7 days, in contrast to the female oedema that had resolved at this time, suggests that rather than a reduced effective UV dose, the male cutaneous mediators for oedema development and resolution, which include IL-6, may respond differently than in females. Our observation is similar to a report by Thomas-Ahner et al. of a reduced male UVB inflammatory response measured in the same strain of Skh hairless mice by both the reduced increase in skinfold thickness and the inhibited induction of myeloperoxidase.18The reduced male inflammatory oedema response to SSUV suggested that immune function would be less disrupted by SSUV in males. However the opposite transpired, and the greater SSUV dose-dependent suppression of CHS in males that correlated with an increased upregulation of immunosuppressive IL-10, was revealed, consistent with these changes we have reported in female Er-β−/− mice.7 In addition, the observation of the reduced level of induction of pro-inflammatory IL-6 in the male oedema reaction suggested a gender-dependent role for UVA radiation, as we have reported in the past that IL-6 expression in mouse skin is essential for induction of the immunoprotective responses by the UVA waveband.11 Therefore we examined the gender-dependence of the UVA/UVB interactions, and confirmed that male mice were compromised in their UVA protection against suppression of CHS by either UVB or cis-urocanic acid. The reduced effect of UVA in males was also evident when the mice were exposed to the UVA-enriched UVB sources, a model of sunbed irradiation or prolonged sun exposure by humans wearing UVB-blocking sunscreening agents.
Interestingly, a male predisposition not only for inflammation but also for photocarcinogenesis was observed in these mice by Thomas-Ahner et al.18 in response to chronic UVB irradiation, thus contrasting with our evidence for a critical role for UVA, and attributing the severe male outcome to a greater accumulation of cutaneous oxidative DNA damage. While that study did not measure immune function, exacerbated suppression of CHS by UVB alone in male mice has been reported by others.19 We have previously found that phytoestrogen treatment was anti-photocarcinogenic in mice20 whereas inhibited Er signalling promoted the growth of transplanted murine skin tumours,21 consistent with the gender bias in photoimmune sensitivity we describe here. As it is evident that the oestrogenic pathway in mice is activated by UVA radiation,22 future studies of the role of gender and the UVA waveband in chronic irradiations for photocarcinogenesis induction are needed.
Whether there is a gender bias in the inflammatory sunburn reaction for humans is unclear at present. The inbred albino mouse avoids the known confounding effects of phenotypic and environmental variations in human subjects that make identification of gender dependence of minimum erythema doses (MEDs) difficult to quantify. A high degree of correlation between erythema and oedema in humans has been reported in an early study.23 However, there are conflicting reports of gender dependence in human UV-induced erythema and inflammation, traditionally assessed at 24 h post-irradiation. At this timepoint we observed in the hairless mice that the gender difference for oedema was very slight. A null effect of gender on the human MED for UVB irradiation has been reported, apparently in agreement with our results for 24 h oedema,24,25 and topical oestradiol applied before SSUV irradiation significantly enhanced the erythema,26 but other clinical studies reported lower MEDs for SSUV in male subjects,23,27 suggesting greater male UV-sensitivity for erythema that is in contrast to our findings.
A gender bias for photoimmune suppression has been reported recently for human subjects,28 males being significantly more immunosuppressed by SSUV than females, using an assay of local elicitation of existing immunity to a tuberculin antigen by the Mantoux test. The gender difference was observed at low sub-erythemogenic SSUV doses in subjects matched for skin colour and MED, and was consistent with our observations in mice exposed to a broader range of SSUV doses that included erythemogenic irradiation, and in which the systemic rather than local changes in the induction of the primary CHS reaction were measured. The human study28 also reported that the MED was negatively related to the degree of immunosuppression, in contrast to the mouse data, and indicating that a greater sensitivity to SSUV inflammation results in a greater suppression of immune function. The equivalent relationship in the mouse appears to be a prolonged sub-acute sunburn inflammation leading to more severe immunosuppression, rather than a faster resolving more acute sunburn.
The increased photoimmune sensitivity in males, mice or human, is consistent with the known increased risk of sunlight-induced skin cancers in male subjects.29–31 Interestingly, very recent reports attempting to define specific gene variants as the determinants of photoimmune suppression and skin cancer sensitivity in humans have indicated a critical role for certain IL-10 variants, and a polymorphism of the histidase gene that produces urocanic acid from histidine, associated with non-melanoma skin cancers, particularly in women.4,32 These studies in chronically photodamaged subjects appear to challenge the older epidemiological data for gender, and may be specific for the north American population that was assessed, or may indicate a new shift in the gender specificity for skin cancer risk in humans.
In summary, our results imply that UVA unresponsiveness in male mice accounts for their lower resistance to photoimmune suppression. Since the UVA waveband is the most effective inducer of oxidative stress in the skin, and one earlier study has already implicated a gender difference in acute UVB-induced antioxidant defences in the skin,18 future studies should question whether male mice have less effective endogenous defence antioxidants inducible by chronic UVA irradiation than females. This is likely, in view of the recent evidence of unresponsiveness of the major UVA-inducible stress protein, haem oxygenase-1, in the skin of both Er-β−/− mice22 and IL6−/− mice.11 This stress enzyme reduces inflammatory and oxidative stress in cells, activates multiple antioxidant genes, and has important photoimmune protective properties.33–35 To conclude, it appears that robust IL-6 responsiveness, UVA responsiveness and female gender provide significant photoimmune protection in mice that may have critical importance in the development of skin carcinogenesis. The role of these factors in humans needs further examination.
Acknowledgements
This study was supported by a grant from the Cancer Council New South Wales. We thank Dr Katie Dixon for advice on statistical analysis. We are grateful to Matthew Jones, Laboratory Animal Services, for careful husbandry of the mice.References
- M. Norval, The mechanisms and consequences of ultraviolet-induced immunosuppression, Prog. Biophys. Mol. Biol., 2006, 92, 108–118 CrossRef CAS.
- S. E. Ullrich, Mechanisms underlying UV-induced immune suppression, Mutat. Res., Fundam. Mol. Mech. Mutagen., 2005, 571, 185–205 CrossRef CAS.
- G. M. Halliday and S. Rana. The effects of solar radiation on the immune response in humans, Biophysical and Physiological Effects of Solar Radiation on Human Skin, ed. P. U. Giacomoni, Royal Society of Chemistry, Cambridge, 2007, pp. 127–163 Search PubMed.
- M. M. Welsh, M. R. Karagas, K. M. Applebaum, S. K. Spencer, A. E. Perry and H. H. Nelson, A role for ultraviolet radiation immunosuppression in non-melanoma skin cancer as evidenced by gene-environment interactions, Carcinogenesis, 2008, 29, 1950–1954 CrossRef CAS.
- S. Widyarini, N. Spinks, A. J. Husband and V. E. Reeve, Isoflavonoid compounds from red clover (Trifolium pratense) protect from inflammation and immune suppression by UV radiation, Photochem. Photobiol., 2001, 74, 465–470 CrossRef CAS.
- S. Widyarini, D. Domanski, N. Painter and V. E. Reeve, Estrogen signaling protects against immune suppression by UV radiation exposure, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 12837–12842 CrossRef CAS.
- J.-L. Cho, M. Allanson, D. Domanski, S. J. Arun and V. E. Reeve, Estrogen receptor-beta signaling protects epidermal cytokine expression and immune function from UVB-induced impairment in mice, Photochem. Photobiol. Sci., 2008, 7, 120–125 RSC.
- V. E. Reeve, M. Bosnic, C. Boehm-Wilcox, N. Nishimura and R. D. Ley, Ultraviolet A radiation (320–400 nm) protects hairless mice from immunosuppression induced by ultraviolet B radiation (280–320 nm) or cis-urocanic acid, Int. Arch. Allergy Immunol., 1998, 115, 316–322 CrossRef CAS.
- J. Shen, S. Bao and V. E. Reeve, Modulation of IL-10, IL-12 and IFN-γ in the epidermis of hairless mice by UVA (320–400 nm) and UVB (280–320 nm) radiation, J. Invest. Dermatol., 1999, 113, 1059–1064 CrossRef CAS.
- V. E. Reeve, D. Domanski and M. Slater, Radiation sources providing increased UVA-UVB ratios induce UVA dose-dependently increased photoprotection in hairless mice, Photochem. Photobiol., 2006, 82, 406–411 CrossRef CAS.
- V. E. Reeve, R. M. Tyrrell, M. Allanson, D. Domanski and L. Blyth, The role of interleukin-6 in UVA protection against UVB-induced immunosuppression, J. Invest. Dermatol., 2008, 129, 1539–1546.
- K. Wade Foster, S. K. Katiyar, N. Yusuf and C. A. Elmets, Inflammation after solar radiation, in Biophysical and Physiological Effects of Solar Radiation on Human Skin, ed. P. U. Giacomoni, Royal Society of Chemistry, Cambridge, 2007, pp. 25–63 Search PubMed.
- Y. Ibuki, M. Allanson, K. M. Dixon and V. E. Reeve, Radiation sources providing increased UVA/UVB ratios attenuate the apoptotic effects of the UVB waveband UVA-dose-dependently, J. Invest. Dermatol., 2007, 127, 2236–2244 CrossRef CAS.
- M. Allanson, D. Domanski and V. E. Reeve, Photoimmunoprotection by UVA (320-400 nm) radiation is determined by UVA dose and is associated with cutaneous cyclic guanosine monophosphate, J. Invest. Dermatol., 2006, 126, 191–197 CrossRef CAS.
- V. E. Reeve, Ultraviolet radiation and the contact hypersensitivity reaction in mice, Methods, 2002, 28, 20–24 CrossRef CAS.
- N. Cole, P. W. Sou, A. Ngo, K. H. Tsang, J. A. Severino, S. J. Arun, C. C. Duke and V. E. Reeve, Topical ‘Sydney’ propolis protects against UV-radiation-induced inflammation, lipid peroxidation and immune suppression in mouse skin, Int. Arch. Allergy Immunol., 2010, 152, 87–97 CrossRef.
- K. Kang, A. C. Gilliam, G. Chen, E. Tootell and K. D. Cooper, In human skin, UVB initiated early induction of IL-10 over IL-12 preferentially in the expanding dermal monocytic/macrophagic population, J. Invest. Dermatol., 1998, 110, 31–38 CrossRef.
- J. M. Thomas-Ahner, B. C. Wulff, K. L. Tober, D. F. Kusewitt, J. A. Riggenbach and T. M. Oberyszyn, Gender differences in UVB-induced skin carcinogenesis, inflammation and DNA damage, Cancer Res., 2007, 67, 3468–3474 CrossRef CAS.
- K. Hiramoto, H. Tanaka, N. Yanagihara, E. F. Sato and M. Inoue, Effect of 17-beta-estradiol on immunosuppression induced by ultraviolet B irradiation, Arch. Dermatol. Res., 2004, 295, 307–311 CrossRef CAS.
- S. Widyarini, A. J. Husband and V. E. Reeve, Protective effect of isoflavonoid equol against hairless mouse skin carcinogenesis induced by UV radiation alone or with a chemical carcinogen, Photochem. Photobiol., 2005, 81, 32–37 CrossRef CAS.
- J.-L. Cho, M. Allanson and V. E. Reeve, Oestrogen receptor-β signalling protects against transplanted skin tumour growth in the mouse, Photochem. Photobiol. Sci., 2010, 9, 608–614 RSC.
- V. E. Reeve, M. Allanson, J.-L. Cho, S. J. Arun and D. Domanski, Interdependence between heme oxygenase-1 induction and estrogen-receptor-β signaling mediates photoimmune protection by UVA radiation in mice, J. Invest. Dermatol., 2009, 129, 2702–2710 CrossRef CAS.
- P. Amblard, Jc. Beani, R. Gautron, Jl. Reymond and B. Doyon, Statistical study of individual variations in sunburn sensitivity in 303 volunteers without photodermatosis, Arch. Dermatol. Res., 1982, 274, 195–206 CrossRef CAS.
- R. Kerb, J. Brockmoeller, T. Remm and I. Roots, Deficiency of glutathione S-transferases T1 and M1 as heritable factors of increased cutaneous UV sensitivity, J. Invest. Dermatol., 1997, 108, 229–232 CrossRef CAS.
- T. Gambichler, G. Moussa, N. S. Tomi, V. Paech, P. Altmeyer and A. Kreuter, Reference limits for erythema-effective ultraviolet doses, Photochem. Photobiol., 2006, 82, 1097–1102 CrossRef CAS.
- G. B. E. Jemec and M. Heidenheim, The influence of sex hormones on UVB induced erythema in man, J. Dermatol. Sci., 1995, 9, 221–224 CrossRef CAS.
- W. M. R. Broekmans, A. A. Vink, E. Boelsma, W. A. A. Klopping-Ketelaars, L. B. M. Tijburg, P. van't Veer, G. Van Poppel and A. F. M. Kardinaal, Determinants of skin sensitivity to solar irradiation, Eur. J. Clin. Nutr., 2003, 57, 1222–1229 CrossRef CAS.
- D. L. Damian, C. R. S. Patterson, M. Stapelberg, J. Park, R. St, C. Barnetson and G. M. Halliday, UV radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide, J. Invest. Dermatol., 2007, 128, 447–454.
- D. S. Preston and R. S. Stern, Nonmelanoma skin cancer, N. Engl. J. Med., 1992, 327, 1649–1662 CAS.
- J. A. Foote, R. B. Harris, A. R. Giuliano, D. J. Roe, T. E. Moon, B. Cartmel and D. S. Alberts, Predictors for cutaneous basal- and squamous-cell carcinoma among actinically damaged adults, Int. J. Cancer, 2001, 95, 7–11 CrossRef CAS.
- R. Molife, P. Lorigan and S. MacNeil, Gender and survival in malignant melanoma, Cancer Treat. Rev., 2001, 27, 201–209 CrossRef CAS.
- M. M. Welsh, M. R. Karagas, J. K. Kuriger, A. Houseman, S. K. Spencer, A. E. Perry and H. H. Nelson, Genetic determinants of UV-susceptibility in non-melanoma skin cancer, PLoS One, 2011, 6, e20019 CrossRef CAS.
- V. E. Reeve and R. M. Tyrrell, Heme oxygenase induction mediates the photoimmunoprotective activity of UVA radiation in the mouse, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 9317–9312 CrossRef CAS.
- R. M. Tyrrell and V. E. Reeve, Potential protection of skin by acute UVA irradiation–From cellular to animal models, Prog. Biophys. Mol. Biol., 2006, 92, 86–91 CrossRef CAS.
- E. J. Collinson, S. Wimmer-Kleikamp, S. K. Gerega, Y. H. Yang, C. R. Parrish, I. W. Dawes and R. Stocker, The yeast homolog of heme oxygenase-1 affords cellular antioxidant protection via the transcriptional regulation of known antioxidant genes, J. Biol. Chem., 2011, 286, 2205–2214 CrossRef CAS.
Footnote |
| † Contribution to the themed issue on the biology of UVA. |
| This journal is © The Royal Society of Chemistry and Owner Societies 2012 |
