Synthesis of chiral hybrid nanotubes of magnetite nanoparticles and conducting polymers†
Masashi
Mitsumori
,
Tsukasa
Nakahodo
and
Hisashi
Fujihara
*
Department of Applied Chemistry, Kinki University, Kowakae, Higashi-Osaka, 577-8502, Japan. E-mail: h-fuji@apch.kindai.ac.jp; Fax: +81-6-6727-2024; Tel: +81-6-6721-2332
First published on 28th October 2011
Abstract
New chiral magnetite nanoparticles with a polymerizable group produced polymer composite films on an electrode surface and the hybrid nanotubes of magnetite nanoparticles and polythiophene from their template-based electropolymerization.
Currently, there has been great interest in the synthesis and functionalization of magnetite nanoparticles (NPs), carbon and polymer nanotubes (NTs), and their nanocomposites due to their unique physical and chemical properties.1–5 Several efforts have been devoted to preparing hybrid materials of carbon NTs and magnetite NPs,6 however, surface modification of the carbon NTs and subsequent assembly with synthesized magnetite NPs via covalent or noncovalent bonding, i.e., decoration of carbon NTs with magnetite NPs, is the most common preparation route. Although there have been many significant developments in the synthesis of magnetic NPs and their nanocomposites, maintaining the stability of these nanoparticles for a long time without aggregation is an important issue. On the other hand, we recently reported new hybrid NTs of metal NPs and polythiophene (PT) by the electropolymerization of thiophene-modified metal NPs and the reusable hybrid nanotube-catalyzed carbon–carbon bond forming reaction under heating conditions, i.e., the hybrid structure prevents aggregation of the metal NPs.7 It is worth pointing out that our approach to prepare the hybrid NTs of metal NPs and polythiophene7 significantly differs from all previous studies of the decoration of metal NPs on the surface of polymer and carbon NTs.3,8 Conducting polythiophenes are indispensable materials for the development of displays, electronic and energy storage devices, actuators, sensors, and neural interfaces. The nanotubular structure of polythiophene is one of the ideal structures that can enhance the device performance by improving the charge-transport rate as well as increasing the surface area. Thus, the nanocomposites based on conducting polymers and metal NPs, such as inorganic nanomaterials, are of interest due to their synergistic and hybrid properties as components. In contrast, so far there have been no reports on the synthesis of nanotube composites consisting of magnetite NPs and a conducting polymer as a constituent of hybrid NTs by the electropolymerization of thiophene-modified magnetite NPs, and their related chiral nanocomposites.9 The combination of conducting polymers with magnetite NPs is the focus of research because materials having both a high conductivity and a high magnetic susceptibility can be used in different applications, such as electromagnetic interference shielding, magneto-optical storage, magnetic electrocatalysis, and drug delivery.
We have developed a new chiral stabilizer (1) which consists of a carboxyl group for the coordination site of the magnetite (Fe3O4) NPs, terthiophene for the polymerization site, and a binaphthyl group as a chiral site (Fig. 1).11,12 It is noteworthy that in many cases the stabilizers not only stabilize the NPs, but can also be used for further functionalization, for instance, with other NPs or various ligands, depending on the desired application. This communication describes the synthesis of chiral 1-modified small size magnetite (Fe3O4) NPs [(R)-, (S)-1-Fe3O4 NPs], the chiral surface modification of an electrode with the chiral magnetite NP–polymer composite films by the electropolymerization of (R)-1-Fe3O4 NPs, and the preparation of the chiral hybrid NTs of magnetite NPs and polythiophene, (R)-, (S)-1-Fe3O4-PT-NTs, from the template-based electropolymerization of (R)-, (S)-1-Fe3O4 NPs in a nanoporous alumina membrane. Much less attention has been paid to the preparation of magnetite NPs modified by an optically active stabilizer and to related chiral nanotube composites.13 In contrast to magnetite NPs, the chirality of extended metal NPs has become an emerging field of research in recent years triggered by potential applications in asymmetric catalysis.14 Despite a number of theoretical and experimental studies on optically active inorganic nanomaterials, such as metal NPs, the origin of the chirality is still under discussion.14,15
![Structure of chiral stabilizer 1 used to derive the (R)- and (S)-1-modified Fe3O4 NPs [(R)-, (S)-1-Fe3O4 NPs].](/image/article/2012/NR/c1nr11312g/c1nr11312g-f1.gif) | ||
| Fig. 1 Structure of chiral stabilizer 1 used to derive the (R)- and (S)-1-modified Fe3O4 NPs [(R)-, (S)-1-Fe3O4 NPs]. | ||
Chiral (R)- or (S)-1-modified magnetite NPs were synthesized as follows. Iron(III) acetylacetonate (0.7 mmol) was mixed in phenyl ether (7 mL) with 1,2-hexadecanediol (3.3 mmol), oleylamine (4.0 mmol), and (R)-1 (1.0 mmol) under argon and then heated at 265 °C for 1 h.11 After cooling to room temperature, the dark-brown mixture was treated with ethanol under air, and a dark-brown material was precipitated from the solution. The product was dissolved in hexane under ultrasonic treatment and reprecipitated with ethanol to give the (R)- or (S)-1-Fe3O4 NPs. The chiral magnetite NPs, (R)-1-Fe3O4 NPs and (S)-1-Fe3O4 NPs, were characterized by transmission electron microscopy (TEM), energy-dispersive X-ray (EDX) spectroscopy, X-ray powder diffraction (XRD), X-ray photoelectron spectroscopy (XPS), UV-vis, and circular dichroism (CD) spectroscopy. The TEM picture of the (R)-1-Fe3O4 NPs shows a 4.4 ± 0.9 nm diameter (Fig. 2a) and their EDX analysis revealed the existence of the O, S, and Fe elements (Fig. S1a, ESI†).12 The XRD spectrum of the (R)-1-Fe3O4 NPs shows that the diffraction peaks at the 2θ values of 30.0°, 35.5°, 43.1°, 53.4°, 57.0° and 62.6° can be assigned to the (220), (311), (400), (422), (511) and (440) crystal planes of the cubic magnetite, respectively (Fig. 3a). XPS was employed to measure the elemental map of the (R)-1-Fe3O4 NPs; as expected, the peaks of sulfur (S 1s), carbon (C 1s), oxygen (O 1s) and iron (Fe 2p3/2 and Fe 2p1/2) were found in the spectrum (Fig. S1b, ESI†). The UV-vis spectrum of the (R)-1-Fe3O4 NPs in CHCl3 shows the absorption due to terthiophene at 340 nm (Fig. 4b). The (R)-1-Fe3O4 NPs exhibit a response to external magnetic fields (Fig. 3b). Interestingly, the CD spectrum of the (R)-1-Fe3O4 NPs in CHCl3 shows positive Cotton effects, while the (S)-1-Fe3O4 NPs spectrum shows negative Cotton effects, as observed in Fig. 2b. Very few examples of the characterization of optically active molecule-modified Fe3O4 NPs by CD spectroscopy are known, although CD has been used as a particularly relevant method for measuring the degree of asymmetry in inorganic nanomaterials, such as metal NPs modified with chiral molecules.14,15 Further studies on this preliminary examination for the chirality (chiroptical activity) of the magnetite NPs modified by an optically active organic molecule are now in progress.
 | ||
| Fig. 2 (a) TEM image of (R)-1-Fe3O4 NPs. (b) CD spectra of (R)-1-Fe3O4 NPs (a: blue) and (S)-1-Fe3O4 NPs (b: red) in CHCl3. | ||
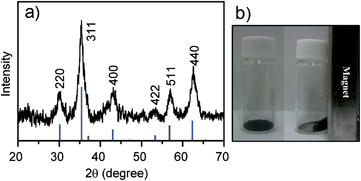 | ||
| Fig. 3 (a) XRD patterns of (R)-1-Fe3O4 NPs. The peak positions and relative intensities recorded in the literature (ICDD) for bulk Fe3O4 samples are indicated by the vertical bars. (b) Photographs of (R)-1-Fe3O4 NPs and their response to a magnet (right). | ||
 | ||
| Fig. 4 (a) Oxidative electropolymerization of (R)-1-Fe3O4 NPs by repeated potential scans on a glassy carbon electrode in CH2Cl2–0.1 M Bu4NPF6; scan rate 100 mV s−1. (b) UV-vis spectra of (R)-1-Fe3O4 NPs (a: blue) and (R)-1-Fe3O4-PT-NTs (b: red) in CHCl3. | ||
In order to fabricate the hybrid NTs by electropolymerization, the electropolymerization of the chiral 1-modified Fe3O4 NPs was studied in the absence or in the presence of a porous alumina membrane. Electrochemical polymerization is an elegant strategy for the immobilization of functional materials on a solid surface. The surface modification of electrodes with polymer films has been performed by the electropolymerization of thiophene-based monomers. Although only one example exists on the preparation of magnetite–polythiophene composite films by the electropolymerization of 3-thiophene-acetic-acid in the presence of Fe3O4 NPs,10 there is no clear-cut example of the electropolymerization of Fe3O4 NPs with an electropolymerizable group, such as thiophene or pyrrole. Accordingly, the electrochemical properties of the (R)-1-Fe3O4 NPs were studied by cyclic voltammetry (CV). The cyclic voltammogram of the (R)-1-Fe3O4 NPs in CH2Cl2–0.1 M Bu4NPF6 at a glassy carbon electrode showed one irreversible oxidation peak at Ep = +0.52 V (vs.Ag/0.1 M AgNO3) due to the oxidation of the terthienyl group (Fig. 4a). In the following scans, an increase in the anodic/cathodic peaks of the (R)-1-Fe3O4 NPs is noted as an indication of the polymer growth. Many scans of the poly[(R)-1-Fe3O4-thiophene] film-modified electrode can be repeated without any change in the voltammetric wave. These results indicate that the chiral (R)- or (S)-1 acts as a protective ligand for magnetite NPs and provides the electropolymerized films. Subsequently, we succeeded in the synthesis of new hybrid nanotubes of magnetite NPs and polythiophene when a nanoporous alumina membrane as the template was used for the electropolymerization of the (R)-, (S)-1-Fe3O4 NPs (vide infra).
A typical procedure for the template synthesis of the chiral hybrid nanotubes, (R)-1-Fe3O4-PT-NTs, is as follows. The electropolymerization was performed by attaching the alumina membrane (Whatman Anodisc; pore diameter of about 200–250 nm, thickness of 60 μm) to a Pt electrode. Another Pt electrode was used as the counter electrode and Ag/0.1 M AgNO3 was used as the reference electrode. The electrolysis solution contained (R)-1-Fe3O4 NPs (15 mg: 4.4 nm) in 0.1 M Bu4NPF6–CH2Cl2 (1 mL). A voltage of 0.55 V (vs.Ag/0.1 M AgNO3) was applied for 20 min. After the electropolymerization, removal of the alumina membranes with 1 M NaOH led to the release of the hybrid nanotubes, (R)-1-Fe3O4-PT-NTs. The UV-vis spectrum of the (R)-1-Fe3O4-PT-NTs in CHCl3 exhibited a broad absorption peak at 440 nm which is ascribed to the polythiophene (Fig. 4b). The TEM image of the (R)-1-Fe3O4-PT-NTs revealed an outer diameter of about 230 nm which corresponds to the pore diameter of the alumina membrane and a wall thickness of around 40 nm (Fig. 5a). The elemental composition of the (R)-1-Fe3O4-PT-NTs was confirmed by the EDX analysis of single nanotubes (Fig. 5a); its EDX spectrum (Fig. 5b) shows the presence of Fe, O, S, and C elements in the hybrid NTs, which confirms that the Fe3O4 NPs have been successfully incorporated in the hybrid NTs during the template-based electropolymerization. The CD spectra of the (R)-1-Fe3O4-PT-NTs/(S)-1-Fe3O4-PT-NTs show intense Cotton effects and a mirror-image relationship in the measured range (Fig. 6a). Fig. 6b shows that the (R)-1-Fe3O4-PT-NTs were attracted towards the external magnetic field located on the backside of the sample vial, demonstrating the high magnetic sensitivity of our hybrid nanotubes.
 | ||
| Fig. 5 (a) TEM image of (R)-1-Fe3O4-PT-NTs. (b) EDX spectrum of (R)-1-Fe3O4-PT-NTs. The Cu peak is from the supporting copper grid. | ||
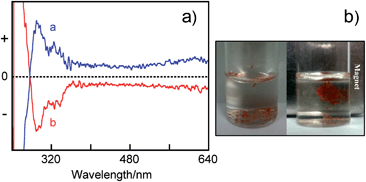 | ||
| Fig. 6 (a) CD spectra of (R)-1-Fe3O4-PT-NTs (a: blue) and (S)-1-Fe3O4-PT-NTs (b: red) in CHCl3. (b) Photographs of (R)-1-Fe3O4-PT-NTs in water (left) and their response to a magnet (right). | ||
In conclusion, we have successfully developed a new multifunctional chiral stabilizer 1 for the synthesis of the chiral 1-modified Fe3O4 NPs with a polymerizable group. The multifunctional chiral 1-modified Fe3O4 NPs with a polymerizable group produce the polymer nanocomposite films on an electrode and new chiral hybrid nanotubes of Fe3O4 NPs and polythiophene by a template-based electropolymerization. The composition of our hybrid NTs is two components of Fe3O4 NPs and polythiophene unlike decorating NTs with Fe3O4 NPs. To the best of our knowledge, this is the first example of chiral hybrid NTs consisting of conducting polythiophene and Fe3O4 NPs. Our developed synthetic method can be extended to the incorporation of other metal oxides in the chiral hybrid nanotubes. The combined functionalities will provide many exciting opportunities in developing applications, such as drug delivery, electro-magnetorheological polymer films, electromagnetic shielding, and magnetically recoverable chiral catalysts. We believe that the chirality of the magnetite NPs and their hybrid NTs is producing more interest because of its novel properties and potential applications. A study on the magnetic and chiral properties of the chiral hybrid NTs is now under way.
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas (no. 21108522 and 23108723, “pi-Space”) and a Scientific Research, no. 21350030, from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Notes and references
- (a) C. N. R. Rao and A. Govindaraj, Nanotubes and Nanowires, RSC Publishing, 2005 Search PubMed; (b) G. A. Ozin, A. C. Arsenault and L. Cademartiri, Nanochemistry, RSC Publishing, 2009 Search PubMed.
- S. Behrens, Nanoscale, 2011, 3, 877 RSC.
- N. Karousis and N. Tagmatarchis, Chem. Rev., 2010, 110, 5366 CrossRef CAS.
- (a) A. N. Aleshin, Adv. Mater., 2006, 18, 17 CrossRef CAS; (b) H. D. Tran, D. Li and R. B. Kaner, Adv. Mater., 2009, 21, 1487 CrossRef CAS , and references cited therein.
- Very few papers have described the preparation of magnetite NTs. For example, Z. Liu, D. Zhang, S. Han, C. Li, B. Lei, W. Lu, J. Fang and C. Zhou, J. Am. Chem. Soc., 2005, 127, 6 CrossRef CAS.
- (a) B. Jia and L. Gao, J. Phys. Chem. B, 2007, 111, 5337 CrossRef CAS; (b) S. W. Ko, M. S. Yang and H. J. Choi, Mater. Lett., 2009, 63, 861 CrossRef CAS.
- R. Umeda, H. Awaji, T. Nakahodo and H. Fujihara, J. Am. Chem. Soc., 2008, 130, 3240 CrossRef CAS.
- (a) X. Zhang and S. K. Manohar, J. Am. Chem. Soc., 2005, 127, 14156 CrossRef CAS; (b) S. Ko and J. Jang, Angew. Chem., Int. Ed., 2006, 45, 7564 CrossRef CAS.
- Although a number of coating and stabilizing magnetite NPs by combining magnetite NPs with polymers have been reported, the electrochemical synthesis of conducting polymer–magnetite NP composites has received much less attention.10Polymers are employed to passivate the surface of the magnetite NPs during or after the synthesis to avoid aggregation of the magnetite NPs (ref. 2).
- C. Janaky, C. Visy, O. Berkesi and E. Tombacz, J. Phys. Chem. C, 2009, 113, 1352 CAS , and references cited therein.
- The adsorption of a carboxyl group as a stabilizer on magnetite NPs is well known: S. Sun and H. Zeng, J. Am. Chem. Soc., 2002, 124, 8204 CrossRef CAS.
- See ESI†.
- A. Hu, G. T. Yee and W. Lin, J. Am. Chem. Soc., 2005, 127, 12486 CrossRef CAS.
- (a) M. Tamura and H. Fujihara, J. Am. Chem. Soc., 2003, 125, 15742 CrossRef CAS; (b) Y. Yanagimoto, Y. Negishi, H. Fujihara and T. Tsukuda, J. Phys. Chem. B, 2006, 110, 11611 CrossRef CAS; (c) K. Sawai, R. Tatumi, T. Nakahodo and H. Fujihara, Angew. Chem., Int. Ed., 2008, 47, 6917 CrossRef CAS; (d) C. Gautier and T. Burgi, ChemPhysChem, 2009, 10, 483 CrossRef CAS.
- D. B. Amabilino, Chirality at the Nanoscale: Nanoparticles, Surfaces, Materials and more, Wiley-VCH, Weinheim, 2009 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis of a chiral stabilizer 1 and the spectral data. See DOI: 10.1039/c1nr11312g |
| This journal is © The Royal Society of Chemistry 2012 |
