Rapid synthesis of highly functionalised α-amino amides and medium ring lactones using multicomponent reactions of amino alcohols and isocyanides†
Martin
Bachman
,
Sam E.
Mann
and
Tom D.
Sheppard
*
Department of Chemistry, University College London, Christopher Ingold Laboratories, 20 Gordon St., London, UK WC1H 0AJ. E-mail: tom.sheppard@ucl.ac.uk
First published on 10th October 2011
Abstract
Four-component reactions between amino alcohols, aldehydes, isocyanides and thiols proceed rapidly under microwave or conventional heating at 60 °C in methanol. The reaction is successful with a wide range of components and gives access to potentially drug-like products containing amine, amide and thioether functionality in moderate to excellent yield. The reaction conditions are also applicable to the synthesis of a range of 8–10 membered medium ring lactonesvia three-component reactions of amino alcohols, isocyanides and acid-aldehydes. Incorporation of L-prolinol as the amino alcohol component in each case gives access to multicomponent products with moderate to high diastereoselectivity.
Introduction
Multicomponent reactions (MCRs)—reactions in which three or more reagents are combined to give a single product—enable the efficient synthesis of complex molecular structures in a single step.1 Reactions of this type which provide access to drug-like structures containing appropriate H-bond donors and acceptors are particularly valuable, as libraries of potentially bioactive compounds can be quickly synthesized. Isocyanides are widely used components in such reactions, as they typically lead to the formation of an amide or aromatic heterocycle in the resulting product.The Ugi reaction,2 the 4-component reaction (4-CR) between an amine, aldehyde, isocyanide and carboxylic acid, is of particular note, as it can provide access to large numbers of diverse amino acid amides from readily available starting materials. Acetals can potentially act as an alternative to the carbonyl component in an Ugi-type MCR, leading to more complex product skeletons and a greater scope for structural diversity (Scheme 1).3–6 In the case of an Ugi (or related) reaction, addition of the isocyanide to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) X bond leads to a molecule containing nucleophilic (XH) and electrophilic (nitrilium cation) sites in a 1,3 relationship (a), which then go on to react with the other components to generate the MCR product. By replacement of the C
X bond leads to a molecule containing nucleophilic (XH) and electrophilic (nitrilium cation) sites in a 1,3 relationship (a), which then go on to react with the other components to generate the MCR product. By replacement of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) X multiple bond with an acetal (b), a spacer is inserted between the nucleophilic (YH) and electrophilic (nitrilium cation) sites in the resulting adduct, providing an opportunity for greater diversity in the resulting MCR product.
X multiple bond with an acetal (b), a spacer is inserted between the nucleophilic (YH) and electrophilic (nitrilium cation) sites in the resulting adduct, providing an opportunity for greater diversity in the resulting MCR product.
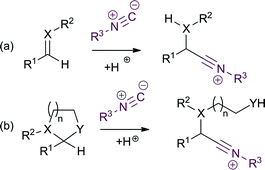 | ||
| Scheme 1 Replacement of a carbonyl derivative (a) with an acetal (b) to give a novel Ugi-like isocyanide MCR. | ||
We have recently reported that 1,3-oxazolidines 1 (simple N,O-acetals derived from ethanolamines) readily undergo 3-CRs with isocyanides and carboxylic acids to give N-acyloxyethylamino acid amides 3, via the cyclic imino ester intermediate 2 (Scheme 2).4,5
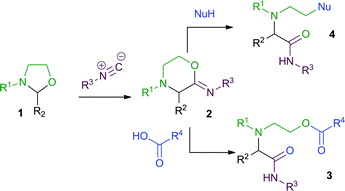 | ||
| Scheme 2 3-CRs of 1,3-oxazolidines. | ||
We also demonstrated that the carboxylic acid component could be replaced by thiophenol, thiobenzoic acid or 4-phenyltetrazole, providing access to different 3-CR products 4via nucleophilic ring-opening of intermediate 2.7 The yields for these latter 3-CRs with sulfur nucleophiles were low, and therefore only limited examples were explored. Given the fact that a large number of functionalised thiols are commercially available, we recognized that this reaction could be potentially valuable. We therefore sought to develop improved conditions for the reaction with a view to developing a more robust method applicable to 4-CRs, which could then be applied to a wide range of aldehydes, isocyanides, amino alcohols and thiols.
Results and discussion
4-CR of amino alcohols, aldehydes, isocyanides and thiols
As a representative reaction, we chose to optimise the 4-CR leading to amide 9a (Scheme 3). Our previous 3-CRs of pre-formed oxazolidines were carried out in MeCN in the presence of catalytic quantities of TsOH, but these conditions gave only a moderate yield of the product from a 4-CR. We subsequently found that MeOH was a superior solvent for this reaction, and that the acid catalyst was unnecessary. Although the reaction did proceed at room temperature, it was somewhat sluggish. However, heating the reaction at 60 °C for 20 min under microwave irradiation led to the formation of 4-CR product 9a in 81% isolated yield.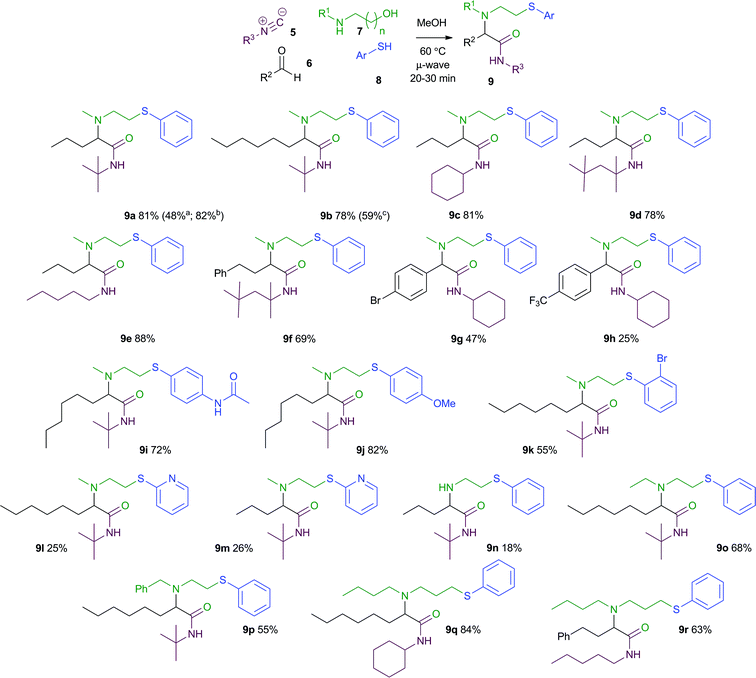 | ||
| Scheme 3 a TsOH, MeCN, reflux, 24 h (NMR yield). b Yield of 4-CR under conventional heating (60 °C, MeOH, 20 min). c Yield obtained under 3-CR conditions from the corresponding 1,3-oxazolidine (TsOH, MeCN, reflux, 24 h).5 | ||
These optimized reaction conditions were then applied to a wide range of isocyanides, aldehydes, thiols and amino alcohols (Scheme 3). The yields of this new 4-CR were superior to those we employed previously in the corresponding 3-CR (9b)5 and could be applied to a range of aldehydes, including both aliphatic systems (e.g.9a, 9b, 9f) and functionalised aryl aldehydes (e.g.9g, 9h). Several different isocyanides could be employed, enabling the nitrogen substituent on the amide to be varied (e.g.9a, 9c, 9d). A variety of commercially available aromatic thiols, including those bearing bromide (9k), methoxy (9j) and acetamide substituents (9i) gave multicomponent products in good yield. 2-Pyridyl thiol could also be employed providing access to MCR products containing heteroaromatic rings, albeit in lower yield (9l and 9m). In terms of the amino alcohol component, a variety of N-substituted ethanolamines could be used (e.g.9a, 9o, 9p). The scaffold of the MCR products could also be extended by incorporating an N-substituted 1,3-propanolamine as one of the components (e.g.9q, 9r), with good yields of the homologous MCR products being obtained. Unsubstituted ethanolamine could also be used, although this gave the corresponding secondary amine product 9n in low yield. Although the reactions were typically carried out under microwave heating, an equally high yield was obtained for one example under thermal conditions (9a).
3-CR of acid-aldehydes, amino alcohols and isocyanides to give medium ring lactones
In our previous report,5 we also carried out a brief examination of the 3-CR of an acid-aldehyde,8amino alcohol and isocyanide to give medium ring lactone products (Scheme 4). Under our original conditions (TsOH, iPrOH), prolonged reaction times were required and only moderate yields of the MCR products were obtained. Pleasingly under our new microwave conditions this reaction was also successful, giving rapid access to a wide range of medium ring lactone products in moderate to excellent yield. Structural variation of the amino alcohol and/or the acid-aldehyde components enabled a variety of eight- (10a–10c), nine- (10d–10f) and ten-membered (10g, 10h) lactones to be accessed. A bis-secondary amine component6 could also be used to synthesise an eight-membered lactam (10i). The synthesis of medium rings is often difficult due to the unfavourable thermodynamics of the ring closing process.9 These reactions are therefore potentially valuable as they offer an efficient one-step route to densely functionalised medium ring systems from readily available starting materials.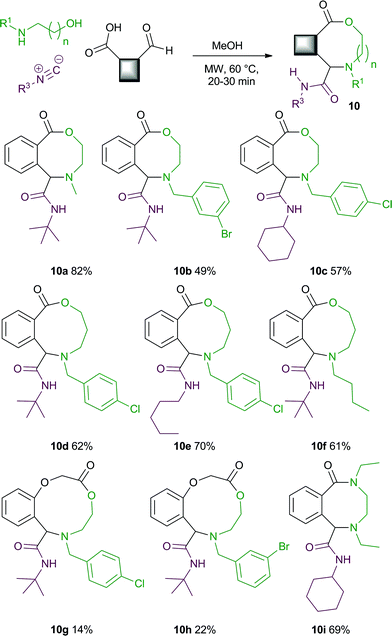 | ||
| Scheme 4 3-CRs of acid-aldehydes, amino alcohols and isocyanides to give medium ring lactones. | ||
We also examined the use of L-prolinol as a chiral amino alcohol component in both of these MCRs (Fig. 1).10 Interestingly, the 4-CR products 11a–11c were obtained as single diastereoisomers in good yield.11 In contrast, the 3-CR of L-prolinol with an acid-aldehyde and an isocyanide led to the diastereomeric eight-membered lactones 12 in a 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Pleasingly, these lactones could be easily separated by chromatography and the relative stereochemistry of each isomer was then assigned using nOe experiments.12
1 ratio. Pleasingly, these lactones could be easily separated by chromatography and the relative stereochemistry of each isomer was then assigned using nOe experiments.12
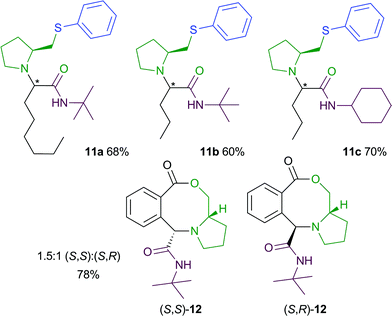 | ||
| Fig. 1 4-CR and 3-CR products obtained using L-prolinol. | ||
Conclusions
In conclusion, we have developed a generally high yielding and rapid procedure for the 4-CR of amino alcohols, aldehydes, thiols and isocyanides to give arylthioalkylamino acid amides. The reaction conditions can also be applied to the synthesis of medium ring 3-CR products by reaction of an amino alcohol and isocyanide with a bifunctional acid-aldehyde. In addition, we have shown that diastereoselective MCRs can be achieved in some cases using L-prolinol as a chiral amino alcohol component. Further work is underway on the development of other novel MCRs which will be reported in due course.Experimental section
All solvents and chemicals were used as obtained from commercial suppliers. Column chromatography was carried out using BDH (40–63 μm) silica gel and analytical thin layer chromatography was carried out using Merck Kieselgel aluminium-backed plates coated with silica gel. Components were visualised using combinations of ultra-violet light, phosphomolybdic acid and potassium permanganate. Melting points were determined using a Reichert hot-stage apparatus and are uncorrected. Optical rotations were measured using a Perkin-Elmer 343 polarimeter (sodium D-line, 529 nm) and αD values are given in 10−1 deg cm2 g−1, concentration (c) in g per 100 mL. Infrared (IR) spectra were recorded on a Perkin-Elmer spectrum 100 FT-IR spectrometer as thin films. 1H and 13C NMR spectra were recorded respectively at 400 MHz and 100 MHz on a Bruker Avance 400 spectrometer or at 600 MHz and 150 MHz on a Bruker Avance 600 spectrometer in the stated solvent. Mass spectra were obtained using either a VG70-SE or MAT 900XP spectrometer at the Department of Chemistry, University College London.General procedure for 4-CRs of amino alcohols, aldehydes, isocyanides and thiols
Aldehyde (1.0 mmol), isocyanide (1.0 mmol) and thiophenol (1.0 mmol) were added to a solution of amino alcohol (1.0 mmol) in methanol (1 ml). The mixture was stirred under microwave irradiation13 at 60 °C for 20 min. The solvent was evaporated and the crude product was purified by flash chromatography (petroleum ether/EtOAc 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford the amide.
1) to afford the amide.
N-tert-Butyl-2-(methyl(2-(phenylthio)ethyl)amino)pentanamide (9a)
Colourless oil. 81% yield. νmax/cm−1 3336 (NH), 2960, 2871 (CH), 1668 (CO), 1584, 1509, 1453 (Ar); δH (600 MHz; CDCl3; Me4Si) 0.90 (3 H, t, J 7.3, CH2CH3), 1.29–1.35 (1 H, m, alkyl), 1.34 (9 H, s, tBu), 1.41–1.47 (1 H, m, alkyl), 1.49–1.54 (1 H, m, alkyl), 1.69–1.75 (1 H, m, alkyl), 2.25 (3 H, s, NCH3), 2.76 (1 H, dt, J 13.4, 6.8, NCHH), 2.78 (1 H, dt, J 13.4, 6.5, NCHH), 2.87 (1 H, dd, J 7.0, 5.3, NCH), 3.04 (1 H, dt, J 12.8, 6.5, SCHH), 3.07 (1 H, dt, J 12.8, 6.8, SCHH), 7.18 (1 H, t, J 7.7, Ar), 7.20 (1 H, s, NH), 7.28 (2 H, app. t, J 7.7, Ar), 7.33 (2 H, dd, J 7.7, 1.2, Ar); δC (150 MHz; CDCl3; Me4Si) 14.4, 21.0, 28.9, 29.4, 32.9, 37.5, 50.6, 53.8, 68.6, 126.2, 129.1, 129.3, 136.5, 172.6; LRMS (CI) 323, 275, 222, 189; HRMS calcd for C18H31N2OS [MH]+ 323.2157, found 323.2153.N-(tert-Butyl)-2-(methyl(2-(phenylthio)ethyl)amino)octanamide (9b)5
Colourless oil. 78% yield. νmax/cm−1 3335 (NH), 2957, 2927; 2856 (CH), 1661 (CO), 1585, 1509, 1453 (Ar); δH (400 MHz; CDCl3; Me4Si) 0.87 (3 H, t, J 6.7, CH2CH3), 1.23–1.32 (7 H, m, alkyl), 1.35 (9 H, s, tBu), 1.37–1.47 (1 H, m, alkyl), 1.49–1.60 (1 H, m, alkyl), 1.69–1.81 (1 H, m, alkyl), 2.26 (3 H, s, NCH3), 2.76 (1 H, dt, J 13.4, 7.0, NCHH), 2.80 (1 H, dt, J 13.4, 6.5, NCHH), 2.87 (1 H, dd, J 7.0, 5.8, NCH), 3.04 (1 H, dt, J 13.0, 6.5, SCHH), 3.08 (1 H, dt, J 13.0, 7.0, SCHH), 7.15–7.22 (2H, m, Ar, NH), 7.28 (2H, br t, J 8.0, Ar), 7.34 (2H, br d, J 8.0, Ar); δC (100 MHz; CDCl3; Me4Si) 14.1, 22.6, 27.2, 27.5, 28.8, 29.6, 31.7, 32.8, 37.4, 50.4, 53.8, 68.7, 126.1, 129.0, 129.2, 136.4, 172.4; LRMS (ES) 363, 279, 227; HRMS calcd for C21H35N2OS [M−H]− 363.2470, found 363.2470.N-Cyclohexyl-2-(methyl(2-(phenylthio)ethyl)amino)pentanamide (9c)
Colourless oil. 81% yield. νmax/cm−1 3314 (NH), 2929, 2853 (CH), 1641 (CO), 1584, 1509, 1450 (Ar); δH (600 MHz; CDCl3; Me4Si) 0.89 (3 H, t, J 7.2, CH2CH3), 1.11–1.20 (3 H, m, cy), 1.28–1.38 (3 H, m, cy), 1.39–1.46 (1 H, m, alkyl), 1.50–1.60 (2 H, m, alkyl), 1.64–1.77 (3 H, m, alkyl), 1.81–1.87 (2 H, m, cy), 2.25 (3 H, s, NCH3), 2.76 (1 H, dt, J 13.1, 6.8, NCHH), 2.78 (1 H, dt, J 13.1, 6.5, NCHH), 2.95 (1 H, t, J 6.4, NCHCO), 3.03 (1 H, dt, J 13.1, 6.5, SCHH), 3.06 (1 H, dt, J 13.1, 6.8, SCHH), 3.68–3.76 (1 H, m, NHCH), 7.18 (1 H, tt, J 7.7, 1.4, Ar), 7.21 (1 H, br d, J 7.8, NH), 7.27 (2 H, app. t, J 7.7, Ar), 7.32 (2 H, dd, J 7.7, 1.4, Ar); δC (150 MHz; CDCl3; Me4Si) 14.4, 20.8, 24.9, 25.7, 29.7, 32.9, 33.3, 37.6, 47.8, 53.9, 68.0, 126.3, 129.1, 129.4, 136.4, 172.3; LRMS (CI) 349, 239, 222, 137; HRMS calcd for C20H33N2OS [MH]+ 349.2314, found 349.2326.2-(Methyl(2-(phenylthio)ethyl)amino)-N-(2,4,4-trimethylpentan-2-yl)pentanamide (9d)
Colourless oil. 78% yield. νmax/cm−1 3334 (NH), 2955, 2871 (CH), 1671 (CO), 1585, 1507, 1480 (Ar); δH (600 MHz; CDCl3; Me4Si) 0.90 (3 H, t, J 7.2, CH2CH3), 1.00 (9 H, s, tBu), 1.28–1.37 (1 H, m, alkyl), 1.40 (6 H, s, 2 × CH3), 1.42–1.52 (2 H, m, alkyl), 1.59 (1 H, d, J 14.9, CHHtBu), 1.72–1.80 (1 H, m, alkyl), 1.83 (1 H, d, J 14.9, CHHtBu), 2.24 (3 H, s, NCH3), 2.78 (1 H, dt, J 13.2, 6.9, NCHH), 2.81 (1 H, dt, J 13.2, 6.4, NCHH), 2.87 (1 H, t, J 6.4, NCH), 3.03 (1 H, dt, J 12.8, 6.4, SCHH), 3.06 (1 H, dt, J 12.8, 6.9, SCHH), 7.17 (1 H, t, J 7.5, Ar), 7.27 (2 H, app. t, J 7.5, Ar), 7.31 (2 H, d, J 7.5, Ar), 7.33 (1 H, br s, NH); δC (150 MHz; CDCl3; Me4Si) 14.4, 21.4, 28.7, 28.9, 29.2, 31.7, 32.9, 37.6, 52.5, 54.0, 54.5, 68.5, 126.2, 129.1, 129.2, 136.5, 172.2; LRMS (CI) 379, 269, 222, 137; HRMS calcd for C22H39N2OS [MH]+ 379.2783, found 379.2777.2-(Methyl(2-(phenylthio)ethyl)amino)-N-pentylpentanamide (9e)
Colourless oil. 88% yield. νmax/cm−1 3334 (NH), 2960, 2932, 2873 (CH), 1655 (CO), 1518, 1481, 1439 (Ar); δH (400 MHz; CDCl3; Me4Si) 0.86 (3 H, t, J 6.9, CH3), 0.88 (3 H, t, J 7.3, CH3), 1.22–1.37 (5 H, m, alkyl), 1.37–1.58 (4 H, m, alkyl), 1.69–1.80 (1 H, m, alkyl), 2.24 (3 H, s, NCH3), 2.76 (2 H, app. t, J 6.5, CH2NMe), 2.96 (1 H, t, J 6.4, NCH), 3.02 (1 H, dt, J 13.1, 6.5, SCHH), 3.05 (1 H, dt, J 13.1, 6.5, SCHH), 3.10–3.26 (2 H, m, CH2NH), 7.14 (1 H, t, J 7.5, Ar), 7.25 (2 H, app. t, J 7.5, Ar), 7.30 (2 H, d, J 7.5, Ar), 7.32 (1 H, br s, NH); δC (100 MHz; CDCl3; Me4Si) 14.0, 14.2, 20.7, 22.3, 29.2, 29.3, 29.5, 32.7, 37.6, 39.0, 53.8, 67.9, 126.0, 128.9, 129.0, 136.3, 173.0; LRMS (CI) 337, 222, 137; HRMS calcd for C19H33N2OS [MH]+ 337.2314, found 337.2314.2-(Methyl(2-(phenylthio)ethyl)amino)-4-phenyl-N-(2,4,4-trimethylpentan-2-yl)butanamide (9f)
Colourless oil. 69% yield. νmax/cm−1 3338 (NH), 2950 (CH), 1672 (CO), 1584, 1507, 1454 (Ar); δH (600 MHz; CDCl3; Me4Si) 1.02 (9 H, s, tBu), 1.42 (3 H, s, NCCH3), 1.44 (3 H, s, NCCH3), 1.60 (1 H, d, J 14.9, CHHtBu), 1.72–1.80 (1 H, m, CHHCH), 1.90 (1 H, d, J 14.9, CHHtBu), 2.10–2.18 (1 H, m, CHHCH), 2.25 (3 H, s, NCH3), 2.64 (1 H, ddd, J 13.5, 9.8, 6.9, CHHPh), 2.71 (1 H, dt, J 12.9, 6.5, NCHH), 2.77 (1 H, dt, J 12.9, 6.5, NCHH), 2.85–3.00 (4 H, m, CHHPh, NCH, SCH2), 7.15–7.21 (4 H, m, Ar), 7.24–7.31 (6 H, m, Ar), 7.31 (1 H, br s, NH); δC (150 MHz; CDCl3; Me4Si) 27.6, 28.8, 29.3, 31.7, 31.8, 32.7, 34.2, 37.6, 52.4, 54.7, 66.9, 126.0, 126.2, 128.5, 128.7, 129.1, 129.2, 136.4, 142.1, 171.9; LRMS (CI) 441, 331, 284; HRMS calcd for C23H32N2OS [MH]+ 441.2940, found 441.2936.2-(4-Bromophenyl)-N-cyclohexyl-2-(methyl(2-(phenylthio)ethyl)amino)acetamide (9g)
White solid. 47% yield. M.p. 114–115 °C (hexanes); νmax/cm−1 3296 (NH), 2928, 2851 (CH), 1645 (CO), 1584, 1521, 1480 (Ar); δH (400 MHz; CDCl3; Me4Si) 1.14–1.29 (3 H, m, cy), 1.30–1.44 (2 H, m, cy), 1.58–1.67 (1 H, m, cy), 1.67–1.78 (2 H, m, cy), 1.80–1.95 (2 H, m, cy), 2.20 (3 H, s, NCH3), 2.66 (2 H, app. t, J 6.7, NCH2), 3.06 (1 H, dt, J 13.2, 6.7, SCHH), 3.09 (1 H, dt, J 13.2, 6.7, SCHH), 3.70–3.82 (1 H, m, NHCH), 3.96 (1 H, s, NCHAr), 7.15 (2 H, d, J 8.3, Ar), 7.19–7.27 (2 H, m, Ar), 7.28–7.32 (4 H, m, Ar, NH), 7.44 (2 H, d, J 8.3, Ar); δC (100 MHz; CDCl3; Me4Si) 24.8, 25.5, 31.9, 33.0, 39.4, 47.8, 53.9, 74.4, 122.1, 126.2, 129.0, 129.2, 130.7, 131.5, 134.9, 136.1, 169.7; LRMS (CI) 463, 461, 352, 336; HRMS calcd for C23H30N2OSBr [MH]+ 461.1262, found 461.1271.N-Cyclohexyl-2-(methyl(2-(phenylthio)ethyl)amino)-2-(4-(trifluoromethyl)phenyl)acetamide (9h)
White solid. 25% yield. M.p. 97–98 °C (hexanes); νmax/cm−1 3302 (NH), 2933, 2853 (CH), 1649 (CO), 1583, 1543, 1481 (Ar); δH (600 MHz; CDCl3; Me4Si) 1.13–1.28 (3 H, m, cy), 1.28–1.40 (2 H, m, cy), 1.56–1.64 (1 H, m, cy), 1.66–1.75 (2 H, m, cy), 1.78–1.92 (2 H, m, cy), 2.18 (3 H, s, NCH3), 2.64 (2 H, app. t, J 6.7, NCH2), 3.06 (1 H, dt, J 13.3, 6.7, SCHH), 3.09 (1 H, dt, J 13.3, 6.7, SCHH), 3.70–3.78 (1 H, m, NHCH), 4.05 (1 H, s, NCHAr), 7.18 (1 H, tt, J 7.7, 1.5, Ar), 7.24–7.30 (4 H, m, Ar), 7.31 (1 H, br s, NH), 7.37 (2 H, d, J 7.7, Ar), 7.44 (2 H, d, J 7.7, Ar); δC (150 MHz; CDCl3; Me4Si) 24.9, 25.6, 32.0, 33.1, 39.4, 48.0, 54.0, 74.6, 124.2 (q, J 272), 125.4 (q, J 3.6), 126.4, 129.2, 129.3, 129.6, 130.2 (q, J 32.4), 136.1, 139.9, 169.6; LRMS (ES) 449, 339, 324, 189; HRMS calcd for C24H28N2OSF3 [M−H]− 449.1874, found 449.1874.2-((2-((4-Acetamidophenyl)thio)ethyl)(methyl)amino)-N-(tert-butyl)octanamide (9i)
Colourless oil. 72% yield. νmax/cm−1 3307 (NH), 2958, 2928, 2857 (CH), 1651 (CO), 1593, 1521, 1493, 1454 (Ar); δH (600 MHz; CDCl3; Me4Si) 0.84 (3 H, t, J 6.6, CH2CH3), 1.17–1.28 (7 H, m, alkyl), 1.33 (9 H, s, tBu), 1.29–1.40 (1 H, m, alkyl), 1.48–1.56 (1 H, m, alkyl), 1.67–1.75 (1 H, m, alkyl), 2.15 (3 H, s, CH3CO), 2.22 (3 H, s, NCH3), 2.72 (2 H, app. t, J 6.6, NCH2), 2.83 (1 H, t, J 6.2, NCH), 2.97 (1 H, dt, J 12.9, 6.6, SCHH), 3.00 (1 H, dt, J 12.9, 6.6, SCHH), 7.24 (1 H, br s, NHtBu), 7.29 (2 H, d, J 8.3, Ar), 7.46 (2 H, d, J 8.3, Ar), 7.93 (1 H, br s, NHAc); δC (150 MHz; CDCl3; Me4Si) 14.2, 22.7, 24.6, 27.4, 27.6, 28.9, 29.7, 31.8, 33.8, 37.4, 50.6, 54.0, 68.9, 120.6, 130.9, 131.1, 137.0, 168.8, 172.8; LRMS (CI) 422, 366, 321, 255; HRMS calcd for C23H40N3O2S [MH]+ 422.2841, found 422.2833.N-(tert-Butyl)-2-((2-((4-methoxyphenyl)thio)ethyl)(methyl)amino)octanamide (9j)
Colourless oil. 82% yield. νmax/cm−1 3331 (NH), 2957, 2926, 2855 (CH), 1671 (CO), 1593, 1493, 1453 (Ar); δH (400 MHz; CDCl3; Me4Si) 0.87 (3 H, t, J 6.7, CH2CH3), 1.24–1.32 (7 H, m, alkyl), 1.35 (9 H, s, tBu), 1.33–1.45 (1 H, m, alkyl), 1.48–1.58 (1 H, m, alkyl), 1.68–1.80 (1 H, m, alkyl), 2.24 (3 H, s, NCH3), 2.72 (2 H, app. t, J 6.7, CH2N), 2.86 (1 H, dd, J 7.0, 5.8, NCH), 2.93 (1 H, dt, J 13.0, 6.7, SCHH), 2.96 (1 H, dt, J 13.0, 6.7, SCHH), 3.80 (3 H, s, OCH3), 6.85 (2 H, d, J 8.8, Ar), 7.24 (1 H, br s, NH), 7.35 (2 H, d, J 8.8, Ar); δC (100 MHz; CDCl3; Me4Si) 14.1, 22.6, 27.2, 27.5, 28.8, 29.6, 31.7, 34.9, 37.4, 50.4, 54.0, 55.3, 68.6, 114.7, 126.2, 133.3, 159.1, 172.5; LRMS (CI) 395, 229, 173, 105; HRMS calcd for C22H39N2O2S [MH]+ 395.2732, found 395.2727.2-((2-((2-Bromophenyl)thio)ethyl)(methyl)amino)-N-(tert-butyl)octanamide (9k)
Colourless oil. 55% yield. νmax/cm−1 3342 (NH), 2956, 2926, 2856 (CH), 1665 (CO), 1576, 1509, 1449 (Ar); δH (400 MHz; CDCl3; Me4Si) 0.89 (3 H, t, J 6.8, CH2CH3), 1.24–1.35 (7 H, m, alkyl), 1.37 (9 H, s, tBu), 1.40–1.50 (1 H, m, alkyl), 1.51–1.63 (1 H, m, alkyl), 1.71–1.84 (1 H, m, alkyl), 2.30 (3 H, s, NCH3), 2.85 (1 H, dt, J 13.0, 6.7, NCHH), 2.89 (1 H, dt, J 13.0, 6.3, NCHH), 2.92 (1 H, dd, J 7.2, 5.4, NCH), 3.07 (1 H, dt, J 12.4, 6.3, SCHH), 3.12 (1 H, dt, J 12.4, 6.7, SCHH), 7.05 (1 H, ddd, J 8.0, 6.4, 2.5, Ar), 7.12 (1 H, br s, NH), 7.26–7.30 (2 H, m, Ar), 7.56 (1 H, dd, J 8.0, 1.1, Ar); δC (100 MHz; CDCl3; Me4Si) 14.1, 22.6, 27.1, 27.6, 28.8, 29.6, 31.7, 31.9, 37.4, 50.5, 53.1, 68.7, 123.6, 126.6, 127.8, 128.0, 133.1, 138.1, 172.3; LRMS (CI) 445, 443, 344, 255; HRMS calcd for C21H36N2OSBr [MH]+ 443.1732, found 443.1715.N-(tert-Butyl)-2-(methyl(2-(pyridin-2-ylthio)ethyl)amino)octanamide (9l)
Colourless oil. 25% yield. νmax/cm−1 3330 (NH), 2956, 2926, 2856 (CH), 1671 (CO), 1578, 1509, 1453, 1414 (Ar); δH (600 MHz; CDCl3; Me4Si) 0.85 (3 H, t, J 6.8, CH2CH3), 1.21–1.29 (7 H, m, alkyl), 1.31 (9 H, s, tBu), 1.36–1.46 (1 H, m, alkyl), 1.53–1.61 (1 H, m, alkyl), 1.69–1.77 (1 H, m, alkyl), 2.28 (3 H, s, NCH3), 2.79 (1 H, dt, J 13.3, 6.5, NCHH), 2.83 (1 H, dt, J 13.3, 6.8, NCHH), 2.89 (1 H, br t, J 5.4, NCH), 3.32 (1 H, dt, J 13.4, 6.5, SCHH), 3.35 (1 H, dt, J 13.4, 6.8, SCHH), 6.97 (1 H, dd, J 6.8, 5.0, Ar), 7.15 (1 H, d, J 8.0, Ar), 7.18 (1 H, br s, NH), 7.46 (1 H, app. td, J 8.0, 1.6, Ar), 8.40 (1 H, br d, J 5.0, Ar); δC (150 MHz; CDCl3; Me4Si) 14.2, 22.8, 27.4, 27.6, 28.6, 28.8, 29.7, 31.8, 37.7, 50.5, 54.1, 68.9, 119.5, 122.4, 136.0, 149.6, 158.8, 172.7; LRMS (CI) 366, 229, 173; HRMS calcd for C20H36N3OS [MH]+ 366.2579, found 366.2564.N-(tert-Butyl)-2-(methyl(2-(pyridin-2-ylthio)ethyl)amino)pentanamide (9m)
Colourless oil. 26% yield. νmax/cm−1 3331 (NH), 2954, 2854 (CH), 1670 (CO), 1578, 1508, 1453, 1415 (Ar); δH (600 MHz, CDCl3) 0.91 (3 H, t, J 7.3, CH2CH3), 1.31–1.36 (10 H, m, CH3CHH, tBu), 1.44–1.47 (1 H, m, CH3CHH), 1.52–1.57 (1 H, m, NCHCHH), 1.69–1.73 (1 H, m, NCHCHH), 2.29 (3 H, s, NCH3), 2.78–2.84 (2 H, m, SCH2), 2.90 (1 H, dd, J 7.2, 5.5, NCH), 3.34 (2 H, m, NCH2), 6.97 (1 H, ddd, J 7.6, 4.8, 0.9, Ar), 7.16 (1 H, br d, J 7.8, Ar), 7.18 (1 H, br s, NH), 7.46 (1 H, ddd, J 7.8, 7.6, 1.8, Ar), 8.40 (1 H, br d, J 4.8, Ar); δC (150 MHz, CDCl3) 14.5, 20.9, 28.6, 28.9, 29.5, 37.8, 50.5, 54.0, 68.6, 119.5, 122.4, 136.0, 149.6, 158.8, 172.7; LRMS (CI) 324, 223, 221, 213, 84; HRMS calcd for C17H30N3OS [MH]+ 324.2110, found 324.2111.N-(tert-Butyl)-2-((2-(phenylthio)ethyl)amino)pentanamide (9n)
Colourless oil. 18% yield. νmax/cm−1 3314 (NH), 2960, 2931, 2872 (CH), 1652 (CO), 1584, 1517, 1453 (Ar); δH (600 MHz; CDCl3; Me4Si) 0.90 (3 H, t, J 7.4, CH2CH3), 1.27–1.42 (2 H, m, alkyl), 1.31 (9 H, s, tBu), 1.44–1.52 (1 H, m, alkyl), 1.61–1.68 (1 H, m, alkyl), 1.77 (1 H, br s, NHtBu), 2.73 (1 H, ddd, J 12.4, 7.0, 5.6, NCHH), 2.81 (1 H, ddd, J 12.4, 6.6, 5.4, NCHH), 2.88 (1 H, dd, J 7.8, 4.8, NCH), 2.99 (1 H, ddd, J 13.1, 6.6, 5.6, SCHH), 3.05 (1 H, ddd, J 13.1, 7.0, 5.4, SCHH), 7.14 (1 H, br s, CHNH), 7.19 (1 H, t, J 7.7, Ar), 7.27 (2 H, app. t, J 7.7, Ar), 7.33 (2 H, d, J 7.7, Ar); δC (150 MHz; CDCl3; Me4Si) 14.1, 19.2, 28.8, 34.6, 36.0, 47.2, 50.4, 63.3, 126.5, 129.1, 129.7, 135.7, 173.5; LRMS (CI) 309, 208, 130; HRMS calcd for C17H29N2OS [MH]+ 309.2001, found 309.2007.N-(tert-Butyl)-2-(ethyl(2-(phenylthio)ethyl)amino)octanamide (9o)
White solid. 68% yield. M.p. 52–53 °C (ether); νmax/cm−1 3334 (NH), 2961, 2926, 2854 (CH), 1674 (CO), 1585, 1508, 1453 (Ar); δH (600 MHz; CDCl3; Me4Si) 0.85 (3 H, t, J 6.9, CH2CH2CH3), 0.99 (3 H, t, J 7.1, NCH2CH3), 1.21–1.30 (7 H, m, alkyl), 1.32 (9 H, s, tBu), 1.41–1.50 (2 H, m, alkyl), 1.74–1.83 (1 H, m, alkyl), 2.49 (1 H, dq, J 13.2, 7.1, NCHHCH3), 2.54 (1 H, dq, J 13.2, 7.1, NCHHCH3), 2.78 (1 H, dt, J 13.5, 7.0, NCHH), 2.81 (1 H, dt, J 13.5, 6.8, NCHH), 2.97–3.05 (3 H, m, NCH, SCH2), 7.16 (1 H, tt, J 6.9, 1.4, Ar), 7.24–7.28 (2 H, m, Ar), 7.29–7.32 (2 H, m, Ar), 7.36 (1 H, br s, NH); δC (150 MHz; CDCl3; Me4Si) 13.6, 14.2, 22.8, 26.6, 28.5, 28.9, 29.7, 31.8, 33.2, 44.4, 49.6, 50.5, 65.3, 126.2, 129.1, 129.2, 136.4, 173.3; LRMS (CI) 379, 243, 159, 143; HRMS calcd for C22H39N2OS [MH]+ 379.2783, found 379.2779.2-(Benzyl(2-(phenylthio)ethyl)amino)-N-(tert-butyl)octanamide (9p)
Colourless oil. 55% yield. νmax/cm−1 3338 (NH), 2957, 2926, 2856 (CH), 1671 (CO), 1584, 1508, 1453 (Ar); δH (400 MHz; CDCl3; Me4Si) 0.92 (3 H, t, J 6.8, CH2CH3), 1.26–1.36 (7 H, m, alkyl), 1.37 (9 H, s, tBu), 1.47–1.64 (2 H, m, alkyl), 1.82–1.94 (1 H, m, alkyl), 2.83–3.11 (5 H, m, NCH2, SCH2, NCH), 3.63 (1 H, d, J 13.8, ArCHH), 3.80 (1 H, d, J 13.8, ArCHH), 6.85 (1 H, br s, NH), 7.14–7.19 (1 H, m, Ar), 7.22–7.37 (9 H, m, Ar); δC (150 MHz; CDCl3; Me4Si) 14.3, 22.8, 25.9, 28.6, 28.9, 29.8, 31.9, 32.7, 49.3, 50.7, 55.3, 64.4, 126.1, 127.5, 128.6, 128.9, 129.0, 129.1, 136.4, 139.1, 172.7; LRMS (CI) 441, 363, 171; HRMS calcd for C27H41N2OS [MH]+ 441.2940, found 441.2940.2-(Butyl(3-(phenylthio)propyl)amino)-N-cyclohexyloctanamide (9q)
Colourless oil. 84% yield. νmax/cm−1 3376 (NH), 2926, 2854 (CH), 1623 (CO), 1583, 1449, 1439 (Ar); δH (600 MHz; CDCl3; Me4Si) 0.78 (3 H, t, J 7.3, CH3), 0.87 (3 H, t, J 7.1, CH3), 0.87–1.66 (21 H, m, alkyl), 1.71–1.81 (4 H, m, alkyl), 1.81–1.89 (1 H, m, alkyl), 2.30 (1 H, ddd, J 12.9, 11.0, 5.2, NCHHPr), 2.47 (1 H, ddd, J 12.9, 11.0, 5.2, NCHHPr), 2.49–2.54 (1 H, m, NCHHCH2CH2S), 2.95 (1 H, ddd, J 12.9, 9.1, 3.5, NCHHCH2CH2S), 3.39 (1 H, dd, J 9.5, 4.3, NCHCO), 3.64–3.73 (2 H, m, SCH2), 3.79–3.86 (1 H, m, NHCH), 5.00 (1 H, br s, NH), 7.28–7.36 (3 H, m, Ar), 7.41–7.47 (2 H, m, Ar); δC (150 MHz; CDCl3; Me4Si) 14.1, 14.2, 20.8, 22.7, 24.5, 25.9, 26.4, 26.5, 28.4, 29.5, 30.2, 31.8, 33.2, 49.9, 50.9, 61.7, 61.9, 64.5, 128.5, 129.3, 132.0, 134.9, 156.7; LRMS (ES) 447, 393, 282; HRMS calcd for C27H47N2OS [MH]+ 447.3409, found 447.3405.2-(Butyl(3-(phenylthio)propyl)amino)-N-pentyl-4-phenylbutanamide (9r)
Colourless oil. 63% yield. νmax/cm−1 3365 (NH), 2955, 2928, 2858 (CH), 1622 (CO), 1583, 1496, 1455 (Ar); δH (600 MHz; CDCl3; Me4Si) 0.78 (3 H, t, J 7.5, CH3), 0.80–0.91 (2 H, m, alkyl), 0.95 (3 H, t, J 7.1, CH3), 1.03–1.16 (2 H, m, alkyl), 1.37–1.49 (5 H, m, alkyl), 1.55–1.63 (1 H, m, alkyl), 1.72–1.81 (3 H, m, alkyl, NCHCHH), 2.17–2.25 (1 H, m, NCHCHH), 2.30 (1 H, ddd, J 12.8, 10.8, 5.3, NCHHPr), 2.41 (1 H, ddd, J 13.8, 10.1, 6.9, NHCHH), 2.46 (1 H, ddd, J 12.8, 10.8, 5.3, NCHHPr), 2.61 (1 H, ddd, J 13.8, 10.1, 4.9, NHCHH), 2.52 (1 H, ddd, J 12.8, 5.4, 4.2, NCHHCH2CH2S), 2.95 (1 H, ddd, J 12.8, 9.0, 4.9, NCHHCH2CH2S), 3.53 (1 H, dd, J 9.3, 4.4, NCHCO), 3.57 (1 H, app. quin, J 7.0, CHHAr), 3.65 (1 H, app. quin, J 7.0, CHHAr), 3.64–3.69 (1 H, m, SCHH), 3.70–3.75 (1 H, m, SCHH), 4.64 (1 H, br s, NH), 7.15 (2 H, d, J 7.0, Ar), 7.19 (1 H, t, J 7.5, Ar), 7.26–7.33 (5 H, m, Ar), 7.37–7.40 (2 H, m, Ar); δC (150 MHz; CDCl3; Me4Si) 14.1, 14.3, 20.7, 22.6, 28.0, 28.7, 30.0, 30.0, 30.4, 32.9, 49.6, 50.9, 53.6, 61.5, 64.1, 126.0, 128.5, 128.6, 128.7, 129.4, 131.5, 134.9, 142.2, 159.6; LRMS (CI) 455, 248, 195; HRMS calcd for C28H43N2OS [MH]+ 455.3096, found 455.3103.General procedure for 3-component reactions of amino alcohols, isocyanides and acid-aldehydes
A solution of acid-aldehyde (1.00 mmol), amino alcohol (1.00 mmol) and isocyanide (1.00 mmol) in methanol (1 ml) was stirred under microwave irradiation13 at 60 °C for 20 min. The solvent was removed in vacuo and the residue purified by column chromatography (petroleum ether/EtOAc 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford the amide.
1) to afford the amide.
N-tert-Butyl-4-methyl-8-oxo-2,3,5-trihydrobenzo[f][1,4]oxazocine-5-carboxamide (10a)
White solid. 82% yield. M.p. 123–125 °C (hexanes); νmax/cm−1 3332 (NH), 2968 (CH), 1704 (CO ester), 1665 (CO amide), 1602, 1515, 1454 (Ar); δH (400 MHz; CDCl3; Me4Si) 1.29 (9 H, s, tBu), 2.43 (3 H, s, NCH3), 2.95–3.00 (1 H, m, NCHH), 3.05–3.09 (1 H, m, NCHH), 3.83–3.87 (1 H, m, OCHH), 4.07–4.11 (1 H, m, OCHH), 4.21 (1 H, s, NCH), 6.79 (1 H, br s, NH), 7.14 (1 H, d, J 7.0, Ar), 7.33–7.36 (3 H, m, Ar); δC (150 MHz; CDCl3; Me4Si) 28.5, 42.2, 51.2, 55.7, 64.8, 71.5, 128.3, 128.5, 128.6, 130.2, 130.8, 134.8, 168.7, 173.3; LRMS (CI) 291, 206, 190; HRMS calcd for C16H23N2O3 [MH]+ 291.1709, found 291.1713.4-(3-Bromobenzyl)-N-(tert-butyl)-8-oxo-2,3,5-trihydrobenzo[f][1,4]oxazocine-5-carboxamide (10b)
Pale yellow solid. 49% yield. M.p. 135–136 °C (hexanes); νmax/cm−1 3344 (NH), 2966 (CH), 1706, 1674 (CO), 1538 (Ar); δH (600 MHz, CDCl3, Me4Si) 1.30 (9 H, s, tBu), 2.80 (1 H, ddd, J 14.2, 6.9 and 4.4, OCH2CHH), 3.15 (1 H, ddd, J 14.2, 6.0 and 4.5, OCH2CHH), 3.64 (1 H, br d, J 14.4, ArCHH), 3.72 (1 H, d, J 14.4, ArCHH), 3.84 (1 H, ddd, J 12.7, 6.0 and 4.5, OCHH), 4.07 (1 H, ddd, J 12.7, 6.9 and 4.4, OCHH), 4.40 (1 H, s, COCH), 6.57 (1 H, br s, NH), 7.20–7.21 (1 H, m, Ar), 7.22 (1 H, t, J 7.7, Ar), 7.27 (1 H, ddd, J 7.7, 1.6 and 1.3, Ar), 7.35–7.36 (3 H, m, Ar), 7.39 (1 H, ddd, J 7.8, 2.0 and 1.2, Ar), 7.44 (1 H, t, J 1.9, Ar); δC (150 MHz, CDCl3) 28.7, 51.1, 51.7, 58.2, 65.0, 71.1 (br), 122.9, 127.0, 128.2, 128.67, 128.74, 130.4, 130.7, 130.8, 130.9, 131.5, 135.5, 140.1, 168.9, 173.8 (br); LRMS (CI) 447, 445, 348, 346, 259, 84; HRMS calcd for C22H26O3N2Br [MH]+ 445.1127, found 445.1116.4-(4-Chlorobenzyl)-N-cyclohexyl-8-oxo-2,3,5-trihydrobenzo[f][1,4]oxazocine-5-carboxamide (10c)
Pale yellow solid. 57% yield. M.p. 117–119 °C (hexanes); νmax/cm−1 3307 (NH), 2929 (CH), 1703, 1665 (CO), 1540 (Ar); δH (600 MHz, CDCl3, Me4Si) 1.06–1.18 (3 H, m, Cy), 1.26–1.35 (2 H, m, Cy), 1.55–1.57 (1 H, br m, Cy), 1.63–1.66 (2 H, m, Cy), 1.73 (1 H, m, Cy), 1.83 (1 H, m, Cy), 2.75 (1 H, ddd, J 13.9, 10.3 and 5.6, OCH2CHH), 3.13 (1 H, ddd, J 13.9, 7.2 and 5.6, OCH2CHH), 3.68–3.81 (3 H, m, ArCH2 and NHCH), 3.85 (1 H, ddd, J 12.4, 7.2 and 5.6 OCHH), 4.08 (1 H, ddd, J 12.4, 10.3 and 5.6, OCHH), 4.45 (1 H, s, COCH), 6.28 (1 H, br d, J 7.2, NH), 7.28–7.30 (3 H, m, Ar), 7.35 (2 H, d, J 8.5, Ar), 7.40–7.43 (3 H, m, Ar); δC (150 MHz, CDCl3) 24.7, 25.5, 32.8, 48.5, 51.6, 58.6, 65.1, 71.5, 128.0, 128.8, 128.9, 129.2, 129.9, 130.4, 130.6, 133.6, 135.5, 135.9, 168.8, 174.1; LRMS (EI) 428, 426, 302, 300, 215; HRMS calcd for C24H27O3N2Cl [M]+ 426.1705, found 426.1703.N-(tert-Butyl)-5-(4-chlorobenzyl)-9-oxo-2,3,4,6-tetrahydrobenzo[g][1,5]oxazonine-6-carboxamide (10d)
Pale yellow foam. 62% yield. νmax/cm−1 3335 (NH), 2970 (CH), 1723, 1660 (CO), 1565 (Ar); δH (600 MHz, CDCl3, Me4Si) 1.42 (9 H, s, tBu), 1.47–1.54 (1 H, m, OCH2CHH) 1.74–1.84 (1 H, m, OCH2CHH), 2.85 (1 H, ddd, J 14.2, 6.2 and 3.3, OCH2CH2CHH), 3.00 (1 H, ddd, J 14.2, 9.1 and 3.4, OCH2CH2CHH), 3.66 (1 H, d, J 13.8, ArCHH), 3.85 (1 H, d, J 13.8, ArCHH), 4.26 (1 H, ddd, J 14.8, 6.1 and 3.4, OCHH), 4.47 (1 H, ddd, J 14.8, 8.4 and 2.4, OCHH), 4.79 (1 H, s, COCH), 6.05 (1 H, br s, NH), 7.23 (2 H, d, J 8.4, Ar), 7.27 (2 H, d, J 8.4, Ar), 7.32–7.42 (3 H, m, Ar), 7.55 (1 H, dt, J 6.6 and 1.7, Ar); δC (150 MHz, CDCl3) 25.6, 28.8, 50.9, 51.8, 55.8, 68.1, 69.4 (br), 127.8, 128.3, 128.4, 128.6, 130.2, 131.1, 133.0, 133.1, 136.8, 139.2, 169.0, 171.6; LRMS (CI) 417, 415, 316, 314, 273, 125; HRMS calcd for C23H28O3N2Cl [MH]+ 415.1789, found 415.1792.5-(4-Chlorobenzyl)-9-oxo-N-pentyl-2,3,4,6-tetrahydrobenzo[g][1,5]oxazonine-6-carboxamide (10e)
Colorless oil. 70% yield. νmax/cm−1 3325 (NH), 2858 (CH), 1710, 1651 (CO), 1491 (Ar); δH (600 MHz, CDCl3, Me4Si) 0.92 (3 H, t, J 7.1, CH3), 1.29–1.39 (4 H, pentyl), 1.48–1.55 (3 H, m, pentyl, OCH2CHH), 1.72–1.78 (1 H, m, OCH2CHH), 2.83 (1 H, ddd, J 14.1, 6.5, 3.3, NCHHCH2), 2.99 (1 H, ddd, J 14.1, 8.6, 3.5, NCHHCH2), 3.26–3.38 (2 H, m, NHCH2), 3.64 (1 H, d, J 13.8, ArCHH), 3.85 (1 H, d, J 13.8, ArCHH), 4.26 (1 H, ddd, J 11.2, 6.4, 3.3, OCHH), 4.48 (1 H, ddd, J 11.2, 8.4, 2.9, OCHH), 4.91 (1 H, s, NCHCO), 6.26 (1 H, br s, NH), 7.21 (2 H, d, J 8.5, Ar), 7.26 (2 H, d, J 8.5, Ar), 7.35–7.40 (3 H, m, Ar), 7.54–7.56 (1 H, m, Ar); δC (150 MHz, CDCl3) 13.8, 22.2, 25.6, 29.0, 29.1, 39.5, 51.1, 56.0, 68.0, 69.3 (br), 127.9, 128.3, 128.50 (br), 128.51 (br), 128.6, 130.3, 131.1, 133.1, 136.8, 139.1, 169.4, 171.7; LRMS (ES) 429, 427, 378, 302, 230; HRMS calcd for C24H28N2O3Cl [M−H]− 427.1788, found 427.1778.N-(tert-Butyl)-5-butyl-9-oxo-2,3,4,6-tetrahydrobenzo[g][1,5]oxazonine-6-carboxamide (10f)
White solid. 61% yield. M.p. 152–154 °C (hexanes); νmax/cm−1 3297 (NH), 2963, 2867 (CH), 1724 (CO ester), 1650 (CO amide), 1548, 1453 (Ar); δH (400 MHz; CDCl3; Me4Si) 0.86 (3 H, t, J 7.3, CH2CH3), 1.17–1.28 (2 H, m, alkyl), 1.38–1.49 (2 H, m, alkyl), 1.40 (9 H, s, tBu), 1.74–1.91 (2 H, m, OCH2CH2), 2.52–2.67 (2 H, m, NCH2Pr), 2.80–2.92 (1 H, m, NCHH), 3.03–3.14 (1 H, m, NCHH), 4.21–4.30 (1 H, m, OCHH), 4.49–4.60 (1 H, m, OCHH), 4.70 (1 H, s, NCHCO), 6.31 (1 H, br s, NH), 7.31–7.43 (3 H, m, Ar), 7.47 (1 H, br d, J 7.1, Ar); δC (100 MHz; CDCl3; Me4Si) 13.7, 20.5, 25.3, 26.0, 28.7, 50.8, 51.2, 51.5, 67.7, 70.4, 127.6, 127.9, 128.5, 130.0, 133.3, 139.5, 169.3, 171.3; HRMS calcd for C20H30N2O3 [MNa]+ 369.2154, found 369.2146.N-(tert-Butyl)-7-(2-chlorobenzyl)-3-oxo-2,3,5,6,7,8-hexahydrobenzo[i][1,4,7]dioxazecine-8-carboxamide (10g)
White solid. 14% yield. M.p. 138–140 °C (hexanes); νmax/cm−1 3378 (NH), 2965 (CH), 1741, 1673 (CO), 1489 (Ar); δH (600 MHz, CDCl3, Me4Si) 1.32 (9 H, s, tBu), 2.54–2.60 (1 H, m, OCH2CHH), 2.75 (1 H, ddd, J 15.0, 11.6 and 3.1, OCH2CHH), 3.85 (1 H, d, J 13.9, NCHHAr), 3.98 (1 H, d, J 13.9, NCHHAr), 4.16 (1 H, ddd, J 11.6, 3.6 and 2.0, CO2CHH), 4.30–4.33 (1 H, m, CO2CHH), 4.54 (1 H, d, J 13.4, ArOCHH), 4.73 (1 H, d, J 13.4, ArOCHH), 5.08 (1 H, s, ArCH), 6.56 (1 H, s, NH), 7.11 (1 H, ddd, J 7.9, 7.3 and 1.1, Ar), 7.17 (1 H, dd, J 8.3 and 1.1, Ar), 7.31 (1 H, ddd, J 8.3, 7.3 and 1.7, Ar), 7.34 (2 H, d, J 8.5, Ar), 7.37 (2 H, d, J 8.5, Ar), 7.42 (1 H, d, J 7.9, Ar); δC (150 MHz, CDCl3) 28.8, 45.7, 51.1, 53.9, 60.8, 65.5, 72.9, 121.4, 124.8, 128.9, 129.0, 129.8, 129.9, 130.7, 133.3, 136.6, 157.0, 168.5, 171.3; LRMS (ES) 433, 431, 330, 224, 208; HRMS calcd for C23H28O4N2Cl [MH]+ 431.1738, found 431.1730.7-(3-Bromobenzyl)-N-(tert-butyl)-3-oxo-2,3,5,6,7,8-hexahydrobenzo[i][1,4,7]dioxazecine-8-carboxamide (10h)
White solid. 22% yield. M.p. 135–136 °C (hexanes); νmax/cm−1 3373 (NH), 2965 (CH), 1742, 1675 (CO), 1506, 1453 (Ar); δH (600 MHz, CDCl3, Me4Si) 1.33 (9 H, s, tBu), 2.56–2.59 (1 H, m, OCH2CHH), 2.78 (1 H, ddd, J 15.4, 11.8 and 3.2, OCH2CHH), 3.84 (1 H, d, J 14.0, NCHHAr), 3.98 (1 H, d, J 14.0, NCHHAr), 4.17 (1 H, ddd, J 11.8, 4.0 and 1.9, CO2CHH), 4.32–4.37 (1 H, m, CO2CHH), 4.55 (1 H, d, J 13.4, ArOCHH), 4.73 (1 H, d, J 13.4, ArOCHH), 5.06 (1 H, s, ArCH), 6.58 (1 H, s, NH), 7.12 (1 H, ddd, J 8.3, 7.5 and 0.9, Ar), 7.17 (1 H, dd, J 8.2 and 0.9, Ar), 7.23–7.26 (1 H, m, Ar), 7.31 (1 H, ddd, J 8.2, 7.5 and 1.7, Ar), 7.37 (1 H, d, J 7.7, Ar), 7.41 (2 H, m, Ar), 7.59 (1 H, s, Ar); δC (150 MHz, CDCl3) 28.8, 45.9, 51.3, 54.1 60.8, 65.5, 73.1, 121.4, 123.0, 124.8, 127.2, 128.9, 130.0, 130.4, 130.6, 130.8, 131.5, 140.5, 157.1, 168.5, 171.3; LRMS (EI) 476, 474, 377, 375, 218, 162; HRMS calcd for C23H27O4N2Br [M]+ 474.1149, found 474.1149.N-Cyclohexyl-1,4-diethyl-8-oxo-2,3,5-trihydrobenzo[f][1,4]diazocine-5-carboxamide (10i)
Colourless oil. 69% yield. νmax/cm−1 3326 (NH), 2859 (CH), 1645 (CO), 1490 (Ar); δH (600 MHz, CDCl3, Me4Si) 0.91–0.95 (1 H, m, Cy), 0.96 (6 H, app. t, J 7.1, CH3), 1.03–1.32 (4 H, m, Cy), 1.57–1.72 (4 H, m, Cy), 1.93–1.95 (1 H, m, Cy), 2.47–2.60 (4 H, m, NCH2CH3), 2.70 (1 H, app. dt, J 13.5, 6.3, CONCHHCH2), 2.81 (1 H, app. dt, J 13.5, 6.2, CONCHHCH2), 3.29 (1 H, app. dt, J 14.1, 6.3, CHNCHHCH2), 3.70 (1 H, tdt, J 11.2, 7.9, 3.9, NHCH), 3.94 (1 H, app. dt, J 14.1, 6.4, CHNCHHCH2), 5.26 (1 H, s, NCHCO), 6.56 (1 H, d, J 7.9, NH), 7.43 (1 H, dd, J 7.6, 7.4, Ar), 7.54 (1 H, ddd, J 7.6, 7.5, 1.1, Ar), 7.68–7.71 (2 H, m, Ar); δC (150 MHz, CDCl3) 11.7, 25.0, 25.1, 25.5, 32.6, 33.1, 41.2, 47.6, 48.9, 50.6, 65.9, 123.0, 123.6, 128.9, 131.0, 132.3, 141.8, 167.3, 170.3; LRMS (CI) 358, 263, 247, 231; HRMS calcd for C21H32N3O2 [MH]+ 358.2495, found 358.2488.N-(tert-Butyl)-2-((S)-2-((phenylthio)methyl)pyrrolidin-1-yl)octanamide (11a)
Colourless oil. 68% yield. [α]25D −44.0 (c 1.0, CHCl3); νmax/cm−1 3336 (NH), 2957, 2926, 2858 (CH), 1656 (CO), 1584, 1514, 1453 (Ar); δH (600 MHz; CDCl3; Me4Si) 0.86 (3 H, t, J 7.1, CH2CH3), 1.19–1.31 (8 H, m, alkyl), 1.29 (9 H, s, tBu), 1.50–1.57 (1 H, m, alkyl), 1.58–1.65 (1 H, m, alkyl), 1.67–1.81 (3 H, m, NCH2CHHCH2), 1.89–1.99 (1 H, m, NCH2CHH), 2.74 (1 H, dt, J 9.0, 7.5, NCHH), 2.82 (1 H, dd, J 12.5, 8.2, SCHH), 2.95 (1 H, dd, J 8.4, 6.1, NCHCO), 2.96–3.00 (1 H, m, NCHCH2), 3.04 (1 H, dd, J 12.5, 3.6, SCHH), 3.14 (1 H, ddd, J 11.8, 8.2, 3.6, SCH2CH), 6.25 (1 H, br s, NH), 7.15 (1 H, t, J 7.6, Ar), 7.26 (2 H, app. t, J 7.6, Ar), 7.32 (2 H, d, J 7.6, Ar); δC (150 MHz; CDCl3; Me4Si) 14.2, 22.7, 23.4, 26.7, 28.9, 29.5, 31.1, 31.5, 31.8, 39.9, 50.7, 52.1, 58.4, 67.2, 126.0, 129.0, 129.2, 136.8, 172.8; LRMS (CI) 391, 290, 267, 137; HRMS calcd for C23H39N2OS [MH]+ 391.2783, found 391.2790.N-(tert-Butyl)-2-((S)-2-((phenylthio)methyl)pyrrolidin-1-yl)pentanamide (11b)
Colourless oil. 70% yield. [α]25D −51.3 (c 1.0, CHCl3); νmax/cm−1 3334 (NH), 2934 (CH), 1663 (CO), 1514 (Ar); δH (600 MHz, CDCl3, Me4Si) 0.87 (3H, t, J 7.4, CH2CH3), 1.28–1.36 (11H, m, tBu, CH3CH2), 1.50–1.55 (1H, m, NCHCHH), 1.58–1.63 (1H, m, NHCHCHH), 1.70–1.80 (3H, m, NCH2CH2, NCH2CH2CHH), 1.93–1.97 (1H, m, NCH2CH2CHH), 2.74 (1H, app. q, J 8.2, NCHH), 2.82 (1H, dd, J 12.6, 8.3, SCHH), 2.96–3.00 (2H, m, NCHCO, NCHH), 3.04 (1H, dd, J 12.6, 3.7, SCHH), 3.13–3.17 (1H, m, NCHCH2), 6.26 (1H, br s, NH), 7.16 (1H, t, J 7.6, Ar), 7.26 (2H, dd, J 7.8, 7.6, Ar), 7.33 (2H, d, J 7.8, Ar); δC (150 MHz, CDCl3) 14.3, 20.0, 23.5, 28.9, 31.1, 33.7, 39.9, 50.8, 52.1, 58.4, 67.0, 126.1, 129.0, 129.2, 136.8, 172.8; LRMS (ES) 349, 248, 239, 180; HRMS calcd for C20H33N2OS [MH]+ 349.2314, found 349.2319.N-(Cyclohexyl)-2-((S)-2-((phenylthio)methyl)pyrrolidin-1-yl)pentanamide (11c)
Colourless oil. 60% yield. [α]25D −30.7 (c 1.0, CHCl3); νmax/cm−1 3306 (NH), 2931 (CH), 1643 (CO), 1520, 1439 (Ar); δH (600 MHz, CDCl3, Me4Si) 0.85 (3H, t, J 7.4, CH2CH3), 1.01–1.14 (3H, m, Cy), 1.29–1.34 (4H, m, CH3CH2, Cy), 1.51–1.79 (10H, m, NCHCH2, NCH2CH2, SCH2CHCHH, Cy), 1.89–1.96 (1H, m, SCH2CHCHH), 2.74 (1H, app. q, J 8.1, NCHH), 2.81 (1H, dd, J 12.7, 8.3, SCHH), 2.95–2.99 (1H, m, NCHH), 3.02–3.07 (2H, m, SCHH, NCHCO), 3.10–3.14 (1H, m, SCH2CH), 3.67–3.74 (1H, m, NHCH), 6.24 (1H, br d, J 7.2, NH), 7.15 (1H, tt, J 7.7, 1.3, Ar), 7.25 (2H, dd, J 8.3, 7.7, Ar), 7.32 (2H, dd, J 8.3, 1.3, Ar); δC (150 MHz, CDCl3) 14.3, 19.9, 22.4, 25.0, 25.6, 31.0, 33.1, 33.4, 39.8, 47.6, 51.9, 58.5, 66.2, 126.1, 129.1, 129.3, 136.8, 172.3; LRMS (ES) 375, 248, 208, 180; HRMS calcd for C22H35N2OS [MH]+ 375.2470, found 375.2465.(3S,8S)-N-(tert-Butyl)-8-oxo-2,5-dihydrobenzo[f]pyrrolo[3,4-c][1,4]oxazocine-5-carboxamide and (3S,8R)-N-(tert-butyl)-8-oxo-2,5-dihydrobenzo[f]pyrrolo[3,4-c][1,4]oxazocine-5-carboxamide (12)
White solid. 78% yield (S,S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S,R 1.5
S,R 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). S,S-Isomer: M.p. 133–135 °C (hexanes); [α]25D −96.1 (c 1.0, CHCl3); νmax/cm−1 3305 (NH), 2968 (CH), 1737, 1662 (CO), 1539, 1455 (Ar); δH (600 MHz, CDCl3, Me4Si) 1.17 (9H, s, tBu), 1.72–1.76 (1H, m, NCH2CHH), 1.81–1.93 (2H, m, NCH2CHH, NCHCHH pyrrolo), 2.04 (1H, dddd, J 16.5, 12.5, 8.0, 4.3, NCHCHH pyrrolo), 2.68 (1H, ddd, J 15.9, 8.7, 7.2, NCHH), 2.78 (1H, ddd, J 15.9, 7.8, 4.3, NCHCH2), 3.20 (1H, app. ddd, J 9.5, 7.5, 5.3, NCHH), 3.72 (1H, dd, J 12.3, 4.4, OCHH), 3.95 (1H, app. d, J 12.3, OCHH), 4.13 (1H, s, NCHCO), 6.13 (1H, br s, NH), 7.26–7.27 (1H, m, Ar), 7.34–7.40 (3H, m, Ar); δC (150 MHz, CDCl3) 22.4, 28.3, 31.4, 51.2, 52.9, 62.6, 68.8, 75.4, 127.1, 127.8, 128.8, 130.0, 130.2, 136.4, 169.6, 174.8; S,R-Isomer: M.p. 144–145 °C (hexanes); [α]25D +73.4 (c 1.0, CHCl3); νmax (film/cm−1) 3305, 2968, 1737, 1662, 1539, 1455; δH (600 MHz, CDCl3, Me4Si) 1.42 (9H, s, tBu), 1.67–1.71 (1H, m, NCH2CHH), 1.78–1.82 (2H, m, NCH2CHH, NCHCHH pyrrolo), 1.96–1.99 (1H, m, NCHCHH pyrrolo), 3.04–3.06 (2H, m, NCH2), 3.60 (1H, app. ddd, J 9.0, 5.9, 3.6, NCHCH2), 3.75 (1H, dd, J 12.1, 3.6, OCHH), 3.85 (1H, app. d, J 12.1, OCHH), 4.23 (1H, s, NCHCO), 6.48 (1H, d, J 7.3, Ar), 7.12 (1H, ddd, J 7.8, 7.6, 1.1, Ar), 7.19 (1H, dd, J 7.6, 1.0, Ar), 7.23 (1H, br s, NH), 7.26 (1H, dd, J 7.8, 7.3, Ar); δC (150 MHz, CDCl3) 23.7, 29.0, 30.7, 52.0, 54.4, 55.8, 65.3, 69.3, 126.8, 127.0, 127.8, 130.2, 131.4, 137.3, 168.3, 174.7; LRMS (ES) 316, 217, 84; HRMS calcd for C18H24N2O3 [MH]+ 317.1865, found 317.1874.
1). S,S-Isomer: M.p. 133–135 °C (hexanes); [α]25D −96.1 (c 1.0, CHCl3); νmax/cm−1 3305 (NH), 2968 (CH), 1737, 1662 (CO), 1539, 1455 (Ar); δH (600 MHz, CDCl3, Me4Si) 1.17 (9H, s, tBu), 1.72–1.76 (1H, m, NCH2CHH), 1.81–1.93 (2H, m, NCH2CHH, NCHCHH pyrrolo), 2.04 (1H, dddd, J 16.5, 12.5, 8.0, 4.3, NCHCHH pyrrolo), 2.68 (1H, ddd, J 15.9, 8.7, 7.2, NCHH), 2.78 (1H, ddd, J 15.9, 7.8, 4.3, NCHCH2), 3.20 (1H, app. ddd, J 9.5, 7.5, 5.3, NCHH), 3.72 (1H, dd, J 12.3, 4.4, OCHH), 3.95 (1H, app. d, J 12.3, OCHH), 4.13 (1H, s, NCHCO), 6.13 (1H, br s, NH), 7.26–7.27 (1H, m, Ar), 7.34–7.40 (3H, m, Ar); δC (150 MHz, CDCl3) 22.4, 28.3, 31.4, 51.2, 52.9, 62.6, 68.8, 75.4, 127.1, 127.8, 128.8, 130.0, 130.2, 136.4, 169.6, 174.8; S,R-Isomer: M.p. 144–145 °C (hexanes); [α]25D +73.4 (c 1.0, CHCl3); νmax (film/cm−1) 3305, 2968, 1737, 1662, 1539, 1455; δH (600 MHz, CDCl3, Me4Si) 1.42 (9H, s, tBu), 1.67–1.71 (1H, m, NCH2CHH), 1.78–1.82 (2H, m, NCH2CHH, NCHCHH pyrrolo), 1.96–1.99 (1H, m, NCHCHH pyrrolo), 3.04–3.06 (2H, m, NCH2), 3.60 (1H, app. ddd, J 9.0, 5.9, 3.6, NCHCH2), 3.75 (1H, dd, J 12.1, 3.6, OCHH), 3.85 (1H, app. d, J 12.1, OCHH), 4.23 (1H, s, NCHCO), 6.48 (1H, d, J 7.3, Ar), 7.12 (1H, ddd, J 7.8, 7.6, 1.1, Ar), 7.19 (1H, dd, J 7.6, 1.0, Ar), 7.23 (1H, br s, NH), 7.26 (1H, dd, J 7.8, 7.3, Ar); δC (150 MHz, CDCl3) 23.7, 29.0, 30.7, 52.0, 54.4, 55.8, 65.3, 69.3, 126.8, 127.0, 127.8, 130.2, 131.4, 137.3, 168.3, 174.7; LRMS (ES) 316, 217, 84; HRMS calcd for C18H24N2O3 [MH]+ 317.1865, found 317.1874.
Acknowledgements
We would like to thank the EPSRC (Advanced Research Fellowship EP/E052789/1 to TDS), AstraZeneca (summer research bursary to MB) and University College London (Studentship to SEM) for supporting this work.Notes and references
- A. Dömling, Curr. Opin. Chem. Biol., 2002, 6, 306–313 CrossRef CAS; A. Dömling, Chem. Rev., 2006, 106, 17–89 CrossRef; L. El Kaïm and L. Grimaud, Tetrahedron, 2009, 65, 2153–2171 CrossRef; B. Ganem, Acc. Chem. Res., 2009, 42, 463–472 CrossRef; M. M. Heravi and S. Moghimi, J. Iranian Chem. Soc., 2011, 8, 306–373 Search PubMed; B. Jiang, T. Rajale, W. Wever, S. J. Tu and G. G. Li, Chem. Asian J., 2010, 5, 2318–2335 CrossRef; V. Nair, C. Rajesh, A. U. Vinod, S. Bindu, A. R. Sreekanth, J. S. Mathen and L. Balagopal, Acc. Chem. Res., 2003, 36, 899–907 CrossRef; E. Ruijter, R. Scheffelaar and R. V. A. Orru, Angew. Chem., Int. Ed., 2011, 50, 6234–6246 CrossRef; M. Syamala, Org. Prep. Proced. Int., 2009, 41, 1–68 CrossRef; B. B. Touré and D. G. Hall, Chem. Rev., 2009, 109, 4439–4486 CrossRef; J. Zhu, Eur. J. Org. Chem., 2003, 1133–1144 CrossRef; J. Zhu and H. Bienaymé, Multicomponent Reactions, Wiley-VCH, Weinheim, 2005 CrossRef; I. Ugi, B. Werner and A. Dömling, Molecules, 2003, 8, 53–66 CrossRef.
- I. Ugi, Angew. Chem., Int. Ed. Engl., 1962, 1, 8–21 CrossRef.
- L. Banfi, A. Basso, G. Guanti, P. Lecinska and R. Riva, Mol. Diversity, 2008, 12, 187–190 CrossRef CAS; Y. B. Kim, E. H. Choi, G. Keum, S. B. Kang, D. H. Lee, H. Y. Koh and Y. Kim, Org. Lett., 2001, 3, 4149–4152 CrossRef; R. Mossetti, T. Pirali and G. C. Tron, J. Org. Chem., 2009, 74, 4890–4892 CrossRef; T. Pirali, G. Callipari, E. Ercolano, A. A. Genazzani, G. B. Giovenzana and G. C. Tron, Org. Lett., 2008, 10, 4199–4202 CrossRef; Y. Ito, H. Imai, K. Segoe and T. Saegusa, Chem. Lett., 1984, 937–940 CrossRef; H. Pellissier and G. Gil, Tetrahedron Lett., 1988, 29, 6773–6774 CrossRef; H. Pellissier, A. Meou and G. Gil, Tetrahedron Lett., 1986, 27, 2979–2980 CrossRef; M. Tobisu, A. Kitajima, S. Yoshioka, I. Hyodo, M. Oshita and N. Chatani, J. Am. Chem. Soc., 2007, 129, 11431–11437 CrossRef; S. Yoshioka, M. Oshita, M. Tobisu and N. Chatani, Org. Lett., 2005, 7, 3697–3699 CrossRef.
- L. J. Diorazio, W. B. Motherwell, T. D. Sheppard and R. W. Waller, Synlett, 2006, 2281–2283 CAS.
- R. W. Waller, L. J. Diorazio, B. A. Taylor, W. B. Motherwell and T. D. Sheppard, Tetrahedron, 2010, 66, 6496–6507 CrossRef CAS.
- G. B. Giovenzana, G. C. Tron, S. Di Paola, I. G. Menegotto and T. Pirali, Angew. Chem., Int. Ed., 2006, 45, 1099–1102 CrossRef CAS.
- For examples of nucleophilic ring opening of closely related systems, see: H. L. Wehrmeister, J. Org. Chem., 1963, 28, 2587–2588 CrossRef CAS.
- For previous examples of the use of acid-aldehydes in Ugi-like reactions, see: R. Passerini, Gazz. Chim. Ital., 1931, 61, 964–969 Search PubMed; C. Hanusch-Kompa and I. Ugi, Tetrahedron Lett., 1998, 2725–2728 CrossRef CAS; J. Zhang, A. Jacobson, J. R. Rusche and W. Herlihy, J. Org. Chem., 1999, 64, 1074–1076 CrossRef; S. V. Ley and S. J. Taylor, Bioorg. Med. Chem. Lett., 2002, 12, 1813–1816 CrossRef; F. Mert-Balci, J. Conrad, K. Meindl, T. Schulz, D. Stalke and U. Beifuss, Synthesis, 2008, 3649–3656 Search PubMed; H. Liu and A. Dömling, J. Org. Chem., 2009, 74, 6895–6898 CrossRef; H. Liu, A. Dömling and E. Herdtweck, Chem. Commun., 2010, 46, 770–772 RSC; C. Faggi, M. García-Valverde, S. Marcaccini and G. Menchi, Org. Lett., 2010, 12, 788–791 CrossRef; A. Ramazani and A. Mahyari, Helv. Chim. Acta, 2010, 93, 2203–2209 CrossRef; W. Wang, S. Ollio, A. Dömling and E. Herdtweck, J. Org. Chem., 2011, 76, 637–644 CrossRef; S. Marcaccini, G. Menchi and A. Trabocchi, Tetrahedron Lett., 2011, 52, 2673–2675 CrossRef.
- N. L. Allinger, M. T. Tribble, M. A. Miller and D. H. Wertz, J. Am. Chem. Soc., 1971, 93, 1637–1648 CrossRef CAS; C. Galli and L. Mandolini, Eur. J. Org. Chem., 2000, 3117–3125 CrossRef; G. Illuminati and L. Mandolini, Acc. Chem. Res., 1981, 14, 95–102 CrossRef; G. A. Molander, Acc. Chem. Res., 1998, 31, 603–609 CrossRef.
- A. Demharter, W. Hörl, E. Herdtweck and I. Ugi, Angew. Chem., Int. Ed. Engl., 1996, 35, 173–175 CrossRef CAS; I. Ugi, A. Demharter, W. Hörl and T. Schmid, Tetrahedron, 1996, 52, 11657–11664 CrossRef.
- The stereochemistry at the newly created chiral centre (*) was not determined.
- An enhancement of the methine signal on the pyrrolidine ring was observed upon irradition of the benzylic methine proton for the (S,S) isomer. No such enhancement was observed for the (S,R) isomer. The nOe difference spectra are provided in the supporting information.
- Microwave reactions were performed using a CEM Explorer microwave with an external IR temperature sensor (150 W power).
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra for all MCR products 9–12 and 2D/nOe spectra for compound 12. See DOI: 10.1039/c1ob06534c |
| This journal is © The Royal Society of Chemistry 2012 |
