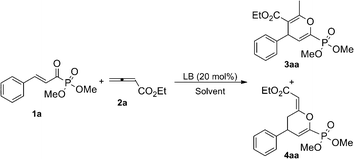
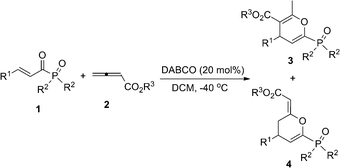
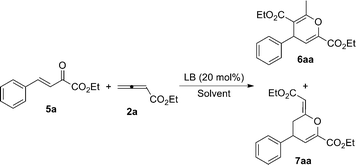
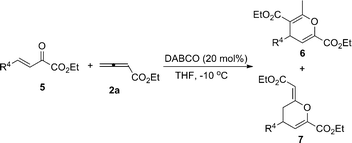
DABCO-catalyzed regioselective cyclization reactions of β,γ-unsaturated α-ketophosphonates or β,γ-unsaturated α-ketoesters with allenic esters†
Received
2nd September 2011
, Accepted 20th September 2011
First published on 21st September 2011
Abstract
Highly efficient DABCO-catalyzed [4 + 2] cycloaddition of β,γ-unsaturated α-ketophosphonates or β,γ-unsaturated α-ketoesters with allenic esters gives the corresponding highly functionalized tetrahydropyran and dihydropyran derivatives in good to excellent yields and moderate to good regioselectivities under mild conditions.
Introduction
Heterocycles are of great value in the design and discovery of new biologically active compounds.1 The development of efficient processes to construct heterocycles, using metal-free catalysts, has been drawing much attention over the past decades.2 Recently, nitrogen-containing Lewis base (LB) catalyzed cyclization reactions of allenoates have emerged as powerful synthetic tools in the rapid construction of cyclic molecular complexity.3 To our surprise, examples of β,γ-unsaturated α-ketophosphonates4 or β,γ-unsaturated α-ketoesters5 as the electrophiles were seldom mentioned in the construction of heterocycles. Herein, we wish to report a novel DABCO-catalyzed regioselective [4 + 2] cycloaddition of β,γ-unsaturated α-ketophosphonates or β,γ-unsaturated α-ketoesters with allenic esters to give the corresponding highly functionalized tetrahydropyran and dihydropyran derivatives, which are structural subunits in many natural products and biologically active molecules.6
Results and discussion
We initially utilized (E)-dimethyl cinnamoylphosphonate 1a (0.1 mmol, 1.0 equiv) and ethyl 2,3-butadienoate 2a (0.12 mmol, 1.2 equiv) as the substrates to investigate their cyclization behavior in tetrahydrofuran (THF) at room temperature in the presence of 20 mol% 1,4-diazabicyclo[2,2,2]octane (DABCO). It was found that the desired [4 + 2] cycloaddition reaction took place smoothly to give the corresponding cyclic products 3aa and 4aa in 87% combined yield but with low regioselectivity as the ratio of 3aa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4aa was 2
4aa was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 within 24 h (Table 1, entry 1). Subsequently, we screened various nitrogen-containing Lewis base catalysts for this reaction, and the results are summarized in Table 1 (entries 2–7). 4-N,N-dimethylpyridine (DMAP) can also efficiently catalyze this reaction to give the product mixture of 3aa and 4aa in 84% combined yield with a ratio of 1
1 within 24 h (Table 1, entry 1). Subsequently, we screened various nitrogen-containing Lewis base catalysts for this reaction, and the results are summarized in Table 1 (entries 2–7). 4-N,N-dimethylpyridine (DMAP) can also efficiently catalyze this reaction to give the product mixture of 3aa and 4aa in 84% combined yield with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 1, entry 2). Other weak nucleophilic nitrogen-containing Lewis base catalysts, such as 1,8-diazabicyclo[5,4,0]-7-undecene (DBU), Et3N and diisopropylethylamine (DIEA) did not catalyze this reaction (Table 1, entries 3–5). We next attempted to screen phosphane-containing Lewis base catalysts, such as PPh3 and tributylphosphine (PBu3), in this reaction, but it was found that no reactions occurred either (Table 1, entries 6 and 7). Of the catalysts examined, DABCO was found to be the best one. Using DABCO as the catalyst, various solvents were examined and dichloromethane (DCM) was found to be the solvent of choice, affording 3aa and 4aa in 90% combined yield with the ratio of 3
1 (Table 1, entry 2). Other weak nucleophilic nitrogen-containing Lewis base catalysts, such as 1,8-diazabicyclo[5,4,0]-7-undecene (DBU), Et3N and diisopropylethylamine (DIEA) did not catalyze this reaction (Table 1, entries 3–5). We next attempted to screen phosphane-containing Lewis base catalysts, such as PPh3 and tributylphosphine (PBu3), in this reaction, but it was found that no reactions occurred either (Table 1, entries 6 and 7). Of the catalysts examined, DABCO was found to be the best one. Using DABCO as the catalyst, various solvents were examined and dichloromethane (DCM) was found to be the solvent of choice, affording 3aa and 4aa in 90% combined yield with the ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 1, entries 8–16). Lowering the reaction temperature to 0 °C or −20 °C, similar results were obtained (Table 1, entries 17 and 18). At −40 °C, the corresponding cyclic adducts 3aa and 4aa were obtained in 86% combined yield along with a 5
1 (Table 1, entries 8–16). Lowering the reaction temperature to 0 °C or −20 °C, similar results were obtained (Table 1, entries 17 and 18). At −40 °C, the corresponding cyclic adducts 3aa and 4aa were obtained in 86% combined yield along with a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 3aa
1 ratio of 3aa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4aa (Table 1, entry 19). Further reducing the reaction temperature did not improve the reaction outcomes (Table 1, entry 20). Thus, we have established the optimal reaction conditions for this reaction: using 20 mol % DABCO as a catalyst and DCM as a solvent to perform the reaction at −40 °C.
4aa (Table 1, entry 19). Further reducing the reaction temperature did not improve the reaction outcomes (Table 1, entry 20). Thus, we have established the optimal reaction conditions for this reaction: using 20 mol % DABCO as a catalyst and DCM as a solvent to perform the reaction at −40 °C.
Table 1 Optimization of the reaction conditions of (E)-dimethyl cinnamoylphosphonate 1a and ethyl 2,3-butadienoate 2aa
Under the optimized reaction conditions, the reaction generality was investigated by using various cinnamoylphosphonates 1 in the reaction with several allenic esters 2, and the results of these experiments are summarized in Table 2. As can be seen from Table 2, changing the ester moiety of allenic esters 2 from OEt to OMe or OiPr provided similar reaction outcomes, affording the desired products in good combined yields (up to 90%) along with moderate regioselectivities (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 = 3
4 = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Table 2, entries 2 and 3). However, we found that the R2 substituent in the phosphonate moiety of cinnamoylphosphonates 1 can significantly affect the reaction outcomes, as that R2group can improve the regioselectivity of the products (up to 3
1) (Table 2, entries 2 and 3). However, we found that the R2 substituent in the phosphonate moiety of cinnamoylphosphonates 1 can significantly affect the reaction outcomes, as that R2group can improve the regioselectivity of the products (up to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 = 10
4 = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) if it had a sterically bulky group, such as OiPr or OtBu (Table 2, entries 4 and 5). As for substrates 1d–1h, electron-withdrawing or electron-donating substituents at the meta- or para-positions of the benzene ring of 1 were equally well-tolerated in the reaction, giving the corresponding products 3 and 4 in good combined yields along with high regioselectivities (up to 3
1) if it had a sterically bulky group, such as OiPr or OtBu (Table 2, entries 4 and 5). As for substrates 1d–1h, electron-withdrawing or electron-donating substituents at the meta- or para-positions of the benzene ring of 1 were equally well-tolerated in the reaction, giving the corresponding products 3 and 4 in good combined yields along with high regioselectivities (up to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 > 20
4 > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Table 2, entries 6–10). The substrate 1i, in which R1 is a 1-naphthyl group, was also able in this reaction to give the corresponding products in 85% combined yield along with good regioselectivity (3ia
1) (Table 2, entries 6–10). The substrate 1i, in which R1 is a 1-naphthyl group, was also able in this reaction to give the corresponding products in 85% combined yield along with good regioselectivity (3ia![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4ia = 14
4ia = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Table 2, entry 11). When R1 is an alkyl group (1j, R1 = Me), the reaction also proceeded smoothly to give the desired products 3ja and 4ja in 75% combined yield but with moderate regioselectivity (3ja
1) (Table 2, entry 11). When R1 is an alkyl group (1j, R1 = Me), the reaction also proceeded smoothly to give the desired products 3ja and 4ja in 75% combined yield but with moderate regioselectivity (3ja![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4ja = 4
4ja = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Table 2, entry 12). The structures of 3 and 4 were determined by the 2-D NMR spectroscopic data (HMQC, HMBC, DEPT and NOESY spectra) of compounds 3ca and 4ca (see the Supporting Information for the details†).
1) (Table 2, entry 12). The structures of 3 and 4 were determined by the 2-D NMR spectroscopic data (HMQC, HMBC, DEPT and NOESY spectra) of compounds 3ca and 4ca (see the Supporting Information for the details†).
Table 2 Substrate scope of the reactions of phosphonates 1 and allenic esters 2a
Encouraged by the above results, β,γ-unsaturated α-ketoesters were also examined under the optimal reaction conditions. Initially, we utilized (E)-ethyl 2-oxo-4-phenylbut-3-enoate 5a (0.10 mmol, 1.0 equiv) and ethyl 2,3-butadienoate 2a (0.12 mmol, 1.2 equiv) as the substrates in DCM (2.00 mL, 0.05 M) at room temperature in the presence of 20 mol% DABCO. We were pleased to find that the reaction proceeded smoothly to give the desired products 6aa and 7aa in 82% combined yield along with good regioselectivity (7aa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6aa = 8
6aa = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Table 3, entry 1), which was contrary to the above results in Table 2. Reducing the concentration to 0.03 M by increasing the amount of solvent (DCM) employed to 3.00 mL can improve the reaction outcome, affording the corresponding products in 85% combined yield along with the 13
1) (Table 3, entry 1), which was contrary to the above results in Table 2. Reducing the concentration to 0.03 M by increasing the amount of solvent (DCM) employed to 3.00 mL can improve the reaction outcome, affording the corresponding products in 85% combined yield along with the 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 7aa
1 ratio of 7aa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6aa (Table 3, entry 1). Inspired by this result, we next screened various nitrogen-containing Lewis base catalysts for this reaction in the optimized amount of solvent (3.00 mL, 0.03 M). DMAP can efficiently catalyze this reaction as well but with low regioselectivity. However, DBU and DIEA did not catalyze this reaction (Table 3, entries 2–4). Accordingly, DABCO was then used as the best catalyst for further investigation of solvent and temperature effects in this reaction. It was found that THF was the solvent of choice in comparison with those reactions carried out in other organic solvents, such as toluene, Et2O, 1,2-dichloroethane (DCE), chloroform, 1,4-dioxane, CH3CN, N,N-dimethylformamide (DMF) and dimethyl sulfoxide (DMSO) (Table 3, entries 5–13). The low temperature (−10 °C) can improve the combined yield of 6aa and 7aa (Table 3, entry 15), but further lowering the reaction temperature to −40 °C reduced the combined yield (Table 3, entry 16). Thus, we have identified the optimal reaction conditions for this reaction: using 20 mol% of DABCO as a catalyst and THF as a solvent to perform the reaction at −10 °C.
6aa (Table 3, entry 1). Inspired by this result, we next screened various nitrogen-containing Lewis base catalysts for this reaction in the optimized amount of solvent (3.00 mL, 0.03 M). DMAP can efficiently catalyze this reaction as well but with low regioselectivity. However, DBU and DIEA did not catalyze this reaction (Table 3, entries 2–4). Accordingly, DABCO was then used as the best catalyst for further investigation of solvent and temperature effects in this reaction. It was found that THF was the solvent of choice in comparison with those reactions carried out in other organic solvents, such as toluene, Et2O, 1,2-dichloroethane (DCE), chloroform, 1,4-dioxane, CH3CN, N,N-dimethylformamide (DMF) and dimethyl sulfoxide (DMSO) (Table 3, entries 5–13). The low temperature (−10 °C) can improve the combined yield of 6aa and 7aa (Table 3, entry 15), but further lowering the reaction temperature to −40 °C reduced the combined yield (Table 3, entry 16). Thus, we have identified the optimal reaction conditions for this reaction: using 20 mol% of DABCO as a catalyst and THF as a solvent to perform the reaction at −10 °C.
Table 3 Optimization of the reaction conditions of (E)-ethyl 2-oxo-4-phenylbut-3-enoate 5a and ethyl 2,3-butadienoate 2aa
Having identified the optimal reaction conditions, we next set out to examine the scope and limitations of the [4 + 2] cycloaddition reaction catalyzed by DABCO using various β,γ-unsaturated α-ketoesters 5 with different substituents on the R4group, and the results are summarized in Table 4. As can be seen from Table 4, when R4 is an aromatic group, whether they have electron-withdrawing or electron-donating substituents at the ortho-, meta- or para-positions on the benzene rings, the reactions proceeded smoothly to give the desired products in good yields along with good regioselectivities (Table 4, entries 2–7). Heterocyclic substrates 5h and 5l were also suitable in this reaction to give the corresponding products in moderate combined yields and regioselectivities (Table 4, entries 8 and 9). The substrate 5j, in which R4 is a 2-naphthyl group, was also tolerable in this reaction to give the corresponding products in 82% combined yield along with good regioselectivity (7ja![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6ja = 8
6ja = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Table 4, entry 10). For substrate 5k, in which R4 is a cyclopropyl group, the reaction also proceeded smoothly to give the desired products in high combined yield (up to 90%) along with high regioselectivity (up to 7ka
1) (Table 4, entry 10). For substrate 5k, in which R4 is a cyclopropyl group, the reaction also proceeded smoothly to give the desired products in high combined yield (up to 90%) along with high regioselectivity (up to 7ka![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6ka = >20
6ka = >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The structure of 6ea was unambiguously determined by X-ray diffraction. The ORTEP drawing is shown in Fig. 1 and its CIF data are summarized in the Supporting Information.†7 The structure of 7 and the E-configuration of the double bond were assigned by the 2-D NMR spectroscopic data (HMQC, HMBC, DEPT and NOESY spectra) of compound 7aa (see the Supporting Information for the details†).
1). The structure of 6ea was unambiguously determined by X-ray diffraction. The ORTEP drawing is shown in Fig. 1 and its CIF data are summarized in the Supporting Information.†7 The structure of 7 and the E-configuration of the double bond were assigned by the 2-D NMR spectroscopic data (HMQC, HMBC, DEPT and NOESY spectra) of compound 7aa (see the Supporting Information for the details†).
Table 4 Substrate scope of the reactions of β,γ-unsaturated α-ketoesters 5 and ethyl 2,3-butadienoate 2aa
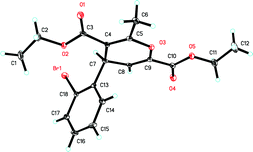 |
| | Fig. 1 An ORTEP drawing of 6ea. | |
The mechanism for the reactions has not been unequivocally established, but one reasonable explanation is shown in Scheme 1 based on earlier reports and our own investigations. Addition of DABCO to allenoate 2a delivers zwitterionic intermediate A, which coexists with its resonance form B.3a,3c The zwitterionic intermediate A reacts with 1 or 5 to give intermediate C, which undergoes enolization to give intermediate E. Subsequent cyclization produces cyclic product G and regenerates the DABCO catalyst. Finally, G isomerizes to give the more stable adducts 3 or 6. At the same time, the zwitterionic intermediate B can also react with 1 or 5 to give intermediate D, which undergoes enolization to give intermediate F. Subsequent cyclization produces cyclic product 4 or 7 and regenerates the DABCO catalyst. We also assumed that the major products in the reaction might be mainly due to the steric interaction between intermediates C and D. When EWG = PO(OiPr)2, which is sterically more bulky than an aromatic ring, the steric repulsion between CO2Et and EWG is larger than that of CO2Et and an aromatic ring. Therefore, intermediate C is more stable than intermediate D, affording 3 as the major product. When EWG = CO2Et, which is sterically smaller than aromatic ring, the steric repulsion between CO2Et and EWG is less than that of CO2Et and aromatic ring. Therefore, intermediate D is more stable than intermediate C, leading to 7 as the major product.
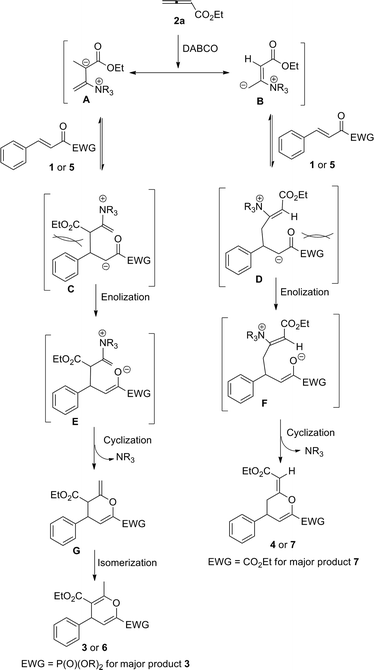 |
| | Scheme 1 A plausible reaction mechanism. | |
In conclusion, we have established a novel DABCO-catalyzed [4 + 2] cycloaddition of β,γ-unsaturated α-ketophosphonates or β,γ-unsaturated α-ketoesters with allenic esters to give the corresponding highly functionalized tetrahydropyran and dihydropyran derivatives in good to excellent yields and moderate to good regioselectivities under mild conditions. The obtained multiple functionalized tetrahydropyran and dihydropyran derivatives are useful building blocks in the organic synthesis of biologically useful compounds. A plausible reaction mechanism has been also proposed on the basis of previous literature and our own investigations. Efforts are in progress to elucidate further mechanistic details of these reactions and to understand their scope and limitations.
Experimental section
General remarks
1H NMR spectra were recorded on a Bruker AM-300 or AM-400 spectrometer for solution in CDCl3 with tetramethylsilane (TMS) as internal standard; J-values are in Hz. Mass spectra were recorded with a HP-5989 instrument. All of the compounds reported in this paper gave satisfactory HRMS analytic data. Melting points were determined on a digital melting point apparatus and temperatures were uncorrected. Infrared spectra were recorded on a Perkin-Elmer PE-983 spectrometer with absorption in cm−1. THF, toluene and Et2O were distilled from sodium (Na) under argon (Ar) atmosphere. CH3CN, 1,2-dichloroethane and dichloromethane were distilled from CaH2 under argon (Ar) atmosphere. Commercially obtained reagents were used without further purification. All reactions were monitored by TLC with Huanghai GF254 silica gel coated plates. Flash column chromatography was carried out using 300–400 mesh silica gel at increased pressure. All the β,γ-unsaturated α-ketophosphonates and β,γ-unsaturated α-ketoesters were prepared according to the literature.4b,8
General procedure for the preparation of 3 and 4 from the reaction of 1a with 2a using 3aa and 4aa as an example in the presence of DABCO
To a mixture of 1a (0.10 mmol, 24.0 mg), 2a (0.12 mmol, 13.6 μL) and DABCO (2.2 mg, 0.02 mmol) was added 2.0 mL of dichloromethane at −40 °C. The reaction solution was monitored by TLC. After the reaction was complete, the solution was concentrated under reduced pressure and the residue was further purified by silica gel column chromatography (EtOAc/PE = 1/6) to give the target products 3aa and 4aa.
Ethyl 6-(dimethoxyphosphoryl)-2-methyl-4-phenyl-4H-pyran-3-carboxylate
3aa.
A colorless oil (16.7 mg, 70%); 1H NMR (400 MHz, CDCl3, TMS) δ 1.09 (t, J = 7.2 Hz, 3H), 2.39 (s, 3H), 3.78 (d, J = 11.2 Hz, 3H), 3.80 (d, J = 11.2 Hz, 3H), 3.96–4.07 (m, 2H), 4.45 (d, J = 5.2 Hz, 1H), 6.12 (dd, J = 10.0 Hz, 5.2 Hz, 1H), 7.19–7.22 (m, 3H), 7.29–7.31 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 13.8, 19.0, 38.1 (d, J = 10.4 Hz), 53.3 (d, J = 6.7 Hz), 53.4 (d, J = 5.9 Hz), 60.1, 104.8, 122.0 (d, J = 20.9 Hz), 126.9, 127.8, 128.5, 137.4, 139.8, 144.0 (d, J = 2.2 Hz), 159.7 (d, J = 8.2 Hz), 166.6; 31P NMR (161.93 MHz, CDCl3, 85% H3PO4): δ 9.734;. IR (CH2Cl2) ν 2966, 2902, 1715, 1659, 1473, 1373, 1260, 1176, 1105, 1026, 947, 800, 741, 700 cm−1; MS (ESI) m/z 353.0 (M+H+). HRMS (ESI) Calcd for C17H22O6P requires (M+H+): 353.1149, found: 353.1156.
(E)-Ethyl 2-(6-(dimethoxyphosphoryl)-4-phenyl-3,4-dihydro-2H-pyran-2-ylidene)acetate
4aa.
A colorless oil (5.5 mg, 16%); 1H NMR (400 MHz, CDCl3, TMS) δ 1.23 (t, J = 7.2 Hz, 3H), 3.10 (dd, J = 10.4 Hz, 6.8 Hz, 1H), 3.67–3.73 (m, 2H), 3.84 (d, J = 11.2 Hz, 3H), 3.85 (d, J = 11.2 Hz, 3H), 4.08–4.14 (m, 2H), 5.67 (s, 1H), 6.28 (dd, J = 10.4 Hz, 2.8 Hz, 1H), 7.19–7.22 (m, 2H), 7.26–7.28 (m, 1H), 7.31–7.35 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 14.1, 29.8, 35.6 (d, J = 11.9 Hz), 53.39 (d, J = 3.7 Hz), 53.40 (d, J = 6.2 Hz), 59.8, 100.8, 122.0 (d, J = 19.3 Hz), 127.0, 127.2, 128.8, 141.0 (d, J = 11.2 Hz), 143.4, 164.6 (d, J = 9.7 Hz), 166.6; 31P NMR (161.93 MHz, CDCl3, 85% H3PO4): δ 9.180; IR (neat) ν 2966, 2902, 1712, 1656, 1494, 1449, 1374, 1261, 1172, 1111, 1026, 824, 764, 700 cm−1; MS (ESI) m/z 353.1 (M+H+); HRMS (ESI) Calcd for C17H22O6P requires (M+H+): 353.1149, found: 353.1159.
Isopropyl 6-(dimethoxyphosphoryl)-2-methyl-4-phenyl-4H-pyran-3-carboxylate
3ab.
A slightly yellow liquid (22.0 mg, 60%); 1H NMR (CDCl3, 400 MHz, TMS) δ 0.91 (d, J = 6.0 Hz, 3H), 1.16 (d, J = 6.0 Hz, 3H), 2.39 (s, 3H), 3.78 (d, J = 11.2 Hz, 3H), 3.81 (d, J = 11.2 Hz, 3H), 4.44 (d, J = 4.8 Hz, 1H), 4.88 (sept, J = 6.0 Hz, 1H), 6.11 (dd, J = 10.4 Hz, 4.8 Hz, 1H), 7.18–7.31 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 19.0, 21.3, 21.8, 38.3 (d, J = 10.6 Hz), 53.29 (d, J = 6.0 Hz), 53.35 (d, J = 6.0 Hz), 67.6, 105.0, 121.9 (d, J = 20.4 Hz), 126.9, 128.0, 128.5, 137.6, 139.9, 144.3, 159.5 (d, J = 9.1 Hz), 166.2; 31P NMR (CDCl3, 161.93 MHz, 85% H3PO4) δ 9.795; IR (CH2Cl2) ν 3061, 2980, 2902, 1713, 1659, 1628, 1374, 1262, 1177, 1104, 1047, 953, 803, 739, 701 cm−1; MS (ESI) m/z 367.1 (M+H+); HRMS (ESI) Calcd for C18H23O6PNa requires (M+Na+): 389.1125, found: 389.1110.
(E)-Isopropyl 2-(6-(dimethoxyphosphoryl)-4-phenyl-3,4-dihydro-2H-pyran-2-ylidene)acetate
4ab.
A slightly yellow liquid (8.1 mg, 22%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.19 (d, J = 6.4 Hz, 3H), 1.22 (d, J = 6.4 Hz, 3H), 3.10 (dd, J = 17.2 Hz, 10.4 Hz, 1H), 3.68–3.72 (m, 2H), 3.84 (d, J = 11.2 Hz, 3H), 3.85 (d, J = 11.2 Hz, 3H), 4.98 (sept, J = 6.4 Hz, 1H), 5.64 (s, 1H), 6.29 (dd, J = 10.0 Hz, 2.8 Hz, 1H), 7.20–7.27 (m, 3H), 7.31–7.34 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 21.8, 29.9, 35.7 (d, J = 11.9 Hz), 53.4 (d, J = 4.4 Hz), 67.1, 101.4, 121.5 (d, J = 19.3 Hz), 127.1, 127.2, 128.8, 141.1 (d, J = 16.4 Hz), 143.6, 164.4 (d, J = 9.7 Hz), 166.2; 31P NMR (CDCl3, 161.93 MHz, 85% H3PO4) δ 9.206; IR (CH2Cl2) ν 3062, 2979, 2905, 1706, 1655, 1452, 1374, 1260, 1170, 1100, 1023, 800, 761, 700 cm−1; MS (ESI) m/z 367.2 (M+H+); HRMS (ESI) Calcd for C18H23O6PNa requires (M+Na+): 389.1125, found: 389.1112.
Methyl 6-(dimethoxyphosphoryl)-2-methyl-4-phenyl-4H-pyran-3-carboxylate
3ac.
A slightly yellow liquid (21.6 mg, 64%); 1H NMR (CDCl3, 400 MHz, TMS) δ 2.39 (s, 3H), 3.57 (s, 3H), 3.78 (d, J = 11.2 Hz, 3H), 3.80 (d, J = 11.2 Hz, 3H), 4.45 (d, J = 4.8 Hz, 1H), 6.13 (dd, J = 10.4 Hz, 4.8 Hz, 1H), 7.20–7.22 (m, 3H), 7.28–7.32 (m, 2H); 13C NMR (CDCl3, 100 MHz, TMS) δ 19.1, 38.1 (d, J = 10.4 Hz), 51.3, 53.27 (d, J = 6.0 Hz), 53.34 (d, J = 5.9 Hz), 104.8, 121.9 (d, J = 20.0 Hz), 127.0, 127.8, 128.6, 137.8, 140.2, 144.0, 160.0 (d, J = 8.9 Hz), 167.2; 31P NMR (CDCl3, 161.93 MHz, 85% H3PO4) δ 9.654. IR (CH2Cl2) ν 3062, 2963, 2904, 1717, 1660, 1627, 1437, 1375, 1262, 1174, 1037, 840, 803, 702 cm−1; MS (ESI) m/z 339.1 (M+H+); HRMS (ESI) Calcd for C16H19O6PNa requires (M+Na+): 361.0812, found: 361.0801.
(E)-Methyl 2-(6-(dimethoxyphosphoryl)-4-phenyl-3,4-dihydro-2H-pyran-2-ylidene)acetate
4ac.
(8.8 mg, 26%): a slightly yellow liquid. 1H NMR (CDCl3, 400 MHz, TMS) δ 3.11 (dd, J = 17.6 Hz, 10.4 Hz, 1H), 3.65 (s, 3H), 3.68–3.73 (m, 2H), 3.85 (d, J = 11.2 Hz, 6H), 5.68 (s, 1H), 6.29 (dd, J = 10.4 Hz, 3.2 Hz, 1H), 7.19–7.27 (m, 3H), 7.33–7.35 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 19.1, 29.9, 35.6 (d, J = 11.9 Hz), 51.1, 53.4 (d, J = 5.2 Hz), 100.4, 122.1 (d, J = 19.4 Hz), 127.1, 127.3, 128.8, 141.1 (d, J = 20.0 Hz), 143.5, 164.5 (d, J = 9.7 Hz), 167.1; 31P NMR (CDCl3, 161.93 MHz, 85% H3PO4) δ 9.165; IR (CH2Cl2) ν 3061, 2963, 2904, 1714, 1655, 1372, 1262, 1168, 1114, 1028, 803, 764, 701, 651 cm−1; MS (ESI) m/z 339.2 (M+H+); HRMS (ESI) Calcd for C16H20O6P requires (M+H+): 339.1001, found: 339.0992.
Ethyl 6-(di-tert-butoxyphosphoryl)-2-methyl-4-phenyl-4H-pyran-3-carboxylate
3ba.
A slightly yellow liquid (27.5 mg, 63%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.08 (t, J = 7.2 Hz, 3H), 1.46 (s, 9H), 1.51 (s, 9H), 2.40 (s, 3H), 3.96–4.06 (m, 2H), 4.42 (d, J = 4.8 Hz, 1H), 5.99 (dd, J = 10.4 Hz, 4.8 Hz, 1H), 7.17–7.20 (m, 3H), 7.26–7.29 (m, 2H); 13C NMR (CDCl3, 100 MHz, TMS) δ 13.7, 19.2, 29.6–31.0 (m, 6C), 38.2 (d, J = 10.4 Hz), 60.1, 83.5, 104.6, 118.3 (d, J = 21.5 Hz), 126.7, 128.0, 128.3, 142.6, 144.6, 144.9, 160.2 (d, J = 8.2 Hz), 167.1; 31P NMR (CDCl3, 161.93 MHz, 85% H3PO4) δ −2.515; IR (CH2Cl2) ν 2980, 2902, 2884, 1714, 1659, 1626, 1476, 1372, 1262, 1171, 1108, 1041, 992, 804, 700 cm−1; MS (ESI) m/z 459.3 (M+Na+); HRMS (ESI) Calcd for C23H33O6PNa requires (M+Na+): 459.1907, found: 459.1919.
(E)-ethyl 2-(6-(di-tert-butoxyphosphoryl)-4-phenyl-3,4-dihydro-2H-pyran-2-ylidene)acetate
4ba.
A slightly yellow liquid (3.1 mg, 7%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.24 (t, J = 7.2 Hz, 3H), 1.54 (s, 18H), 3.05 (dd, J = 13.6 Hz, 6.8 Hz, 1H), 3.61–3.70 (m, 2H), 4.09–4.14 (m, 2H), 5.65 (s, 1H), 6.19 (dd, J = 11.2 Hz, 2.8 Hz, 1H), 7.19–7.21 (m, 2H), 7.22–7.26 (m, 1H), 7.30–7.33 (m, 2H); 31P NMR (CDCl3, 121 MHz, 85% H3PO4) δ −3.003; IR (CH2Cl2) ν 2977, 2903, 1713, 1655, 1373, 1261, 1170, 1114, 1042, 1014, 801, 739, 700 cm−1; MS (ESI) m/z 459.2 (M+Na+); HRMS (ESI) Calcd for C23H33O6PNa requires (M+Na+): 459.1907, found: 459.1915.
Ethyl 6-(diisopropoxyphosphoryl)-2-methyl-4-phenyl-4H-pyran-3-carboxylate
3ca.
A slightly yellow liquid (31.4 mg, 77%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.09 (t, J = 6.8 Hz, 3H), 1.31 (d, J = 6.4 Hz, 6H), 1.35 (d, J = 6.4 Hz, 3H), 1.37 (d, J = 6.4 Hz, 3H), 2.40 (s, 3H), 3.97–4.06 (m, 2H), 4.44 (d, J = 4.8 Hz, 1H), 4.65–4.73 (m, 2H), 6.10 (dd, J = 10.4 Hz, 4.8 Hz, 1H), 7.18–7.22 (m, 3H), 7.28–7.30 (m, 2H); 13C NMR (CDCl3, 100 MHz, TMS) δ 13.8, 19.0, 23.60 (d, J = 3.8 Hz), 23.65 (d, J = 4.4 Hz), 23.9 (d, J = 4.5 Hz), 38.2 (d, J = 9.9 Hz), 60.1, 71.8 (d, J = 4.9 Hz), 104.8, 120.7 (d, J = 19.8 Hz), 126.8, 127.9, 128.0, 128.4, 139.7, 142.0, 144.3, 159.9 (d, J = 9.4 Hz), 166.8; 31P NMR (CDCl3, 161.93 MHz, 85% H3PO4) δ 4.537; IR (CH2Cl2) ν 2982, 2905, 2876, 1769, 1720, 1658, 1376, 1261, 1098, 1009, 800, 701 cm−1; MS (ESI) m/z 409.2 (M+H+); HRMS (ESI) Calcd for C21H29O6PNa requires (M+Na+): 431.1594, found: 431.1595.
(E)-Ethyl 2-(6-(diisopropoxyphosphoryl)-4-phenyl-3,4-dihydro-2H-pyran-2-ylidene)acetate
4ca.
A slightly yellow liquid (3.2 mg, 8%); 1H NMR (CDCl3, 300 MHz, TMS) δ 1.23 (t, J = 7.2 Hz, 3H), 1.35 (d, J = 6.3 Hz, 6H), 1.39 (d, J = 6.3 Hz, 6H), 3.10 (dd, J = 16.8 Hz, 9.9 Hz, 1H), 3.64–3.71 (m, 2H), 4.07–4.16 (m, 2H), 4.71–4.80 (m, 2H), 5.65 (s, 1H), 6.27 (dd, J = 10.2 Hz, 6.9 Hz, 1H), 7.19–7.26 (m, 3H), 7.30–7.35 (m, 2H); 13C NMR (CDCl3, 75 MHz, TMS) δ 14.2, 23.7 (d, J = 4.6 Hz), 24.0 (d, J = 3.5 Hz), 29.9, 35.6 (d, J = 11.4 Hz), 59.8, 71.9 (d, J = 5.7 Hz), 100.4, 120.6 (d, J = 19.4 Hz), 127.1, 127.2, 128.7, 141.3, 142.8, 145.9, 165.1 (d, J = 9.7 Hz), 166.8; 31P NMR (CDCl3, 121.453 MHz, 85% H3PO4) δ 5.107; IR (CH2Cl2) ν 3063, 2980, 2937, 2902, 1711, 1654, 1352, 1259, 1170, 1110, 1044, 985, 886, 762, 700 cm−1; MS (ESI) m/z 409.2 (M+H+); HRMS (ESI) Calcd for C21H29O6PNa requires (M+Na+): 431.1594, found: 431.1579.
Ethyl 4-(4-chlorophenyl)-6-(diisopropoxyphosphoryl)-2-methyl-4H-pyran-3-carboxylate
3da.
A slightly yellow liquid (34.9 mg, 79%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.11 (t, J = 7.2 Hz, 3H), 1.31 (d, J = 6.0 Hz, 6H), 1.36 (d, J = 6.0 Hz, 3H), 1.37 (d, J = 6.0 Hz, 3H), 2.40 (s, 3H), 4.00–4.08 (m, 2H), 4.43 (d, J = 4.8 Hz, 1H), 4.64–4.75 (m, 2H), 6.06 (dd, J = 10.0 Hz, 4.8 Hz, 1H), 7.14 (d, J = 7.2 Hz, 2H), 7.28 (d, J = 7.2 Hz, 2H); 13C NMR (CDCl3, 100 MHz, TMS) δ 14.2, 19.1, 23.0–24.5 (m, 4C), 37.6 (d, J = 10.4 Hz), 60.2, 71.8 (d, J = 5.2 Hz), 71.9 (d, J = 5.2 Hz), 104.4, 119.9 (d, J = 20.0 Hz), 128.6, 129.2, 132.6, 140.1, 142.4, 142.9, 160.2 (d, J = 8.9 Hz), 166.6; 31P NMR (CDCl3, 161.93 MHz, 85% H3PO4) δ 4.175; IR (CH2Cl2) ν 2980, 2942, 2904, 1714, 1663, 1625, 1488, 1376, 1259, 1176, 1104, 986, 946, 804, 685 cm−1; MS (ESI) m/z 443.3 (M+H+); HRMS (ESI) Calcd for C21H28O6PNa requires (M+Na+): 465.1204, found: 465.1193.
(E)-Ethyl 2-(4-(4-chlorophenyl)-6-(diisopropoxyphosphoryl)-3,4-dihydro-2H-pyran-2-ylidene)acetate
4da.
A slightly yellow liquid (3.5 mg, 8%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.24 (t, J = 7.2 Hz, 3H), 1.34 (d, J = 6.4 Hz, 3H), 1.35 (d, J = 6.4 Hz, 3H), 1.39 (d, J = 6.0 Hz, 6H); 3.16 (dd, J = 15.2 Hz, 7.6 Hz, 1H), 3.55 (dd, J = 15.2 Hz, 6.0 Hz, 1H); 3.64–3.67 (m, 1H), 4.09–4.13 (m, 2H), 4.73–4.79 (m, 2H), 5.65 (s, 1H), 6.22 (dd, J = 10.0 Hz, 4.0 Hz, 1H), 7.13 (d, J = 8.4 Hz, 2H), 7.29 (d, J = 8.4 Hz, 2H); 31P NMR (CDCl3, 161.93 MHz, 85% H3PO4) δ 3.723; IR (CH2Cl2) ν 2980, 2942, 2902, 1713, 1657, 1492, 1375, 1261, 1172, 1116, 1045, 994, 820, 739, 703 cm−1; MS (ESI) m/z 443.2 (M+H+); HRMS (ESI) Calcd for C21H28O6PNa requires (M+Na+): 465.1204, found: 465.1213.
Ethyl 6-(diisopropoxyphosphoryl)-2-methyl-4-(4-nitrophenyl)-4H-pyran-3-carboxylate
3ea.
A slightly yellow liquid (36.2 mg, 80%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.11 (t, J = 7.2 Hz, 3H), 1.32 (d, J = 6.4 Hz, 6H), 1.36 (d, J = 6.4 Hz, 3H), 1.38 (d, J = 6.4 Hz, 3H), 2.43 (s, 3H), 4.00–4.06 (m, 2H), 4.58 (d, J = 4.8 Hz, 1H), 4.67–4.74 (m, 2H), 6.04 (dd, J = 10.0 Hz, 4.8 Hz, 1H), 7.38 (d, J = 8.8 Hz, 2H), 8.17 (d, J = 8.8 Hz, 2H); 13C NMR (CDCl3, 100 MHz, TMS) δ 14.0, 19.3, 23.7 (d, J = 4.6 Hz), 23.9 (d, J = 2.3 Hz), 38.3 (d, J = 9.9 Hz), 60.5, 72.1 (d, J = 5.3 Hz), 103.8, 118.6 (d, J = 19.5 Hz), 123.9, 128.7 141.1, 143.5, 146.9, 151.6, 161.2 (d, J = 8.3 Hz), 166.3; 31P NMR (CDCl3, 161.93 MHz, 85% H3PO4) δ 3.597; IR (CH2Cl2) ν 3074, 2981, 2906, 2874, 1715, 1662, 1626, 1521, 1375, 1347, 1176, 1105, 988, 854, 806, 700 cm−1; MS (ESI) m/z 454.2 (M+H+); HRMS (ESI) Calcd for C21H28NO8PNa requires (M+Na+): 476.1445, found: 476.1461.
Ethyl 4-(4-bromophenyl)-6-(diisopropoxyphosphoryl)-2-methyl-4H-pyran-3-carboxylate
3fa.
A slightly yellow liquid (38.3 mg, 79%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.11 (t, J = 6.8 Hz, 3H), 1.31 (d, J = 6.4 Hz, 6H), 1.35 (d, J = 6.4 Hz, 3H), 1.37 (d, J = 6.4 Hz, 3H), 2.39 (s, 3H), 4.00–4.06 (m, 2H), 4.41 (d, J = 4.8 Hz, 1H), 4.65–4.73 (m, 2H), 6.05 (dd, J = 10.4 Hz, 4.8 Hz, 1H), 7.08 (d, J = 8.0 Hz, 2H), 7.61 (d, J = 8.0 Hz, 2H); 13C NMR (CDCl3, 100 MHz, TMS) δ 14.0, 19.1, 23.64 (d, J = 2.3 Hz), 23.68 (d, J = 2.3 Hz), 23.9 (d, J = 3.8 Hz), 37.7 (d, J = 10.6 Hz), 60.3, 71.9 (d, J = 5.3 Hz), 104.4 (d, J = 1.5 Hz), 119.8 (d, J = 19.8 Hz), 120.8, 129.6, 131.6, 140.2, 142.5, 143.5 (d, J = 2.3 Hz), 160.2 (d, J = 8.4 Hz), 166.6; 31P NMR (CDCl3, 161.93 MHz, 85% H3PO4) δ 4.165; IR (CH2Cl2) ν 2979, 2934, 2906, 1713, 1657, 1488, 1448, 1261, 1170, 1111, 1010, 802, 740, 702, 668 cm−1; MS (ESI) m/z 487.3 (M+H+); HRMS (ESI) Calcd for C21H28O6PBrNa requires (M+Na+): 509.0699, found: 509.0705.
(E)-Ethyl 2-(4-(4-bromophenyl)-6-(diisopropoxyphosphoryl)-3,4-dihydro-2H-pyran-2-ylidene)acetate
4fa.
A slightly yellow liquid (3.1 mg, 6%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.24 (t, J = 7.2 Hz, 3H), 1.35 (d, J = 6.4 Hz, 3H), 1.36 (d, J = 6.4 Hz, 3H), 1.39 (d, J = 6.4 Hz, 6H); 3.16 (dd, J = 15.2 Hz, 7.6 Hz, 1H), 3.57 (dd, J = 15.2 Hz, 6.4 Hz, 1H), 3.62–3.66 (m, 1H), 4.09–4.14 (m, 2H), 4.72–4.79 (m, 2H), 5.65 (s, 1H), 6.21 (dd, J = 10.0 Hz, 4.0 Hz, 1H), 7.08 (d, J = 8.0 Hz, 2H), 7.45 (d, J = 8.0 Hz, 2H); 31P NMR (CDCl3, 161.93 MHz, 85% H3PO4) δ 3.697; IR (CH2Cl2) ν 2980, 2939, 2902, 1714, 1661, 1626, 1485, 1376, 1260, 1176, 1106, 992, 805, 740, 664 cm−1; MS (ESI) m/z 487.3 (M+H+); HRMS (ESI) Calcd for C21H28O6PBrNa requires (M+Na+): 509.0699, found: 509.0708.
Ethyl 6-(diisopropoxyphosphoryl)-2-methyl-4-m-tolyl-4H-pyran-3-carboxylate
3ga.
A slightly yellow liquid (32.6 mg, 77%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.11 (t, J = 7.2 Hz, 3H), 1.32 (d, J = 6.4 Hz, 6H), 1.35 (d, J = 6.4 Hz, 3H), 1.37 (d, J = 6.4 Hz, 3H), 2.31 (s, 3H), 2.39 (s, 3H), 4.00–4.07 (m, 2H), 4.40 (d, J = 4.8 Hz, 1H), 4.66–4.73 (m, 2H), 6.09 (dd, J = 10.0 Hz, 4.8 Hz, 1H), 7.00–7.02 (m, 3H), 7.17 (t, J = 8.0 Hz, 1H); 13C NMR (CDCl3, 100 MHz, TMS) δ 13.9, 19.0, 21.3, 23.6 (d, J = 5.2 Hz), 23.7 (d, J = 5.9 Hz), 24.0 (d, J = 3.7 Hz), 38.1 (d, J = 9.7 Hz), 60.1, 71.7 (d, J = 5.2 Hz), 104.9, 120.8 (d, J = 20.1 Hz), 125.0, 127.6, 128.3, 128.7, 138.0, 139.7, 142.0, 144.3, 159.8 (d, J = 8.2 Hz), 166.9; 31P NMR (CDCl3, 161.93 MHz, 85% H3PO4) δ 5.259; IR (CH2Cl2) ν 2979, 2901, 2884, 1715, 1660, 1626, 1475, 1376, 1261, 1171, 1107, 1011, 801, 739, 703 cm−1; MS (ESI) m/z 423.3 (M+H+); HRMS (ESI) Calcd for C22H31O6PNa requires (M+Na+): 445.1751, found: 445.1743.
(E)-Ethyl 2-(6-(diisopropoxyphosphoryl)-4-m-tolyl-3,4-dihydro-2H-pyran-2-ylidene)acetate
4ga.
A slightly yellow liquid (3.3 mg, 8%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.24 (t, J = 7.2 Hz, 3H), 1.35 (d, J = 6.4 Hz, 3H), 1.36 (d, J = 6.4 Hz, 3H), 1.39 (d, J = 6.4 Hz, 6H), 2.33 (s, 3H), 3.03 (dd, J = 15.2 Hz, 8.4 Hz, 1H), 3.62–3.64 (m, 1H), 3.70 (dd, J = 15.2 Hz, 6.0 Hz, 1H), 4.08–4.14 (m, 2H), 4.73–4.79 (m, 2H), 5.64 (s, 1H), 6.26 (dd, J = 10.4 Hz, 3.6 Hz, 1H), 6.99–7.00 (m, 2H), 7.06 (d, J = 7.6 Hz, 1H), 7.21 (t, J = 7.6 Hz, 1H); 31P NMR (CDCl3, 161.93 MHz, 85% H3PO4) δ 4.008; IR (CH2Cl2) ν 2979, 2903, 1713, 1656, 1375, 1260, 1175, 1112, 1044, 1013, 800, 739, 703 cm−1; MS (ESI) m/z 423.3 (M+H+); HRMS (ESI) Calcd for C22H31O6PNa requires (M+Na+): 445.1751, found: 445.1744.
Ethyl 6-(diisopropoxyphosphoryl)-4-(4-methoxyphenyl)-2-methyl-4H-pyran-3-carboxylate
3ha.
A slightly yellow liquid (29.2 mg, 67%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.12 (t, J = 7.2 Hz, 3H), 1.30 (d, J = 6.4 Hz, 3H), 1.32 (d, J = 6.4 Hz, 3H), 1.35 (d, J = 6.4 Hz, 3H), 1.37 (d, J = 6.4 Hz, 3H), 2.37 (s, 3H), 3.78 (s, 3H), 4.00–4.08 (m, 2H), 4.38 (d, J = 4.8 Hz, 1H), 4.65–4.73 (m, 2H), 6.08 (dd, J = 10.0 Hz, 4.8 Hz, 1H), 6.82 (d, J = 8.0 Hz, 2H), 7.12 (d, J = 8.0 Hz, 2H); 13C NMR (CDCl3, 100 MHz, TMS) δ 14.0, 19.0, 23.6 (d, J = 1.4 Hz), 23.7 (d, J = 1.4 Hz), 24.0 (d, J = 4.6 Hz), 37.3 (d, J = 9.9 Hz), 55.2, 60.1, 71.7 (d, J = 5.3 Hz), 105.1 (d, J = 2.3 Hz), 113.8, 120.8 (d, J = 19.8 Hz), 129.0, 136.7 (d, J = 2.3 Hz), 139.6, 141.9, 158.5, 159.6 (d, J = 9.2 Hz), 167.0; 31P NMR (CDCl3, 161.93 MHz, 85% H3PO4) δ 4.631; IR (CH2Cl2) ν 2980, 2937, 2906, 1714, 1626, 1510, 1376, 1259, 1176, 1106, 1070, 991, 946, 805, 745 cm−1; MS (ESI) m/z 439.3 (M+H+); HRMS (ESI) Calcd for C22H31O7PNa requires (M+Na+): 461.1700, found: 461.1699.
(E)-ethyl 2-(6-(diisopropoxyphosphoryl)-4-(4-methoxyphenyl)-3,4-dihydro-2H-pyran-2-ylidene)acetate
4ha.
A slightly yellow liquid (3.7 mg, 8%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.24 (t, J = 7.2 Hz, 3H), 1.35 (d, J = 6.4 Hz, 3H), 1.36 (d, J = 6.4 Hz, 3H), 1.38 (d, J = 6.0 Hz, 6H), 3.11 (dd, J = 10.4 Hz, 4.8 Hz, 1H), 3.57–3.63 (m, 2H), 3.79 (s, 3H), 4.08–4.14 (m, 2H), 4.72–4.78 (m, 2H), 5.64 (s, 1H), 6.24 (dd, J = 10.4 Hz, 4.4 Hz, 1H), 6.85 (d, J = 8.4 Hz, 2H), 7.12 (d, J = 8.4 Hz, 2H); 31P NMR (CDCl3, 161.93 MHz, 85% H3PO4) δ 4.061; IR (CH2Cl2) ν 2968, 2902, 2839, 1713, 1657, 1513, 1474, 1447, 1375, 1172, 1109, 1046, 1016, 803, 738, 702 cm−1; MS (ESI) m/z 439.3 (M+H+); HRMS (ESI) Calcd for C22H31O7PNa requires (M+Na+): 461.1700, found: 461.1694.
Ethyl 6-(diisopropoxyphosphoryl)-2-methyl-4-(naphthalen-1-yl)-4H-pyran-3-carboxylate
3ia.
A slightly yellow liquid (36.2 mg, 79%); 1H NMR (CDCl3, 400 MHz, TMS) δ 0.74 (t, J = 7.2 Hz, 3H), 1.25 (d, J = 6.4 Hz, 3H), 1.29 (d, J = 5.2 Hz, 3H), 1.30 (d, J = 6.0 Hz, 3H), 1.36 (d, J = 6.4 Hz, 3H), 2.49 (s, 3H), 3.85 (q, J = 7.2 Hz, 2H), 4.53–4.62 (m, 1H), 4.65–4.73 (m, 1H), 5.34 (d, J = 4.8 Hz, 1H), 6.21 (dd, J = 10.4 Hz, 4.8 Hz, 1H), 7.32 (d, J = 6.8 Hz, 1H), 7.42 (t, J = 8.0 Hz, 1H), 7.47–7.56 (m, 2H), 7.71 (d, J = 8.0 Hz, 1H), 7.86 (d, J = 8.0 Hz, 1H), 8.19 (d, J = 8.0 Hz, 1H); 13C NMR (CDCl3, 100 MHz, TMS) δ 13.6, 18.9, 23.5 (d, J = 4.4 Hz), 23.6 (d, J = 5.2 Hz), 23.87 (d, J = 4.4 Hz), 23.91 (d, J = 4.4 Hz), 33.1 (d, J = 10.5 Hz), 60.0, 71.66 (d, J = 5.2 Hz), 71.72 (d, J = 6.0 Hz), 104.3 (d, J = 1.5 Hz), 120.2 (d, J = 21.1 Hz), 122.5, 125.60, 125.61, 126.3, 127.3, 128.7, 130.4, 133.7, 139.5, 140.8 (d, J = 2.2 Hz), 141.8, 160.4 (d, J = 8.2 Hz), 166.8; 31P NMR (CDCl3, 161.93 MHz, 85% H3PO4) δ 4.637; IR (CH2Cl2) ν 3059, 2980, 2939, 1713, 1665, 1627, 1375, 1327, 1258, 1106, 984, 798, 776, 740, 702 cm−1; MS (ESI) m/z 459.3 (M+H+); HRMS (ESI) Calcd for C25H31O6PNa requires (M+Na+): 481.1751, found: 481.1760.
(E)-Ethyl 2-(6-(diisopropoxyphosphoryl)-4-(naphthalen-1-yl)-3,4-dihydro-2H-pyran-2-ylidene)acetate
4ia.
A slightly yellow liquid (2.6 mg, 6%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.16 (t, J = 7.2 Hz, 3H), 1.38 (d, J = 6.0 Hz, 6H), 1.40 (d, J = 6.0 Hz, 6H), 3.22 (dd, J = 15.2 Hz, 8.4 Hz, 1H), 3.87 (dd, J = 15.2 Hz, 5.6 Hz, 1H), 4.00–4.08 (m, 2H), 4.47–4.50 (m, 1H), 4.77–4.84 (m, 2H), 5.68 (s, 1H), 6.38 (dd, J = 10.4 Hz, 3.6 Hz, 1H), 7.32 (d, J = 6.8 Hz, 1H), 7.42 (t, J = 7.6 Hz, 1H), 7.49–7.58 (m, 2H), 7.77 (d, J = 7.6 Hz, 1H), 7.89 (d, J = 7.6 Hz, 1H), 8.04 (d, J = 8.4 Hz, 1H); 31P NMR (CDCl3, 161.93 MHz, 85% H3PO4) δ 3.937; IR (CH2Cl2) ν 2981, 2901, 1712, 1657, 1474, 1374, 1261, 1175, 1116, 1046, 1013, 88, 801, 739 cm−1; MS (ESI) m/z 459.3 (M+H+); HRMS (ESI) Calcd for C25H31O6PNa requires (M+Na+): 481.1751, found: 481.1743.
Ethyl 6-(diisopropoxyphosphoryl)-2,4-dimethyl-4H-pyran-3-carboxylate
3ja.
A slightly yellow liquid (20.8 mg, 60%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.16 (d, J = 6.4 Hz, 3H), 1.30 (t, J = 7.2 Hz, 3H), 1.30 (d, J = 6.0 Hz, 3H), 1.34 (d, J = 6.0 Hz, 3H), 1.36 (d, J = 6.0 Hz, 3H), 1.38 (d, J = 6.0 Hz, 3H), 2.27 (s, 3H), 3.29–3.35 (m, 1H), 4.16–4.26 (m, 2H), 4.64–4.76 (m, 2H), 6.07 (dd, J = 10.0 Hz, 5.2 Hz, 1H); 13C NMR (CDCl3, 100 MHz, TMS) δ 14.2, 19.1, 23.4 (d, J = 2.7 Hz), 23.6 (d, J = 4.5 Hz), 24.0 (d, J = 3.8 Hz), 26.7 (d, J = 10.2 Hz), 60.1, 71.6 (d, J = 5.3 Hz), 71.7 (d, J = 5.3 Hz), 106.4, 122.6 (d, J = 20.1 Hz), 140.5, 142.8, 160.0 (d, J = 8.4 Hz), 167.3; 31P NMR (CDCl3, 161.93 MHz, 85% H3PO4) δ 4.672; IR (CH2Cl2) ν 2980, 2937, 2905, 2874, 1713, 1665, 1625, 1375, 1259, 1163, 1056, 989, 945, 801, 662, 611 cm−1; MS (ESI) m/z 347.2 (M+H+); HRMS (ESI) Calcd for C16H27O6PNa requires (M+Na+): 369.1438, found: 369.1439.
(E)-Ethyl 2-(6-(diisopropoxyphosphoryl)-4-methyl-3,4-dihydro-2H-pyran-2-ylidene)acetate
4ja.
A slightly yellow liquid (5.2 mg, 15%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1H NMR (CDCl3, 400 MHz, TMS) δ 1.13 (d, J = 7.2 Hz, 3H), 1.28 (t, J = 7.2 Hz, 3H), 1.32 (d, J = 6.4 Hz, 3H), 1.33 (d, J = 6.4 Hz, 3H), 1.37 (d, J = 5.6 Hz, 6H), 2.45–2.92 (m, 1H), 2.68 (dd, J = 14.8 Hz, 8.4 Hz, 1H), 3.44 (dd, J = 14.8 Hz, 5.6 Hz, 1H), 4.16 (q, J = 7.2 Hz, 2H), 4.66–4.74 (m, 2H), 5.61 (s, 1H), 6.08 (dd, J = 10.4 Hz, 4.0 Hz, 1H); 31P NMR (CDCl3, 161.93 MHz, 85% H3PO4) δ 4.380; IR (CH2Cl2) ν 2978, 2906, 1713, 1657, 1375, 1351, 1261, 1114, 1048, 1014, 803, 739, 703 cm−1; MS (ESI) m/z 347.2 (M+H+); HRMS (ESI) Calcd for C16H27O6PNa requires (M+Na+): 369.1438, found: 369.1421.
General procedure for the preparation of 6 and 7 from the reaction of 5a with 2a using 6aa and 7aa as an example in the presence of DABCO
To a mixture of 1a (0.10 mmol, 20.4 mg), 2a (0.12 mmol, 13.6 μL) and DABCO (2.2 mg, 0.02 mmol) was added 3.0 mL of THF at −10 °C. The reaction solution was monitored by TLC. After the reaction was complete, the solution was concentrated under reduced pressure and the residue was further purified by silica gel column chromatography (EtOAc/PE = 1/6) to give the target products 6aa and 7aa.
Diethyl 6-methyl-4-phenyl-4H-pyran-2,5-dicarboxylate
6aa.
A slightly yellow liquid (2.7 mg, 7%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.08 (t, J = 7.2 Hz, 3H), 1.30 (t, J = 7.2 Hz, 3H), 2.44 (s, 3H), 3.99–4.06 (m, 2H), 4.21–4.30 (m, 2H), 4.51 (d, J = 5.2 Hz, 1H), 6.26 (d, J = 5.2 Hz, 1H), 7.20–7.24 (m, 3H), 7.29–7.33 (m, 2H); 13C NMR (CDCl3, 100 MHz, TMS) δ 13.9, 14.1, 19.1, 38.9, 60.2, 61.5, 104.6, 116.2, 127.0, 128.1, 128.6, 138.9, 144.4, 159.8, 161.1, 166.9; IR (CH2Cl2) ν 2980, 2903, 1736, 1714, 1658, 1448, 1374, 1260, 1118, 1018, 860, 802, 761, 739, 700 cm−1; MS (ESI) m/z 317.2 (M+H+); HRMS (ESI) Calcd for C18H20O5Na requires (M+Na+): 339.1203, found: 339.1206.
(E)-Ethyl 2-(2-ethoxy-2-oxoethylidene)-4-phenyl-3,4-dihydro-2H-pyran-6-carboxylate
7aa.
A slightly yellow liquid (30.0 mg, 95%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.23 (t, J = 7.2 Hz, 3H), 1.34 (t, J = 7.2 Hz, 3H), 3.11 (dd, J = 8.0 Hz, 4.8 Hz, 1H), 3.68–3.72 (m, 2H), 4.07–4.14 (m, 2H), 4.28–4.34 (m, 2H), 5.75 (s, 1H), 6.45 (d, J = 4.8 Hz, 1H), 7.20–7.22 (m, 2H), 7.26–7.28 (m, 1H), 7.31–7.35 (m, 2H); 13C NMR (CDCl3, 100 MHz, TMS) δ 14.1, 14.2, 29.7, 35.8, 59.8, 61.6, 101.0, 116.5, 127.2, 128.8, 141.3, 141.7, 161.1, 164.6, 166.9; IR (CH2Cl2) ν 2981, 2903, 2875, 1736, 1711, 1660, 1493, 1373, 1258, 1170, 1118, 1046, 1015, 821, 760 cm−1; MS (ESI) m/z 317.2 (M+H+). HRMS (ESI) Calcd for C18H20O5 requires (M+Na+): 339.1203, found: 339.1206.
Diethyl 4-(4-chlorophenyl)-6-methyl-4H-pyran-2,5-dicarboxylate
6ba.
A slightly yellow liquid (2.0 mg, 6%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.11 (t, J = 7.2 Hz, 3H), 1.31 (t, J = 7.2 Hz, 3H), 2.44 (s, 3H), 4.00–4.08 (m, 2H), 4.22–4.31 (m, 2H), 4.49 (d, J = 4.8 Hz, 1H), 6.21 (d, J = 4.8 Hz, 1H), 7.16 (d, J = 8.8 Hz, 2H), 7.27 (d, J = 8.8 Hz, 2H); 13C NMR (CDCl3, 100 MHz, TMS) δ 14.00, 14.04, 19.2, 38.4, 60.3, 61.6, 104.3, 115.5, 128.7, 129.4, 132.8, 139.1, 142.9, 160.1, 161.0, 166.7; IR (CH2Cl2) ν 2981, 2904, 1739, 1715, 1629, 1488, 1372, 1327, 1268, 1106, 1049, 1015, 946, 805, 739 cm−1; MS (ESI) m/z 373.2 (M+Na+); HRMS (ESI) Calcd for C18H19ClNaO5 requires (M+Na+): 373.0816, found: 373.0813.
(E)-Ethyl 4-(4-chlorophenyl)-2-(2-ethoxy-2-oxoethylidene)-3,4-dihydro-2H-pyran-6-carboxylate
7ba.
A slightly yellow liquid (28.1 mg, 80%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.24 (t, J = 7.2 Hz, 3H), 1.35 (t, J = 7.2 Hz, 3H), 3.18 (dd, J = 15.2 Hz, 7.6 Hz, 1H), 3.57 (dd, J = 15.2 Hz, 6.0 Hz, 1H), 3.68–3.72 (m, 1H), 4.08–4.14 (m, 2H), 4.29–4.35 (m, 2H), 5.75 (s, 1H), 6.40 (d, J = 4.0 Hz, 1H), 7.14 (d, J = 8.8 Hz, 2H), 7.30 (d, J = 8.8 Hz, 2H); 13C NMR (CDCl3, 100 MHz, TMS) δ 14.1, 14.2, 29.5, 35.2, 59.9, 61.7, 101.3, 115.6, 128.6, 128.9, 133.1, 139.7, 142.0, 161.0, 164.0, 166.8; IR (CH2Cl2) ν 2981, 2903, 2875, 1736, 1711, 1660, 1493, 1373, 1258, 1170, 1118, 1046, 1015, 821, 760 cm−1; MS (ESI) m/z 373.2 (M+Na+); HRMS (ESI) Calcd for C18H19ClO5 requires (M+Na+): 373.0813, found: 373.0821.
Diethyl 4-(4-bromophenyl)-6-methyl-4H-pyran-2,5-dicarboxylate
6ca.
A slightly yellow liquid (3.1 mg, 8%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.12 (t, J = 7.2 Hz, 3H), 1.31 (t, J = 7.2 Hz, 3H), 2.44 (s, 3H), 3.99–4.08 (m, 2H), 4.23–4.30 (m, 2H), 4.48 (d, J = 5.2 Hz, 1H), 6.21 (d, J = 5.2 Hz, 1H), 7.11 (d, J = 8.8 Hz, 2H), 7.43 (d, J = 8.8 Hz, 2H); 13C NMR (CDCl3, 100 MHz, TMS) δ 14.0, 14.1, 19.2, 38.4, 60.3, 61.6, 104.2, 115.4, 120.9, 129.8, 131.7, 139.1, 143.4, 160.1, 160.9, 166.6; IR (CH2Cl2) ν 2979, 2902, 1716, 1660, 1373, 1263, 1173, 1107, 1048, 1013, 803, 762, 738, 703 cm−1; MS (ESI) m/z 395.2 (M+H+); HRMS (ESI) Calcd for C18H19BrO5Na requires (M+Na+): 417.0308, found: 417.0320.
(E)-Ethyl 4-(4-bromophenyl)-2-(2-ethoxy-2-oxoethylidene)-3,4-dihydro-2H-pyran-6-carboxylate
7ca.
A slightly yellow liquid (31.2 mg, 80%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.24 (t, J = 7.2 Hz, 3H), 1.35 (t, J = 7.2 Hz, 3H), 3.18 (dd, J = 15.2 Hz, 7.2 Hz, 1H), 3.56 (dd, J = 15.2 Hz, 6.4 Hz, 1H), 3.66–3.71 (m, 1H), 4.09–4.13 (m, 2H), 4.30–4.34 (m, 2H), 5.75 (s, 1H), 6.40 (d, J = 5.2 Hz, 1H), 7.09 (d, J = 8.0 Hz, 2H), 7.45 (d, J = 8.0 Hz, 2H); 13C NMR (CDCl3, 100 MHz, TMS) δ 14.1, 14.2, 29.4, 35.3, 59.9, 61.7, 101.3, 115.4, 121.1, 129.0, 131.9, 140.2, 142.0, 161.0, 164.0, 166.8; IR (CH2Cl2) ν 3084, 2982, 2904, 1736, 1711, 1659, 1489, 1394, 1300, 1167, 1117, 1011, 859, 818, 760 cm−1; MS (ESI) m/z 417.2 (M+Na+); HRMS (ESI) Calcd for C18H19BrO5Na requires (M+Na+): 417.0308, found: 417.0315.
Diethyl 4-(3-bromophenyl)-6-methyl-4H-pyran-2,5-dicarboxylate
6da.
A slightly yellow liquid (3.6 mg, 9%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.12 (t, J = 7.2 Hz, 3H), 1.32 (t, J = 7.2 Hz, 3H), 2.44 (s, 3H), 4.00–4.09 (m, 2H), 4.25–4.30 (m, 2H), 4.48 (d, J = 5.2 Hz, 1H), 6.21 (d, J = 5.2 Hz, 1H), 7.16–7.18 (m, 2H), 7.34–7.26 (m, 2H); 13C NMR (CDCl3, 100 MHz, TMS) δ 14.0, 14.1, 19.2, 38.7, 60.3, 61.7, 104.1, 115.3, 122.7, 126.8, 130.1, 130.2, 131.2, 139.3, 146.7, 160.4, 160.9, 166.5; IR (CH2Cl2) ν 3058, 2980, 2873, 1714, 1627, 1590, 1472, 1328, 1267, 1096, 964, 839, 764, 739, 696 cm−1; MS (ESI) m/z 395.2 (M+H+); HRMS (ESI) Calcd for C18H19BrO5Na requires (M+Na+): 417.0308, found: 417.0315.
(E)-Ethyl 4-(3-bromophenyl)-2-(2-ethoxy-2-oxoethylidene)-3,4-dihydro-2H-pyran-6-carboxylate
7da.
A slightly yellow liquid (29.2 mg, 74%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.24 (t, J = 7.2 Hz, 3H), 1.35 (t, J = 7.2 Hz, 3H), 3.12 (dd, J = 14.8 Hz, 6.8 Hz, 1H), 3.61–3.69 (m, 2H), 4.09–4.14 (m, 2H), 4.29–4.35 (m, 2H), 5.76 (s, 1H), 6.39 (d, J = 3.6 Hz, 1H), 7.13–7.16 (m, 1H), 7.21 (t, J = 8.0 Hz, 1H), 7.35 (s, 1H), 7.39–7.41 (m, 1H); 13C NMR (CDCl3, 100 MHz, TMS) δ 14.1, 14.2, 29.5, 35.6, 59.9, 61.8, 101.4, 115.3, 122.8, 126.0, 130.38, 130.41, 130.5, 142.1, 143.6, 161.0, 164.0, 166.8; IR (CH2Cl2) ν 3082, 2981, 2903, 2873, 1710, 1658, 1593, 1475, 1393, 1349, 1256, 1111, 1045, 1021, 855, 790, 676 cm−1; MS (ESI) m/z 419.1 (M+Na+); HRMS (ESI) Calcd for C18H19BrO5Na requires (M+Na+): 417.0308, found: 417.0307.
Diethyl 4-(2-bromophenyl)-6-methyl-4H-pyran-2,5-dicarboxylate
6ea.
A white solid (3.7 mg, 9%); m.p. 122–124 °C; 1H NMR (CDCl3, 400 MHz, TMS) δ 1.00 (t, J = 7.2 Hz, 3H), 1.30 (t, J = 7.2 Hz, 3H), 2.50 (s, 3H), 3.93–4.03 (m, 2H), 4.20–4.29 (m, 2H), 5.06 (d, J = 4.8 Hz, 1H), 6.27 (d, J = 4.8 Hz, 1H), 7.05–7.10 (m, 1H), 7.20 (d, J = 8.0 Hz, 1H), 7.27–7.30 (m, 1H), 7.53 (d, J = 8.0 Hz, 1H); 13C NMR (CDCl3, 100 MHz, TMS) δ 13.8, 14.1, 19.0, 38.3, 60.2, 61.6, 103.6, 114.4, 122.7, 128.2, 128.4, 130.0, 132.7, 139.2, 143.4, 161.0, 161.2, 166.5; IR (CH2Cl2) ν 3058, 2980, 2873, 1714, 1627, 1590, 1472, 1328, 1267, 1096, 964, 839, 764, 739, 696 cm−1; MS (ESI) m/z 395.2 (M+H+); HRMS (ESI) Calcd. for C18H19BrO5Na requires (M+Na+): 417.0308, Found: 417.0315.
(E)-Ethyl 4-(2-bromophenyl)-2-(2-ethoxy-2-oxoethylidene)-3,4-dihydro-2H-pyran-6-carboxylate
7ea.
A slightly yellow liquid (29.8 mg, 76%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.23 (t, J = 7.2 Hz, 3H), 1.35 (t, J = 7.2 Hz, 3H), 3.20 (dd, J = 15.6 Hz, 7.6 Hz, 1H), 3.59 (dd, J = 15.6 Hz, 6.4 Hz, 1H), 4.07–4.14 (m, 2H), 4.18–4.23 (m, 1H), 4.29–4.35 (m, 2H), 5.75 (s, 1H), 6.42 (d, J = 4.4 Hz, 1H), 7.13–7.17 (m, 2H), 7.27–7.30 (m, 1H), 7.58 (d, J = 8.0 Hz, 1H); 13C NMR (CDCl3, 100 MHz, TMS) δ 14.09, 14.14, 28.1, 35.2, 59.9, 61.7, 101.5, 115.1, 124.0, 127.9, 128.4, 128.8, 133.1, 140.0, 142.4, 161.1, 163.8, 166.6; IR (CH2Cl2) ν 3060, 2980, 2902, 1711, 1659, 1470, 1372, 1298, 1258, 1197, 1108, 1022, 856, 803, 754, 670 cm−1; MS (ESI) m/z 395.0 (M+H+); HRMS (ESI) Calcd for C18H19BrO5Na requires (M+Na+): 417.0308, found: 417.0312.
Diethyl 4-(2,4-dichlorophenyl)-6-methyl-4H-pyran-2,5-dicarboxylate 6fa.
A slightly yellow liquid (4.2 mg, 11%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.05 (t, J = 7.2 Hz, 3H), 1.31 (t, J = 7.2 Hz, 3H), 2.49 (s, 3H), 3.97–4.03 (m, 2H), 4.22–4.29 (m, 2H), 5.03 (d, J = 4.8 Hz, 1H), 6.22 (d, J = 4.8 Hz, 1H), 7.14 (d, J = 8.4 Hz, 1H), 7.22 (d, J = 8.4 Hz, 1H), 7.37 (s, 1H). 13C NMR (CDCl3, 100 MHz, TMS) δ 13.8, 14.1, 19.1, 35.1, 60.4, 61.7, 103.0, 113.9, 127.8, 129.1, 130.7, 132.8, 133.2, 139.4, 140.4, 160.8, 161.6, 166.3; IR (CH2Cl2) ν 3086, 2982, 2906, 1716, 1630, 1586, 1561, 1372, 1270, 1189, 1172, 1097, 1074, 964, 918, 767 cm−1; MS (ESI) m/z 385.3. (M+H+); HRMS (ESI) Calcd for C18H18Cl2NaO5 requires (M+Na+): 407.0424, found: 407.0435.
(E)-Ethyl 4-(2,4-dichlorophenyl)-2-(2-ethoxy-2-oxoethylidene)-3,4-dihydro-2H-pyran-6-carboxylate
7fa.
A slightly yellow liquid (29.2 mg, 76%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.23 (t, J = 7.2 Hz, 3H), 1.35 (t, J = 7.2 Hz, 3H), 3.30 (dd, J = 15.2 Hz, 6.8 Hz, 1H), 3.47 (dd, J = 15.2 Hz, 6.4 Hz, 1H), 4.07–4.19 (m, 3H), 4.30–4.36 (m, 2H), 5.76 (s, 1H), 6.37 (d, J = 4.4 Hz, 1H), 7.09 (d, J = 8.4 Hz, 1H), 7.22 (d, J = 8.4 Hz, 1H), 7.41 (s, 1H); 13C NMR (CDCl3, 100 MHz, TMS) δ 14.11, 14.15, 27.7, 32.2, 60.0, 61.8, 101.8, 114.2, 127.5, 129.1, 129.6, 133.7, 134.1, 136.9, 142.7, 160.9, 163.5, 166.6; IR (CH2Cl2) ν 3086, 2982, 2905, 1660, 1587, 1561, 1474, 1372, 1299, 1256, 1170, 1104, 1020, 861, 818, 760 cm−1; MS (ESI) m/z 385.3. (M+H+); HRMS (ESI) Calcd for C18H18Cl2NaO5 requires (M+Na+): 407.0424, found: 407.0435.
Diethyl 6-methyl-4-p-tolyl-4H-pyran-2,5-dicarboxylate 6ga.
A slightly yellow liquid (2.2 mg, 7%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.11 (t, J = 7.2 Hz, 3H), 1.29 (t, J = 7.2 Hz, 3H), 2.31 (s, 3H), 2.43 (s, 3H), 3.98–4.07 (m, 2H), 4.21–3.31 (m, 2H), 4.46 (d, J = 4.8 Hz, 1H), 5.74 (s, 1H), 6.25 (d, J = 4.8 Hz, 1H), 7.08–7.13 (m, 4H); 13C NMR (CDCl3, 100 MHz, TMS) δ 14.0, 14.1, 19.1, 21.0, 38.5, 60.2, 61.5, 104.8, 116.4, 128.0, 129.3, 136.7, 138.9, 141.5, 159.6, 161.2, 167.0; IR (CH2Cl2) ν 2979, 2902, 1715, 1659, 1629, 1511, 1446, 1373, 1324, 1263, 1172, 1106, 1021, 865, 803, 739 cm−1; MS (ESI) m/z 353.1 (M+Na+); HRMS (ESI) Calcd for C19H22O5Na requires (M+Na+): 353.1359, found: 353.1371.
(E)-Ethyl 2-(2-ethoxy-2-oxoethylidene)-4-p-tolyl-3,4-dihydro-2H-pyran-6-carboxylate
7ga.
A slightly yellow liquid (25.9 mg, 78%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.23 (t, J = 7.2 Hz, 3H), 1.34 (t, J = 7.2 Hz, 3H), 2.33 (s, 3H), 3.06–3.13 (m, 1H), 3.62–3.69 (m, 2H), 4.08–4.14 (m, 2H), 4.27–4.33 (m, 2H), 5.74 (s, 1H), 6.43 (d, J = 4.4 Hz, 1H), 7.09 (d, J = 8.4 Hz, 2H), 7.14 (d, J = 8.4 Hz, 2H); 13C NMR (CDCl3, 100 MHz, TMS) δ 14.1, 14.2, 21.0, 29.7, 35.5, 59.8, 61.6, 100.9, 116.8, 127.1, 129.5, 136.9, 138.3, 141.6, 161.2, 164.8, 166.9; IR (CH2Cl2) ν 2980, 2902, 1736, 1712, 1658, 1514, 1373, 1297, 1167, 1113, 1045, 1020, 847, 808, 762 cm−1; MS (ESI) m/z 353.1 (M+Na+); HRMS (ESI) Calcd for C19H22O5Na requires (M+Na+): 353.1359, found: 353.1371.
Diethyl 4-(furan-2-yl)-6-methyl-4H-pyran-2,5-dicarboxylate 6ha.
A slightly yellow liquid (4.6 mg, 15%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.18 (t, J = 7.2 Hz, 3H), 1.32 (t, J = 7.2 Hz, 3H), 2.41 (s, 3H), 4.06–4.18 (m, 2H), 4.25–4.31 (m, 2H), 4.67 (d, J = 5.2 Hz, 1H), 6.05 (d, J = 5.2 Hz, 1H), 6.28–6.29 (m, 2H), 7.27–7.29 (m, 1H); 13C NMR (CDCl3, 100 MHz, TMS) δ 14.06, 14.12, 19.1, 32.3, 60.3, 61.6, 102.2, 106.1, 110.5, 112.9, 140.1, 141.6, 156.1, 160.5, 160.9, 166.7; IR (CH2Cl2) ν 2980, 2903, 2857, 1738, 1716, 1660, 1475, 1372, 1266, 1173, 1107, 1048, 1020, 798, 763, 739 cm−1; MS (ESI) m/z 329.1 (M+Na+); HRMS (ESI) Calcd for C16H18O6Na requires (M+Na+): 329.0996, found: 329.1000.
(E)-Ethyl 2-(2-ethoxy-2-oxoethylidene)-4-(furan-2-yl)-3,4-dihydro-2H-pyran-6-carboxylate
7ha.
A slightly yellow liquid (13.8 mg, 45%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.27 (t, J = 7.2 Hz, 3H), 1.34 (t, J = 7.2 Hz, 3H), 3.36 (dd, J = 15.2 Hz, 8.0 Hz, 1H), 3.56 (dd, J = 15.2 Hz, 5.2 Hz, 1H), 3.78–3.83 (m, 1H), 4.15 (q, J = 7.2 Hz, 2H), 4.30 (q, J = 7.2 Hz, 2H), 5.76 (s, 1H), 6.13–6.14 (m, 1H), 6.30–6.31 (m, 1H), 6.45 (d, J = 4.0 Hz, 1H), 7.35–7.36 (m, 1H); 13C NMR (CDCl3, 100 MHz, TMS) δ 14.1, 14.3, 26.3, 29.7, 59.9, 61.7, 101.4, 105.7, 110.3, 113.4, 141.8, 142.1, 153.4, 161.1, 164.1, 166.9; IR (CH2Cl2) ν 2980, 2906, 2874, 1737, 1712, 1660, 1446, 1373, 1298, 1174, 1120, 1046, 1017, 801, 761, 739 cm−1; MS (ESI) m/z 329.1 (M+Na+); HRMS (ESI) Calcd for C16H18O6Na requires (M+Na+): 329.0996, found: 329.1007.
Diethyl 6-methyl-4-(thiophen-2-yl)-4H-pyran-2,5-dicarboxylate
6ia.
A slightly yellow liquid (2.6 mg, 8%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.19 (t, J = 7.2 Hz, 3H), 1.32 (t, J = 7.2 Hz, 3H), 2.41 (s, 3H), 4.07–4.17 (m, 2H), 4.25–4.31 (m, 2H), 4.83 (d, J = 5.2 Hz, 1H), 6.34 (d, J = 5.2 Hz, 1H), 6.86–6.87 (m, 1H), 6.91 (dd, J = 4.8 Hz, 3.2 Hz, 1H), 7.17 (dd, J = 4.8 Hz, 1.2 Hz, 1H); 13C NMR (CDCl3, 100 MHz, TMS) δ 14.03, 14.1, 19.1, 33.4, 60.4, 61.6, 104.8, 115.1, 124.6, 124.9, 126.9, 139.4, 148.3, 159.7, 161.0, 166.7; IR (CH2Cl2) ν 2980, 2903, 2857, 1738, 1716, 1660, 1475, 1372, 1266, 1173, 1107, 1048, 1020, 798, 763, 739 cm−1; MS (ESI) m/z 345.1 (M+Na+); HRMS (ESI) Calcd for C16H18O5SNa requires (M+Na+): 345.0767, found: 345.0775.
(E)-Ethyl 2-(2-ethoxy-2-oxoethylidene)-4-(thiophen-2-yl)-3,4-dihydro-2H-pyran-6-carboxylate
7ia.
A slightly yellow liquid (15.7 mg, 49%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.25 (t, J = 7.2 Hz, 3H), 1.34 (t, J = 7.2 Hz, 3H), 3.41 (dd, J = 15.2 Hz, 8.0 Hz, 1H), 3.56 (dd, J = 15.2 Hz, 5.2 Hz, 1H), 3.99–4.03 (m, 1H), 4.13 (q, J = 7.2 Hz, 2H), 4.31 (q, J = 7.2 Hz, 2H), 5.77 (s, 1H), 6.48 (d, J = 4.0 Hz, 1H), 6.90 (d, J = 3.6 Hz, 1H), 6.95 (dd, J = 4.8 Hz, 3.6 Hz, 1H), 7.20 (d, J = 4.8 Hz, 1H); 13C NMR (CDCl3, 100 MHz, TMS) δ 14.15, 14.24, 29.8, 31.2, 59.9, 61.7, 101.7, 115.7, 124.4, 124.5, 126.9, 141.5, 144.1, 161.2, 163.9, 166.8; IR (CH2Cl2) ν 3075, 2966, 2934, 1736, 1713, 1660, 1373, 1259, 1173, 1259, 1119, 1046, 1020, 802, 762, 738, 701 cm−1; MS (ESI) m/z 345.2 (M+Na+); HRMS (ESI) Calcd for C16H18O5SNa requires (M+Na+): 345.0767, found: 345.0777.
Diethyl 6-methyl-4-(naphthalen-2-yl)-4H-pyran-2,5-dicarboxylate
6ja.
A slightly yellow liquid (3.3 mg, 9%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.07 (t, J = 7.2 Hz, 3H), 1.29 (t, J = 7.2 Hz, 3H), 2.48 (s, 3H), 3.98–4.00 (m, 2H), 4.24–4.27 (m, 2H), 4.68 (d, J = 5.2 Hz, 1H), 6.31 (d, J = 5.2 Hz, 1H), 7.38 (dd, J = 8.4 Hz, 1.6 Hz, 1H), 7.44–7.47 (m, 2H), 7.65 (s, 1H), 7.78–7.80 (m, 3H); 13C NMR (CDCl3, 100 MHz, TMS) δ 14.0, 14.1, 19.2, 39.1, 60.2, 61.6, 104.6, 116.0, 125.8, 126.1, 126.3, 126.9, 127.6, 127.8, 128.3, 132.6, 133.5, 139.0, 141.8, 159.9, 161.1, 166.9; IR (CH2Cl2) ν 3057, 2980, 2902, 1715, 1474, 1373, 1262, 1106, 1048, 1021, 956, 859, 801, 746, 702, 667 cm−1; MS (ESI) m/z 389.2 (M+Na+); HRMS (ESI) Calcd for C22H22O5Na requires (M+Na+): 389.1359, found: 389.1374.
(E)-ethyl 2-(2-ethoxy-2-oxoethylidene)-4-(naphthalen-2-yl)-3,4-dihydro-2H-pyran-6-carboxylate
7ja.
A slightly yellow liquid (26.7 mg, 73%); 1H NMR (CDCl3, 400 MHz, TMS) δ 1.21 (t, J = 7.2 Hz, 3H), 1.36 (t, J = 7.2 Hz, 3H), 3.27 (dd, J = 14.8 Hz, 8.4 Hz, 1H), 3.71 (dd, J = 14.8 Hz, 5.2 Hz, 1H), 3.86–3.89 (m, 1H), 4.05–4.12 (m, 2H), 4.31–4.36 (m, 2H), 5.76 (s, 1H), 6.54 (d, J = 4.0 Hz, 1H), 7.34 (dd, J = 8.4 Hz, 1.2 Hz, 1H), 7.46–7.49 (m, 2H), 7.64 (s, 1H), 7.78–7.83 (m, 3H); 13C NMR (CDCl3, 100 MHz, TMS) δ 14.17, 14.20, 29.6, 36.0, 59.8, 61.7, 101.3, 116.3, 125.5, 125.8, 125.9, 126.3, 127.6, 127.7, 128.7, 132.6, 133.4, 138.6, 142.0, 161.2, 164.5, 166.9; IR (CH2Cl2) ν 3056, 2982, 2904, 1734, 1709, 1659, 1508, 1474, 1445, 1372, 1298, 1254, 1172, 1115, 854, 817, 760 cm−1; MS (ESI) m/z 389.2 (M+Na+); HRMS (ESI) Calcd for C22H22O5Na requires (M+Na+): 389.1359, found: 389.1364.
(E)-Ethyl 4-cyclopropyl-2-(2-ethoxy-2-oxoethylidene)-3,4-dihydro-2H-pyran-6-carboxylate
7ka.
A slightly yellow liquid (27.3 mg, 90%); 1H NMR (CDCl3, 400 MHz, TMS) δ 0.23–0.26 (m, 2H), 0.52–0.56 (m, 2H), 0.70–0.76 (m, 1H), 1.28 (t, J = 7.2 Hz, 3H), 1.35 (t, J = 7.2 Hz, 3H), 1.72–1.79 (m, 1H), 2.92 (dd, J = 15.2 Hz, 8.4 Hz, 1H), 3.48 (dd, J = 15.2 Hz, 5.6 Hz, 1H), 4.16 (q, J = 7.2 Hz, 2H), 4.27–4.32 (m, 2H), 5.70 (s, 1H), 6.32 (d, J = 4.0 Hz, 1H). 13C NMR (CDCl3, 100 MHz, TMS) δ 3.2, 3.6, 14.1, 14.3, 15.3, 27.7, 34.9, 59.8, 61.5, 100.4, 117.1, 141.1, 161.3, 165.7, 167.2. IR (CH2Cl2) ν 3081, 2982, 2903, 1737, 1713, 1659, 1372, 1335, 1298, 1256, 1120, 1048, 1021, 854, 761, 740 cm−1. MS (ESI) m/z 281.1 (M+H+). HRMS (ESI) Calcd for C15H20O5Na requires (M+Na+): 303.1203, found: 303.1209.
Acknowledgements
Financial support from the National Basic Research Program of China (973)-2010CB833302, the Fundamental Research Funds for the Central Universities and the National Natural Science Foundation of China for financial support (21072206, 20472096, 20902019, 20872162, 20672127, 20821002, 20732008, 20772030 and 20702059) is greatly acknowledged.
References
-
(a) E. A. Couladouros and A. T. Strongilos, Angew. Chem., Int. Ed., 2002, 41, 3677 CrossRef CAS and references therein;
(b) K. Moonen, I. Laureyn and C. Stevens, Chem. Rev., 2004, 104, 6177 CrossRef CAS and references therein.
-
(a) X. Lu, C. Zhang and Z. Xu, Acc. Chem. Res., 2001, 34, 535 CrossRef CAS;
(b) P. I. Dalko and L. Moisan, Angew. Chem., Int. Ed., 2004, 43, 5138 CrossRef CAS;
(c) G. C. Fu, Acc. Chem. Res., 2004, 37, 542 CrossRef CAS.
-
(a) X. Wang, T. Fang and X. Tong, Angew. Chem., Int. Ed., 2011, 50, 5361 Search PubMed;
(b) J. Peng, X. Huang, L. Jiang, H.-L. Cui and Y.-C. Chen, Org. Lett., 2011, 13, 4584 Search PubMed;
(c) J.-B. Denis, G. Masson, P. Retailleau and J. Zhu, Angew. Chem., Int. Ed., 2011, 50, 5356 Search PubMed;
(d) Y.-L. Shi and M. Shi, Org. Lett., 2005, 7, 3057 CrossRef CAS;
(e) X.-C. Zhang, S.-H. Cao, Y. Wei and M. Shi, Org. Lett., 2011, 13, 1142 Search PubMed;
(f) G.-L. Zhao, Y.-L. Shi and M. Shi, Org. Lett., 2005, 7, 4527 CrossRef CAS;
(g) G.-L. Zhao, J.-W. Huang and M. Shi, Org. Lett., 2003, 5, 4737 CrossRef CAS;
(h) C.-K. Pei and M. Shi, Tetrahedron: Asymmetry, 2011, 22, 1239 Search PubMed.
- Selected papers on the cyclization of β,γ-unsaturated α-ketophosphonates:
(a) D. A. Evans and J. S. Johnson, J. Am. Chem. Soc., 1998, 120, 4895 CrossRef CAS;
(b) D. A. Evans, J. S. Johnson and E. J. Olhava, J. Am. Chem. Soc., 2000, 122, 1635 CrossRef CAS;
(c) S. Hanessian and P. Compain, Tetrahedron, 2002, 58, 6521 CrossRef CAS;
(d) K. Srinivasan and B. C. Gibb, Org. Lett., 2007, 9, 745 CrossRef CAS;
(e) Y. J. Shin, C.-E. Yeom, M. J. Kim and B. M. Kim, Synlett, 2008, 1, 89 Search PubMed.
- Selected papers on the cyclization of β,γ-unsaturated α-ketoesters:
(a) D. A. Evans, J. S. Johnson and E. J. Olhava, J. Am. Chem. Soc., 2000, 122, 1635 CrossRef CAS;
(b) D. A. Evans and J. M. Janey, Org. Lett., 2001, 3, 2125 CrossRef CAS;
(c) W. Yao, Y. Wu, G. Wang, Y. Zhang and C. Ma, Angew. Chem., Int. Ed., 2009, 48, 9713 CAS;
(d) M. A. Calter and J. Wang, Org. Lett., 2009, 11, 2205 CrossRef CAS;
(e) F. Gallier, H. Hussain, A. Martel, A. Kirschning and G. Dujaedin, Org. Lett., 2009, 11, 3060 CrossRef CAS;
(f) W. Yao, L. Pan, Y. Wu and C. Ma, Org. Lett., 2010, 12, 2422 CrossRef CAS;
(g) D. Xu, Y. Zhang and D. Ma, Tetrahedron Lett., 2010, 51, 3827 CrossRef CAS;
(h) M. Terada and H. Nii, Chem.–Eur. J., 2011, 17, 1760 CrossRef CAS;
(i) C.-L. Cao, X.-L. Sun, Y.-B. Kang and Y. Tang, Org. Lett., 2007, 9, 4151 CrossRef CAS;
(j) J. Wang, F. Yu, X. Zhang and D. Ma, Org. Lett., 2008, 10, 2561 CrossRef CAS;
(k) K. Juhl and K. A. Jørgensen, Angew. Chem., Int. Ed., 2003, 42, 1498 CrossRef CAS;
(l) J. Zhou and Y. Tang, Org. Biomol. Chem., 2004, 2, 429 RSC;
(m) D. Belmessieri, L. C. Morrill, C. Simal, A. M. Z. Slawin and A. D. Smith, J. Am. Chem. Soc., 2011, 133, 2714 CrossRef CAS;
(n) Y. Zhu, C. Zhai, Y. Yue, L. Yang and W. Hu, Chem. Commun., 2009, 1362 RSC;
(o) D. T. Cohen, B. C. David and K. A. Scheidt, Angew. Chem., Int. Ed., 2011, 50, 1678 CrossRef CAS;
(p) Y.-C. Wu, L. Liu, H.-J. Li, D. Wang and Y.-J. Chen, J. Org. Chem., 2006, 71, 6592 CrossRef CAS;
(q) A. Martel, S. Leconte, G. Dujardin, E. Brown, V. Maisonneuve and R. Retoux, Eur. J. Org. Chem., 2002, 514 Search PubMed;
(r) S. Leconte, G. Dujardin and E. Brown, Eur. J. Org. Chem., 2000, 639 CrossRef CAS;
(s) X.-K. Chen, C.-W. Zheng, S.-L. Zhao, Z. Chai, Y.-Q. Yang, G. Zhao and W.-G. Cao, Adv. Synth. Catal., 2010, 352, 1648 CrossRef CAS;
(t) J. Thorhauge, M. Johannsen and K. A. Jørgensen, Angew. Chem., Int. Ed., 1998, 37, 2404 CrossRef CAS;
(u) Y. Ying, Z. Chai, H.-F. Wang, P. Li, C.-W. Zheng, G. Zhao and Y.-P. Cai, Tetrahedron, 2011, 67, 3337 CrossRef CAS;
(v) Z. Dong, J. Feng, W. Cao, X. Liu, L. Lin and X. Feng, Tetrahedron Lett., 2011, 52, 3433 Search PubMed;
(w) W. Li, K. T. Mead and L. T. Smith, Tetrahedron Lett., 2003, 44, 6351 Search PubMed;
(x) Y. Gao, Q. Ren, L. Wang and J. Wang, Chem.–Eur. J., 2010, 16, 13068 CrossRef;
(y) H. L. van Lingen, W. Zhuang, T. Hansen, F. P. J. T. Rutjes and K. A. Jørgensen, Org. Biomol. Chem., 2003, 1, 1953 RSC.
-
(a) A. A. L. Gunatilaka, J. Nat. Prod., 2006, 69, 509 CrossRef CAS;
(b) K.-S. Yeung and I. Paterson, Chem. Rev., 2005, 105, 4237 CrossRef CAS;
(c) E. J. Kang and E. Lee, Chem. Rev., 2005, 105, 4348 CrossRef CAS;
(d) M. Inoue, Chem. Rev., 2005, 105, 4379 CrossRef CAS;
(e) J. E. Aho, P. M. Pihko and T. K. Rissa, Chem. Rev., 2005, 105, 4406 CrossRef CAS;
(f) T. Nakata, Chem. Rev., 2005, 105, 4314 CrossRef CAS;
(g) W. P. D. Goldring and G. Pattenden, Acc. Chem. Res., 2006, 39, 354 CrossRef CAS;
(h) L. Yet, Chem. Rev., 2003, 103, 4283 CrossRef CAS.
- The crystal data of compound 6ea have been deposited in CCDC with number 833190. Empirical Formula: C18H19BrO5; Formula Weight: 395.24; Crystal Color, Habit: colorless; Crystal Dimensions: 0.28 × 0.15 × 0.10 mm; Crystal System: Triclinic; Lattice Type: Primitive; Lattice Parameters: a = 8.5579(17) Å, b = 9.7108(19) Å, c = 11.568(3) Å, = 73.985(4)°, β = 68.692(3)°, γ = 73.448(3)°, V = 842.4(3) Å3; Space group: P
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ; Z = 2; Dc = 1.558 g cm−3; F000 = 404; Final R indices [I > 2σ(I)]: R1 = 0.0438; wR2 = 0.1321.
; Z = 2; Dc = 1.558 g cm−3; F000 = 404; Final R indices [I > 2σ(I)]: R1 = 0.0438; wR2 = 0.1321.
-
(a) A. Kumar, S. Sharma, V. D. Tripathi, R. A. Maurya, S. P. Srivastava, G. Bhatia, A. K. Tamrakar and A. K. Srivastava, Bioorg. Med. Chem., 2010, 18, 4138 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2012 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4aa was 2
4aa was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 within 24 h (Table 1, entry 1). Subsequently, we screened various nitrogen-containing Lewis base catalysts for this reaction, and the results are summarized in Table 1 (entries 2–7). 4-N,N-dimethylpyridine (DMAP) can also efficiently catalyze this reaction to give the product mixture of 3aa and 4aa in 84% combined yield with a ratio of 1
1 within 24 h (Table 1, entry 1). Subsequently, we screened various nitrogen-containing Lewis base catalysts for this reaction, and the results are summarized in Table 1 (entries 2–7). 4-N,N-dimethylpyridine (DMAP) can also efficiently catalyze this reaction to give the product mixture of 3aa and 4aa in 84% combined yield with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 1, entry 2). Other weak nucleophilic nitrogen-containing Lewis base catalysts, such as 1,8-diazabicyclo[5,4,0]-7-undecene (DBU), Et3N and diisopropylethylamine (DIEA) did not catalyze this reaction (Table 1, entries 3–5). We next attempted to screen phosphane-containing Lewis base catalysts, such as PPh3 and tributylphosphine (PBu3), in this reaction, but it was found that no reactions occurred either (Table 1, entries 6 and 7). Of the catalysts examined, DABCO was found to be the best one. Using DABCO as the catalyst, various solvents were examined and dichloromethane (DCM) was found to be the solvent of choice, affording 3aa and 4aa in 90% combined yield with the ratio of 3
1 (Table 1, entry 2). Other weak nucleophilic nitrogen-containing Lewis base catalysts, such as 1,8-diazabicyclo[5,4,0]-7-undecene (DBU), Et3N and diisopropylethylamine (DIEA) did not catalyze this reaction (Table 1, entries 3–5). We next attempted to screen phosphane-containing Lewis base catalysts, such as PPh3 and tributylphosphine (PBu3), in this reaction, but it was found that no reactions occurred either (Table 1, entries 6 and 7). Of the catalysts examined, DABCO was found to be the best one. Using DABCO as the catalyst, various solvents were examined and dichloromethane (DCM) was found to be the solvent of choice, affording 3aa and 4aa in 90% combined yield with the ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 1, entries 8–16). Lowering the reaction temperature to 0 °C or −20 °C, similar results were obtained (Table 1, entries 17 and 18). At −40 °C, the corresponding cyclic adducts 3aa and 4aa were obtained in 86% combined yield along with a 5
1 (Table 1, entries 8–16). Lowering the reaction temperature to 0 °C or −20 °C, similar results were obtained (Table 1, entries 17 and 18). At −40 °C, the corresponding cyclic adducts 3aa and 4aa were obtained in 86% combined yield along with a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 3aa
1 ratio of 3aa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4aa (Table 1, entry 19). Further reducing the reaction temperature did not improve the reaction outcomes (Table 1, entry 20). Thus, we have established the optimal reaction conditions for this reaction: using 20 mol % DABCO as a catalyst and DCM as a solvent to perform the reaction at −40 °C.
4aa (Table 1, entry 19). Further reducing the reaction temperature did not improve the reaction outcomes (Table 1, entry 20). Thus, we have established the optimal reaction conditions for this reaction: using 20 mol % DABCO as a catalyst and DCM as a solvent to perform the reaction at −40 °C.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4aa (%)c
4aa (%)c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 = 3
4 = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Table 2, entries 2 and 3). However, we found that the R2 substituent in the phosphonate moiety of cinnamoylphosphonates 1 can significantly affect the reaction outcomes, as that R2group can improve the regioselectivity of the products (up to 3
1) (Table 2, entries 2 and 3). However, we found that the R2 substituent in the phosphonate moiety of cinnamoylphosphonates 1 can significantly affect the reaction outcomes, as that R2group can improve the regioselectivity of the products (up to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 = 10
4 = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) if it had a sterically bulky group, such as OiPr or OtBu (Table 2, entries 4 and 5). As for substrates 1d–1h, electron-withdrawing or electron-donating substituents at the meta- or para-positions of the benzene ring of 1 were equally well-tolerated in the reaction, giving the corresponding products 3 and 4 in good combined yields along with high regioselectivities (up to 3
1) if it had a sterically bulky group, such as OiPr or OtBu (Table 2, entries 4 and 5). As for substrates 1d–1h, electron-withdrawing or electron-donating substituents at the meta- or para-positions of the benzene ring of 1 were equally well-tolerated in the reaction, giving the corresponding products 3 and 4 in good combined yields along with high regioselectivities (up to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 > 20
4 > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Table 2, entries 6–10). The substrate 1i, in which R1 is a 1-naphthyl group, was also able in this reaction to give the corresponding products in 85% combined yield along with good regioselectivity (3ia
1) (Table 2, entries 6–10). The substrate 1i, in which R1 is a 1-naphthyl group, was also able in this reaction to give the corresponding products in 85% combined yield along with good regioselectivity (3ia![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4ia = 14
4ia = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Table 2, entry 11). When R1 is an alkyl group (1j, R1 = Me), the reaction also proceeded smoothly to give the desired products 3ja and 4ja in 75% combined yield but with moderate regioselectivity (3ja
1) (Table 2, entry 11). When R1 is an alkyl group (1j, R1 = Me), the reaction also proceeded smoothly to give the desired products 3ja and 4ja in 75% combined yield but with moderate regioselectivity (3ja![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4ja = 4
4ja = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Table 2, entry 12). The structures of 3 and 4 were determined by the 2-D NMR spectroscopic data (HMQC, HMBC, DEPT and NOESY spectra) of compounds 3ca and 4ca (see the Supporting Information for the details†).
1) (Table 2, entry 12). The structures of 3 and 4 were determined by the 2-D NMR spectroscopic data (HMQC, HMBC, DEPT and NOESY spectra) of compounds 3ca and 4ca (see the Supporting Information for the details†).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4c
4c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4aa
4aa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4ab
4ab![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4ac
4ac![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4ba
4ba![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4ca
4ca![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4da
4da![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4ea
4ea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4fa
4fa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4ga
4ga![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4ha
4ha![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4ia
4ia![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4ja
4ja![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6aa = 8
6aa = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Table 3, entry 1), which was contrary to the above results in Table 2. Reducing the concentration to 0.03 M by increasing the amount of solvent (DCM) employed to 3.00 mL can improve the reaction outcome, affording the corresponding products in 85% combined yield along with the 13
1) (Table 3, entry 1), which was contrary to the above results in Table 2. Reducing the concentration to 0.03 M by increasing the amount of solvent (DCM) employed to 3.00 mL can improve the reaction outcome, affording the corresponding products in 85% combined yield along with the 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 7aa
1 ratio of 7aa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6aa (Table 3, entry 1). Inspired by this result, we next screened various nitrogen-containing Lewis base catalysts for this reaction in the optimized amount of solvent (3.00 mL, 0.03 M). DMAP can efficiently catalyze this reaction as well but with low regioselectivity. However, DBU and DIEA did not catalyze this reaction (Table 3, entries 2–4). Accordingly, DABCO was then used as the best catalyst for further investigation of solvent and temperature effects in this reaction. It was found that THF was the solvent of choice in comparison with those reactions carried out in other organic solvents, such as toluene, Et2O, 1,2-dichloroethane (DCE), chloroform, 1,4-dioxane, CH3CN, N,N-dimethylformamide (DMF) and dimethyl sulfoxide (DMSO) (Table 3, entries 5–13). The low temperature (−10 °C) can improve the combined yield of 6aa and 7aa (Table 3, entry 15), but further lowering the reaction temperature to −40 °C reduced the combined yield (Table 3, entry 16). Thus, we have identified the optimal reaction conditions for this reaction: using 20 mol% of DABCO as a catalyst and THF as a solvent to perform the reaction at −10 °C.
6aa (Table 3, entry 1). Inspired by this result, we next screened various nitrogen-containing Lewis base catalysts for this reaction in the optimized amount of solvent (3.00 mL, 0.03 M). DMAP can efficiently catalyze this reaction as well but with low regioselectivity. However, DBU and DIEA did not catalyze this reaction (Table 3, entries 2–4). Accordingly, DABCO was then used as the best catalyst for further investigation of solvent and temperature effects in this reaction. It was found that THF was the solvent of choice in comparison with those reactions carried out in other organic solvents, such as toluene, Et2O, 1,2-dichloroethane (DCE), chloroform, 1,4-dioxane, CH3CN, N,N-dimethylformamide (DMF) and dimethyl sulfoxide (DMSO) (Table 3, entries 5–13). The low temperature (−10 °C) can improve the combined yield of 6aa and 7aa (Table 3, entry 15), but further lowering the reaction temperature to −40 °C reduced the combined yield (Table 3, entry 16). Thus, we have identified the optimal reaction conditions for this reaction: using 20 mol% of DABCO as a catalyst and THF as a solvent to perform the reaction at −10 °C.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6aa (%)c
6aa (%)c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (8
1 (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)d
1)d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6ja = 8
6ja = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Table 4, entry 10). For substrate 5k, in which R4 is a cyclopropyl group, the reaction also proceeded smoothly to give the desired products in high combined yield (up to 90%) along with high regioselectivity (up to 7ka
1) (Table 4, entry 10). For substrate 5k, in which R4 is a cyclopropyl group, the reaction also proceeded smoothly to give the desired products in high combined yield (up to 90%) along with high regioselectivity (up to 7ka![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6ka = >20
6ka = >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The structure of 6ea was unambiguously determined by X-ray diffraction. The ORTEP drawing is shown in Fig. 1 and its CIF data are summarized in the Supporting Information.†7 The structure of 7 and the E-configuration of the double bond were assigned by the 2-D NMR spectroscopic data (HMQC, HMBC, DEPT and NOESY spectra) of compound 7aa (see the Supporting Information for the details†).
1). The structure of 6ea was unambiguously determined by X-ray diffraction. The ORTEP drawing is shown in Fig. 1 and its CIF data are summarized in the Supporting Information.†7 The structure of 7 and the E-configuration of the double bond were assigned by the 2-D NMR spectroscopic data (HMQC, HMBC, DEPT and NOESY spectra) of compound 7aa (see the Supporting Information for the details†).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6c
6c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6aa
6aa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6ba
6ba![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6ca
6ca![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6da
6da![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6ea
6ea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6fa
6fa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6ga
6ga![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6ha
6ha![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6ia
6ia![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6ja
6ja![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6ka
6ka![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1

![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ; Z = 2; Dc = 1.558 g cm−3; F000 = 404; Final R indices [I > 2σ(I)]: R1 = 0.0438; wR2 = 0.1321.
; Z = 2; Dc = 1.558 g cm−3; F000 = 404; Final R indices [I > 2σ(I)]: R1 = 0.0438; wR2 = 0.1321.