Mechanism of nanoparticle actuation by responsive polymer brushes: from reconfigurable composite surfaces to plasmonic effects†
Yuri
Roiter
a,
Iryna
Minko
a,
Dmytro
Nykypanchuk
b,
Ihor
Tokarev
*a and
Sergiy
Minko
*a
aDepartment of Chemistry and Biomolecular Science, Clarkson University, Potsdam, New York 13699-5810, USA. E-mail: itokarev@clarkson.edu; sminko@clarkson.edu
bCenter for Functional Nanomaterials, Brookhaven National Laboratory, Upton, New York 11973-5000, USA
First published on 14th November 2011
Abstract
The mechanism of nanoparticle actuation by stimuli-responsive polymer brushes triggered by changes in the solution pH was discovered and investigated in detail in this study. The finding explains the high spectral sensitivity of the composite ultrathin film composed of a poly(2-vinylpyridine) (P2VP) brush that tunes the spacing between two kinds of nanoparticles—gold nanoislands immobilized on a transparent support and gold colloidal particles adsorbed on the brush. The optical response of the film relies on the phenomenon of localized surface plasmon resonances in the noble metal nanoparticles, giving rise to an extinction band in visible spectra, and a plasmon coupling between the particles and the islands that has a strong effect on the band position and intensity. Since the coupling is controlled by the interparticle spacing, the pH-triggered swelling–shrinking transition in the P2VP brush leads to pronounced changes in the transmission spectra of the hybrid film. It was not established in the previous publications how the actuation of gold nanoparticles within a 10–15 nm interparticle distance could result in the 50–60 nm shift in the absorbance maximum in contrast to the model experiments and theoretical estimations of several nanometer shifts. In this work, the extinction band was deconvoluted into four spectrally separated and overlapping contributions that were attributed to different modes of interactions between the particles and the islands. These modes came into existence due to variations in the thickness of the grafted polymeric layer on the profiled surface of the islands. In situatomic force microscopy measurements allowed us to explore the behavior of the Au particles as the P2VP brush switched between the swollen and collapsed states. In particular, we identified an interesting, previously unanticipated regime when a particle position in a polymer brush was switched between two distinct states: the particle exposed to the surface of the collapsed layer and the particle engulfed by the swollen brush. On average, the characteristic distance between the particles and the islands increased upon the brush swelling. The observed behavior was a result of the anchoring of the particles to polymeric chains that limited the particles' vertical motion range. The experimental findings will be used to design highly sensitive optical nanosensors based on a polymer-brush-modulated interparticle plasmon coupling.
Introduction
The mechanism of nanoparticle actuation by stimuli-responsive polymer brushes is discovered and investigated in detail in this study. Reconfigurable ultrathin composite films constituted of polymer brushes and nanoparticles have recently attracted great interest due to the unique properties of controlled tuning and switching of optical properties, transport of nanoparticles, and interactions with biological molecules and cells.1–6In this work, we explain the previously unknown mechanism of the pronounced optical response of a dense monolayer of noble metal nanoclusters (known as nanoislands) on a transparent substrate and colloid nanoparticles of the same metal adsorbed onto a stimuli-responsive polymer brush placed between the particles and the nanoislands (Scheme 1). In this design, the particles and the islands reside in close proximity, enabling their electromagnetic interactions. The responsive brush modulates the characteristic distance between the particles anchored to the polymer chains and the immobile nanoislands on the solid substrate, leading to changes in the interparticle interactions. This architecture was used to engineer a transduction device for the nanosensor platform,7,8 however, it was not understood from the previous publications how the actuation of gold particles at a rather large distance from nanoislands could result in such a substantial (ca. 60 nm versus predicted several nanometer) shift in the absorbance maximum of the plasmonic spectra.
 | ||
| Scheme 1 The ultrathin composite layer is assembled on the glass substrate (1) from the following ingredients: Au nanoislands (2), PGMA anchoring layer (3) coating and stabilizing the islands, carboxyl-terminated P2VP chains (4) tethered to the PGMA layer to form a brush, and Au nanoparticles adsorbed on the brush (5). | ||
The optical response of the noble metal nanoparticles originates from localized surface plasmon resonance (LSPR)-coupled resonant oscillations of electron density and evanescent electromagnetic field (collectively known as plasmons) excited near the particle's surface by the incident light of specific wavelengths.9 The phenomenon leads to characteristic extinction bands (called LSPR bands) in the visible and ultraviolet parts of the transmission spectrum of the particles. The particles interact with their local dielectric environment and with other particles through the evanescent field. The interparticle interactions via overlapping evanescent fields are known as plasmon coupling.10 Plasmon coupling comes into effect when particles are brought into close vicinity to each other and causes profound changes in the transmission spectra. This specific property of noble metal nanoparticles has been broadly exploited for analytical applications.11–17 Compared to quantum dots and dyes, the important advantages of noble metal nanoparticles as sensing probes are high stability and biocompatibility as well as more pronounced absorption compared to organic dyes.
Coupling noble metal nanoparticles with a responsive polymeric material can be used to probe polymer volume phase transitions triggered by environmental stimuli and control the particles' aggregation state. This approach has been explored to create diverse plasmonic devices sensitive to pH and ionic strength,7,18–22solvent quality,23–25 temperature,26–30 and other external triggers.8,31 The devices that relied solely on changes in the dielectric permittivity of the environment typically suffered from low sensitivity that originated from fairly small variations in the refractive index of many polymeric materials undergoing volume phase transitions. However, the use of responsive polymers to change the interparticle spacing has been proven to dramatically enhance the sensitivity of such hybrid plasmonic devices. In this case, polymer chains act as tunable linkers between the nanoparticles. The optical response in this configuration depends on the relative range of interparticle motion during the conformational transition in the polymer chains. The optical response also depends on how close (on average) the particles can be pulled together by the collapsed chains. The maximum spectral changes can be attained when the motion range spans between the zero interparticle spacing (i.e., the particles are in direct contact), at which plasmon coupling is the strongest, and the interparticle distance exceeding ∼2.5 times the particle size, at which the particles behave as essentially isolated.10
A polymer brush provides an attractive platform for building plasmonic sensing devices owing to significant conformational changes in the polymer chains and the possibility of precisely positioning nanoparticles in the brush layer.32,33 By definition, the polymer brush refers to a dense layer of surface-tethered polymeric chains that stretch away from the grafting surface in order to reduce their excluded-volume interactions. The behavior of nanosized particles brought into contact with polymer brushes has been a subject of intense experimental4–6,34–39 and theoretical research40–42 over the past two decades. Many parameters define this behavior, including the particle size, r, the physical characteristics of the brush, such as the length of the grafted polymeric chains, N, and their grafting density, σ, the polymer–polymer, polymer–solvent and polymer–particle interactions, among others. The inclusion of a particle in the brush incurs the entropic energy penalty associated with the osmotic pressure in the brush and local perturbation of the chain conformation due to the excluded volume effect. The deformation of the chains produces a steric repulsion force that opposes van der Waals interactions and, in the absence of other attractive interactions, forms the basis for engineering surfaces with antifouling properties. It turns out, however, that particles that are smaller than the threshold value, r* ≈ σ−2/3, are capable of infiltrating the brush if they are miscible with the polymer.40 In this size range, the entropy of mixing becomes the dominant contribution, and the particles distribute uniformly within the brush. In the case of larger particles, it has been theoretically demonstrated that they are capable of penetrating the brush only at the limited depth determined by balancing the free energy penalty due to excluded volume effects with free energy gains due to mixing entropy contributions. On the other side, the particles become completely expelled from the brush above a specific critical size, rmax ≈ (N/σ)1/4.40 The tendency to expel relatively large particles to the brush surface is referred to as the “buoyancy” effect.
In this work, we adopted the previously developed system7 (Scheme 1) in which brushes were made of poly(2-vinyl pyridine) (P2VP)—a weak cationic polyelectrolyte with reported pKa values between 3.2 and 5.2.43,44 Carboxyl-terminated P2VP chains were tethered to the surface of a glass slide with gold nanoislands via a polyglycidylmethacrylate (PGMA) anchoring layer, and the resulting P2VP brush served as a substrate for the adsorption of gold colloidal nanoparticles whose size was comparable to the thickness of the collapsed brush. The adsorbed particles became permanently anchored to the polymeric layer due to the strong affinity of P2VP to gold surfaces. The layer thickness was chosen in such a way that strong plasmon coupling existed between the Au nanoparticles and nanoislands. The grafted layer exhibited a profound swelling–shrinking transition in response to the solution pH. At pH values below the pKa, the P2VP layer was protonated and highly swollen due to the osmotic pressure of the entrapped counterions. As the solution pH was increased above the pKa, the layer underwent hydrophobic collapse from the swollen to nearly solvent-free state. This transition was concluded to lead to a considerable change in the characteristic spacing between the brush-actuated Au nanoparticles and the immobile Au nanoislands as evidenced from the drastic shift of the LSPR extinction band by 50 nm between pH 2 and pH 5.
However, the mechanism of how the swelling–shrinking transition affects particles' location in the grafted layer has not been established. Here, we revealed the involved mechanisms by analyzing the positions of the individual Au nanoparticles in the swollen and collapsed P2VP layer with in situatomic force microscopy (AFM). The AFM data were complemented by UV-vis spectroscopy and X-ray reflectivity measurements to create a detailed picture of how nanoparticles are actuated by the responsive polymer brush and how the energy of the chemical reaction is translated into an optical signal.
Experimental
Assembly of the sensing platform
Gold nanoislands were prepared on BK7 glass slides by evaporating gold shots at 10−7 mbar in an Edwards AUTO 306 high vacuum evaporator. Layers with a nominal thickness of 4.0 nm as measured in situ with a quartz crystal microbalance were obtained. The consequent annealing of the layers in a vacuum oven (∼1 mbar) at 140 °C overnight resulted in the formation of a monolayer of gold nanoislands with an average height of 22.4 ± 3.2 nm and a dominant center-to-center inter-island distance of ∼47 nm (both parameters were determined using AFM).A 2.4 ± 0.3 nm thick layer of PGMA (Sigma-Aldrich) was deposited onto the annealed gold nanoislands to serve as an anchoring layer for the polymer brush. The spin-coating deposition was carried out from a 0.06% solution in dehydrated methyl ethyl ketone (MEK) in a chamber with controlled humidity (less than 10% relative humidity) to prevent dewetting of PGMA from the surface. PGMA was crosslinked by heating at 120 °C for 20 minutes in a vacuum oven (at 1 mbar) and washed three times in dry MEK to remove the ungrafted polymer. This led to the formation of a stable and strongly adhered layer. The crosslinked PGMA layer was found to significantly improve the stability of gold nanoislands on glass substrates. Although some fraction of the reactive epoxy groups in the PGMA layer was involved in the crosslinking, the remaining groups were available in sufficient quantity for the grafting of a polymeric brush.45
A thin film of carboxyl-terminated poly(2-vinylpyridine), P2VP (Mn = 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 g mol−1, Mw = 41
200 g mol−1, Mw = 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1, Polymer Source, Inc., Canada), was spin-coated on the PGMA-modified substrate from a 0.5% solution in chloroform and subsequently annealed at 140 °C for 8 h in a vacuum oven (at 1 mbar) to enable the reaction between the epoxy groups of the PGMA anchoring layer and the terminal carboxyl groups of P2VP. The ungrafted P2VP was removed by multiple washing in chloroform, ethanol, and water (pH 2.0), yielding a layer of end-tethered P2VP chains (a polymer brush) with a thickness in the dry state of 7.4 ± 0.6 nm (as measured with ellipsometry). This brush thickness corresponds to the grafting density value of ∼0.1 nm−2.
500 g mol−1, Polymer Source, Inc., Canada), was spin-coated on the PGMA-modified substrate from a 0.5% solution in chloroform and subsequently annealed at 140 °C for 8 h in a vacuum oven (at 1 mbar) to enable the reaction between the epoxy groups of the PGMA anchoring layer and the terminal carboxyl groups of P2VP. The ungrafted P2VP was removed by multiple washing in chloroform, ethanol, and water (pH 2.0), yielding a layer of end-tethered P2VP chains (a polymer brush) with a thickness in the dry state of 7.4 ± 0.6 nm (as measured with ellipsometry). This brush thickness corresponds to the grafting density value of ∼0.1 nm−2.
In the final step, the citrate-capped Au nanoparticles were adsorbed onto the grafted P2VP layer from aqueous dispersion (pH 5.5). The synthesis of the gold nanoparticles is described elsewhere.46 The Au nanoparticles had a diameter of 10.7 ± 1.4 nm as measured with dynamic light scattering (90Plus particle size analyzer, Brookhaven Instruments, NY, USA) and exhibited the LSPR extinction peak at 518 nm.
Characterization
The UV-vis transmission spectra of the samples were recorded on a single-beam, microprocessor-controlled diode array spectrophotometer with collimating optics (Hewlett-Packard 8452A). The processing of the LSPR spectral data included the removal of the baseline followed by deconvolution of the LSPR band into several components using the Gaussian function. The fitting was carried out with the minimum number of Gaussian components needed to achieve the coefficient of determination R2 greater than 0.999.A Multiskop null-ellipsometer (Optrel, Germany) equipped with a He–Ne laser (λ = 633 nm) and set at an angle of incidence of 70° was used for ellipsometric characterization of P2VP brushes in the dry and wet states. The P2VP brushes prepared on PGMA-modified silicon wafers were used for the measurements. Since gold nanoislands and nanoparticles are strong light scatterers, they were eliminated in this case to avert significant measurement errors associated with incoherent scattering. The measurements were performed for each sample after each step of the modification in order to use the measurements of the previous steps as a reference for the ellipsometric data simulation. The following refractive indices were used in calculating the thicknesses of polymer layers in the dry state: 1.525 for PGMA45 and 1.59 for P2VP. The in situ characterization of the brush swelling was carried out using a set-up in which light guide extension tubes were attached to the laser and detector arms of the ellipsometer and dipped into a sample liquid cell. In this case, both the refractive index and thickness were calculated from experimental ellipsometric data.
AFM imaging of the layer topography was performed using a MultiMode scanning probe microscope (Bruker Nano, NY, USA) operating in the tapping mode in liquid. NPS silicon nitride probes (Bruker Nano, CA, USA) with a spring constant of 0.32 N m−1, a resonance frequency in aqueous media of ∼9 kHz, and the radius of the tip curvature of 20 nm (nominal) were used for scanning. A sample was fixed on the holder of the MultiMode and carefully covered by the fluid cell (MTFML, Bruker Nano, CA, USA) to minimize the mechanical drift effects caused by the silicone rubber O-ring. Afterwards, the fluid cell was filled with 50 μl of Millipore water adjusted to the targeted pH value. The microscope was then equilibrated for 0.5–2.0 h to minimize the thermal drift of the scanner. Scanning was performed with different tapping forces ranging between 70% and 98% from the initial set point (it defines the minimal force to be exerted upon a sample by an AFM tip in order to establish the contact; the experimental values varied from 1.75 to 1.90 V), a scanning rate of 0.4 Hz, and a temperature of 28.0 ± 0.5 °C (the temperature in the fluid cell of the MultiMode microscope was measured with a calibrated thermistor placed in the fluid cell). The pH in the fluid cell was changed between 2.5 and 5.5 according to the following procedure. The set point was raised to 5 V to avoid damaging the AFM tip. Then 20 μl of water (from the total amount of 50 μl) was carefully removed from the fluid cells and replaced with the same amount of water at a different pH value. This step was repeated multiple times: at least five times when the pH was changed from 5.5.to 2.5 and twenty times when the pH moved from 2.5 to 5.5 (the calculated pH value corresponds to pH 5.485). Each addition was followed by a ∼10 s pause allowing for equilibration of the pH throughout the cell. After this procedure, the set point was returned to the original value. A typical lateral sample displacement was 100–1000 nm and was compensated by the lateral offset command.
The average thicknesses of the layer after each fabrication step and the height of individual features were determined with the AFM “scratch test.” In this procedure, a sharp steel needle was used to scratch the layer down to the surface of the glass (or silicon) substrate, producing a step with a height equal to the layer thickness (note that the needle does not damage the harder substrate surface).
AFM images were processed using the WSxM software.47 Superposition of the images was performed using software developed in-house that enables relative lateral skewing, scaling, positioning of AFM data matrices, as well as linear vertical offset (for a detailed description, see ref. 48).
X-Ray reflectivity was measured in air on an Ultima III multipurpose diffractometer (Rigaku Corp.) in parallel beam geometry with a 100 μm vertical beam size and Cu-tube as the X-ray source. For the X-ray reflectivity measurements, P2VP brushes with adsorbed Au particles were prepared on PGMA-modified silicon wafers. The reflectivity data, corrected for beam spillover at small angles, were analyzed with MOTOFIT software that accounts for material absorbance.49X-Ray reflectivity measurements provide statistically averaged information over a large surface area (typically in the millimetre–centimetre range).
Results and discussion
AFM characterization at different stages of the sample preparation was conducted to determine the morphology and the averaged thicknesses (from the scratch test) of individual layers. Since an AFM tip can probe only the limited space between Au nanoislands (the penetration depth does not exceed 6 nm for AFM probes of the given sharpness), we carried out measurements in the positions corresponding to the island apices instead. The layer thickness after the grafting of P2VP increased on average by ∼7 nm, which is close to the value 7.4 ± 0.6 nm obtained with ellipsometry for a reference sample of a P2VP brush on a PGMA-modified Si wafer.Citric acid stabilized ∼10.7 nm in diameter Au nanoparticles with a narrow size distribution were adsorbed on the P2VP brush from aqueous dispersion at pH 5.5. At this pH value, the grafted P2VP chains adopt a compact conformation, and the layer is expected to be impermeable for the particles. The AFM topography image in Fig. 1A shows that the particles form a random monolayer of intermediate density (∼5 × 1014 m−2). The monolayer consists of isolated particles, dimers, trimers, and in rare cases larger associates. The adsorbed particles reside on the P2VP surface as follows from the comparison of the average vertical position of the particle apices (41.0 nm) and the average thickness of the grafted P2VP layer on the islands (30.4 nm); the height increment by 10.6 nm corresponds to the mean particle size. The conclusion is also corroborated with X-ray reflectivity measurements of a reference sample with no islands (flat geometry). Fig. 2 shows the X-ray reflectivity plot acquired for the dry sample, the electron density profile obtained by fitting the experimental data,49 and the vertical arrangement of the substrate, polymeric layer, and particles identified based on the electron density profile. The positioning of the Au nanoparticles on the surface with no or little penetration into the brush can be concluded from these data.
 | ||
| Fig. 1 The AFM topography images (A–E) of the hybrid layer (near the edge of the needle scratch) acquired in succession under the following conditions: at pH 5.5, SP 98% (A), pH 2.5, SP 88% (B), pH 2.5, SP 85% (C), pH 2.5, SP 70% (D), and pH 5.5, SP 98% (E). The cross-sectional profiles (F) obtained from the images in the locations denoted by the line; the profiles are shifted with respect to each other for clarity; the vertical dot lines show the positions of selected particles. The histogram of the particle rise vs. the baseline obtained in the scratched area (G) was produced based on 550 measurements. | ||
 | ||
| Fig. 2 Measured (circles) and simulated (solid line) X-ray reflectivity, R, vs. scattering vector, q, for a P2VP layer (grafted to a surface-functionalized Si substrate with no gold islands) with adsorbed Au colloidal particles in the dry state. Inset: electron density profile (solid line) corresponding to the simulated X-ray reflectivity curve; the dashed line represents the electron density profile at the solid–polymer interface as acquired by fitting the X-ray reflectivity curve of the substrate (not shown); the gray rectangle, purple rectangle, and red semicircle denote the estimated arrangement of the substrate, polymeric layer, and gold nanoparticles, respectively, as defined in the direction normal to the substrate surface (Z-axis). | ||
Unlike the reference sample in which the adsorbed particles are concentrated on the same plane, the particles adsorbed on the P2VP-coated islands exhibit a relatively broad vertical distribution with a standard deviation from the average position of 5.6 nm. To explain such a distribution, we examined the surface topography of the grafted P2VP layer and identified two levels of height heterogeneity. The smaller-scale features had a periodicity of ∼50 nm and are attributed to the P2VP grafted layer conforming to the islands' surface (see Scheme 2). The larger-scale irregularities (visible as elevated features on the AFM image of the layer in Fig. 1A and outlined with a dashed line in Scheme 2A) have lateral dimensions of ∼100 to 200 nm and a peak-to-valley height of ca. 8 nm. These features arise from variations in the brush thickness, which, in turn, is a result of uneven distribution of the PGMA layer on the profiled surface of the island film. It was established earlier that the thickness of the anchoring PGMA layer controls the thickness of a tethered polymer layer.50 The irregularities of both types were found to be important in attaining the high sensitivity of the plasmonic sensing device as discussed in detail below.
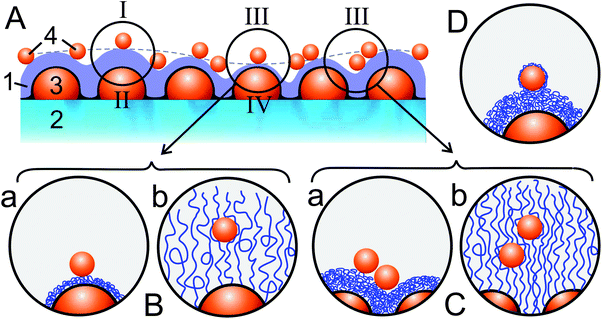 | ||
| Scheme 2 (A) Schematic showing a hybrid layer composed of P2VP (1) grafted to a glass substrate (2) with a PGMA-coated Au island layer (3) and gold colloid particles (4) adsorbed on the P2VP surface. The hybrid layer exhibits two levels of heterogeneity in the surface topography with the smaller-scale features corresponding to the P2VP grafted layer conforming to the islands' surface and the larger-scale features (outlined by a dashed line) corresponding to variations in the thickness of the grafted P2VP layer. These heterogeneities are responsible for the different contributions of the Au nanoparticles and nanoislands to the LSPR spectra (see Fig. 3 and explanations in the text): single particles (I) and islands (II) separated by a thick P2VP layer and hence experiencing weak plasmon coupling; III—particles experiencing strong plasmon coupling due to the proximity of islands (in the regions corresponding to a thin P2VP layer, panel B) and other particles (in dimers and larger particle associates, panel C); IV—islands experiencing strong plasmon coupling due to the proximity of particles (in the regions corresponding to a thin P2VP grafted layer, panel B). The particles adsorbed on the P2VP layer at pH 5.5 reside on its surface (case a in panels B and C). The grafted P2VP layer undergoes the transition into the swollen state in water at pH 2.5 when the particles are pushed away from the grafting surface and at the same time enveloped by highly stretched polymeric chains (case b in panels B and C). (D) The P2VP layer re-immersed in water at pH 5.5 undergoes hydrophobic collapse into the nearly solvent-free state in which the particles become enveloped into a thin layer of adsorbed polymeric chains. | ||
We conducted in situAFM experiments to study the swelling properties of the grafted layer and the behavior of the adsorbed nanoparticles at pH 5.5 and pH 2.5, i.e., above and below the pKa of P2VP.43,44 All the AFM measurements were carried out in the same location near the edge of a scratch. The average thickness of the hybrid layer was found to be 30.4 nm at pH 5.5 and 63.4 nm at pH 2.5. The former value corresponds to the thickness of the dry layer, indicating that it is essentially solvent-free at this pH value. The increased thickness at pH 2.5 can be attributed to the osmotic swelling of the P2VP layer due to the protonized pyridine groups of P2VP and counterions. The increased thickness corresponds to 6.6-fold swelling of the grafted P2VP layer (the degree of swelling is defined as a ratio of the P2VP layer thickness in the swollen state to that in the collapsed state). The in situ ellipsometric measurements of a reference sample of a P2VP brush (for this experiment, the brush was prepared on a Si wafer) yielded 4.7-fold swelling. The discrepancy between the AFM and ellipsometric values is a consequence of the parabolic profile of the chain density in the highly swollen brush (for additional discussion, see ESI†).32 The switching of the hybrid layer between the collapsed and swollen states was fully reversible as confirmed by cycling of the solution pH four times between pH 5.5 and 2.5.
The AFM measured thickness of the swollen P2VP layer at pH 2.5 depended strongly on the force an AFM tip exerted upon the sample. As the set point (SP) value was progressively lowered (implying an increase in the probing force) from 98% (used in the AFM experiments above) to 70%, the thickness exhibited a monotonous decrease. Two different factors might be responsible for such a decrease: penetration of the AFM tip into the grafted layer and layer compression under the tip. The former factor is probably dominant at small penetration depths where the density of polymeric chains is low and they can escape the penetrating sharp tip. When plotted as a function of the film thickness vs. the SP (Fig. S1 in the ESI†), the experimental data are well fitted with a sigmoid function; its upper plateau value of 64.3 nm can be attributed to the thickness of the swollen layer under the condition of no penetration of an AFM tip into the grafted layer. This value is very close to the thickness measured at SP 98% (63.4 nm), indicating that the layer experienced minor perturbation at this SP value (the regime called hereafter “light tapping”). The lower plateau value of 42.2 nm corresponds to the maximum compression of the grafted layer under the given experimental conditions, such as the tip radius and cantilever's spring constant. The results (Fig. S2 in the ESI†) suggest that the perturbation of the grafted layer by the AFM tip is reversible, i.e., the layer completely recovered as we switched from the hard to light tapping regime.
We observed interesting behavior of the adsorbed particles as the grafted layer underwent the transition between the collapsed and swollen states. The particles remained on the P2VP-layer surface at pH 5.5 (Fig. 1A). The vertical distribution of the particles was the same as in the dry layer. In contrast, no particles could be seen on the surface of the swollen layer at pH 2.5 (see Fig. S2B–D in the ESI†), pointing out that the particles penetrated into the swollen layer. As the sample was dried or transferred into water at pH 5.5, the particles were expelled to the layer surface (Fig. 1E). The AFM measurements show that, after being treated with acidic water, the particle apices were slightly further away from the layer surface than after initial deposition. Further cycling between pH 2.5 and 5.5 did not lead to any noticeable changes in the average particle position. The increment after the first pH 2.5–pH 5.5 cycle can be rationalized by the formation of an adsorption layer of P2VP around the nanoparticles (Scheme 2D).
Although no particles were identified on the AFM image of the swollen layer acquired at SP 98% (i.e., at light tapping, see Fig. S2B–D in the ESI†), they became visible at lower SP values. The first particles emerged at SP 88% (Fig. 1B). As the AFM tip probed harder into the swollen layer, an increasing number of particles came into sight (Fig. 1C and D). At the maximum applied force (SP 70%), nearly all the particles were visible due to penetration of the AFM tip into the brush (Fig. 1D). To analyze the vertical distribution of the Au nanoparticles in the layer, we aligned the images in Fig. 1B–E with respect to the image in Fig. 1A in such a way that the lateral positions of the particles in all the images coincide with one-pixel accuracy (see the Experimental section for details). The resulting AFM images allowed rapid and precise processing of the positions of individual particles in the layer and study of how these particles respond to changes in the probing force. We found that the Au particles at pH 2.5 resided at raised positions compared to those at pH 5.5. The particle rise analysis for the selected 550 particles is presented in Fig. 1G in the form of a histogram. The average particle rise between the studied pH values is 9.4 ± 4.7 nm. Therefore, the AFM measurements supported our hypothesis7 that the grafted layer undergoing the swelling–shrinking transition produced significant changes in the characteristic spacing between adsorbed Au nanoparticles and immobile Au nanoislands.
An important finding was that the strength of an applied force had a minor effect on the particles' positions in the swollen layer. This finding implies that the Au nanoparticles are anchored by several P2VP chains as shown schematically in Scheme 2B that limit the particles' vertical movement range to the extent the free segments connecting the particles to the grafting surface can stretch upon swelling. As soon as the tip reaches the particle surface, further propagation into the brush layer requires pushing of the particle toward the grafting surface. Inserting the particle into the grafted layer involves local compression and perturbation of the polymeric chains that leads to the mounting entropic energy penalty.42 It turns out that the force exerted by the tip is insufficient to cause significant dislocation of the anchored particles.
In the next set of experiments, we measured and analyzed the UV-vis spectra of the hybrid layer under water. For the sample in Fig. 3, the position of the LSPR extinction band shifts by 60 nm between pH 2.5 and pH 5.5. Careful analysis of the spectroscopic data provides further insight into the mechanism of the optical response of the plasmonic sensing device. The extinction band of the hybrid layer can be deconvoluted into four Gaussian peaks (shown with the dashed lines in Fig. 3; see also Table S1 in the ESI†). We attribute these peaks to individual contributions from single nanoparticles (peak I), nanoislands (peak II), particles experiencing strong plasmon coupling with islands and other particles (peak III), and nanoislands experiencing strong plasmon coupling with particles and particle associates (peak IV); we also refer the reader to Scheme 2A–C for a graphic interpretation. Peaks I and II arise from the layer regions where the particles and islands are separated by a thick P2VP layer and hence experience weak plasmon coupling (Scheme 2A). Their peak positions are close to those of individual components (Table S1 in the ESI†). In contrast, peaks III and IV arise from the regions where the particles and islands are separated by a thin P2VP layer (Scheme 2A and B). Peaks III and IV are dramatically red-shifted and broadened compared to peaks I and II, respectively, indicating that strong plasmonic coupling exists between the particles and the islands. An additional important contribution to peak III comes from the inter-particle plasmon coupling. The particle associates (mainly dimers) are the source of this contribution (Scheme 2C). A similar peak is observed in a reference sample with no islands (Table S1 in the ESI†). Strongly red-shifted LSPR peaks have been also reported for associates of gold nanoparticles of a comparable size in the literature.51–53
 | ||
| Fig. 3 LSPR bands (circles) obtained from the transmission absorption spectra of the plasmonic sensing device in water at pH 5.5 (A) and 2.5 (B) after the removal of the baseline. The best fits (solid lines) to the experimental plots consist of four Gaussian components (dashed lines). The individual components are numbered for reference in the text. | ||
The magnitude of the shifts and intensities of the peaks depend on the solution pH. The observed behavior is a result of the swelling–shrinking transition in the P2VP layer that changes the characteristic spacing between the particles and the islands (Scheme 2B and C) and the dielectric permittivity of their local environment. All the peaks are blue-shifted when switching from pH 5.5 to pH 2.5, implying that the coupling strength is decreased when the grafted layer undergoes swelling. In particular, peaks I and II, produced by the weakly interacting particles and islands, exhibit moderate shifts by 6 and 13 nm, respectively. In contrast, peaks III and IV, attributed to the strongly coupled particles and islands, yield substantially stronger shifts by 21 and 45 nm, respectively. These spectral shifts are accompanied by almost twofold changes in the peak intensities. All the constituent peaks exhibit smaller shifts between the pH values compared to the shift of the LSPR extinction band (by 60 nm). It turns out that the enhanced spectral sensitivity of this plasmonic platform requires the coexistence of the surface regions in which the plasmon coupling between the particles and the islands is weak (peaks I and II exhibit small spectral changes with the pH and act as reference peaks) and strong (peaks III and IV are effectively offset compared to peaks I and II and undergo considerable changes with the pH). In other words, the pronounced spectral shift of the LSPR band originates from a synergetic effect of changes in the positions and intensities of the spectrally separated constituent peaks.
The in situAFM measurements indicate that the vertical positions of the nanoparticles in associates vary independently with the pH. This point is exemplified in Fig. 4, which shows the AFM images and cross-sectional profiles of selected dimers and trimers in two different sample locations. In particular, the particle denoted as 1 in Fig. 4A–C occupies a lower position in the collapsed layer than the adjacent particle 2. The situation is inversed at pH 2.5: particle 1, pushed toward the brush surface by swollen polymeric chains, occupies a higher position than particle 2. We attribute this behavior to the embossed surface relief of the polymeric layer (arising from the underlying island layer) and associated variations in the grafting density of P2VP chains. The grafting density is possibly higher in the regions between the islands than on top of the islands due to the concave geometry.
 | ||
| Fig. 4 AFM topography images (A, B, D, and E) of the hybrid layer showing two examples of a rise of nanoparticles in associates (dimers and trimers) at different pH values. The image pairs (A and B) and (D and E) were recorded in two different locations. The AFM images were acquired under the following conditions: at pH 5.5, SP 98% (A and D), and pH 2.5, SP 88% (B and E). The cross-sectional profiles obtained from the pair (A and B) are shown in panel C and those from the pair (D and E) in panel F. The numbers denote the same particles on the images and profiles (no number is assigned if a particle is hidden in the layer); the arrows on the profiles denote the particles that exhibited a noticeable rise in the swollen layer. | ||
The local variations in the grafting density combined with the random character of the anchoring of the particles to polymeric chains cause the particles to rise to different heights as the layer swells. The possible arrangement of particles 1 and 2 in the collapsed and swollen polymeric layers (Fig. 4A–C) is illustrated in Scheme 2C. Obviously, other modes of particles' movement are also possible. The important aspect here is that the strength of the inter-particle plasmon coupling in the particle associates depends on the layer swelling state. The coupling is strongest when the particles, expelled to the surface by the collapsed P2VP layer, are in close vicinity (Scheme 2Ca) and weakens as the particles become separated by stretched polymeric chains (Scheme 2Cb). Such changes are reflected in the shift of peak III (Fig. 3). In a reference sample with no islands (Table S1 in the ESI†), the peak attributed to the inter-particle interactions has weak dependence on the pH. The flat geometry of the sample in which all the particles are concentrated on the same plane does not lead to the efficient dissociation of particle associates upon the polymer brush swelling.
Conclusions
We studied in detail the behavior of Au colloidal particles adsorbed on the layer of end-grafted polymeric chains. The key distinction of this particle-brush system from those described in the literature is that the particles are strongly anchored to the chains' fragments exposed to the surface of the initially collapsed layer. Such linkage limits the vertical motion range of the particle to the extent the chain segments connecting the particles to the grafting surface can stretch upon swelling. The distance the particles can travel is in fact smaller than the length of the chains in the swollen layer. According to the theoretical prediction,40 a polymer brush of the given degree of polymerization and grafting density (0.1 nm−2 was used in the calculations) has to expel spherical particles whose size exceeds ∼2 nm to the brush surface; note that the nanoparticles in our study are fivefold larger than the threshold particle size. The Au nanoparticles indeed segregated to the surface of the collapsed layer as confirmed by the experiments above. However, as the polymeric layer swells, the aforementioned linkage forces the grafted chains to engulf the particles despite the large entropic energy penalty associated with this process. It turns out, however, that the particles in this study are small enough to allow such a transition; we observed no sign of particle desorption. The engulfed particles are pushed toward the near-surface region (due to the “buoyancy” effect40), where the layer is much softer, resulting in a rise in the average position of the particles. The actuation of nanoparticles anchored to a responsive polymeric layer leads to a previously unanticipated effect when the particle positions in the layer can be controlled by environmental signals.This study revealed morphological aspects of the mechanism of plasmonic response that can be used to develop new materials. In particular, specific functionalities, such as catalysts or recognition centers, attached to the particle carrier can be either exposed to the surface or concealed inside the layer surface on demand. This behavior can be exploited to create ‘smart’ interfaces for chemical and biochemical catalysis as well as for tissue engineering and cell research. Furthermore, the revealed mode of autonomous motion of the particles in associates on the profiled polymer-brush surfaces can be useful in designing plasmonic sensors that operate without an island layer. This will be the subject of our future study.
An important aspect of the studied sensing platform is that the modulation of plasmon coupling between noble metal nanoparticles and nanoislands with a stimuli-responsive polymer brush will lead to a high spectral response when the following requirements are met. First, the polymeric layer has to be thin enough to provide conditions for strong plasmon coupling in the collapsed state and, at the same time, thick enough to enable the actuation of the particles anchored to this layer and hence the efficient modulation of the coupling strength. In the studied system, strong plasmon coupling exists when the interparticle distance is below ∼10 nm. Since the system is characterized by the large-scale variations in the thickness of the grafted P2VP layer, these contradictory requirements are fulfilled in some sample regions where the layer approaches the optimal thickness. Second, the platform has to be designed to produce two (or more) spectrally separated contributions to LSPR spectra. A shift of the LSPR extinction band composed of several peaks whose mutual positions and intensities change in response to stimulus-triggered changes in the properties of the responsive layer may significantly exceed the shifts in the constituent peaks. In the studied system, the spectrally offset peaks were produced by two different sample regions where weakly interacting Au particles and islands were separated by a thick P2VP layer and where strongly interacting particles and islands were separated by a thin layer. The relative changes in the position and intensities of these peaks (caused by the swelling–shrinking transition in the P2VP layer) led to the pronounced shift of the LSPR band by 60 nm exceeding those of the individual contributions. The acquired knowledge will be used to design highly sensitive optical sensors based on polymer-brush-modulated inter-particle plasmon coupling.
Acknowledgements
The authors acknowledge the support of the U.S. Army via Grant W911NF-05-1-0339. Research was carried out in part at the Center for Functional Nanomaterials, Brookhaven National Laboratory, which is supported by the U.S. Department of Energy, Office of Basic Energy Sciences, under contract no. DE-AC02-98CH10886. The authors express special thanks to Dr Oleg Gang (Brookhaven National Laboratory) for helpful discussions.Notes and references
- L. Ionov, J. Mater. Chem., 2010, 20, 3382–3390 RSC.
- S. Minko, O. Hoy, B. Zdyrko, R. Lupitskyy, R. Sheparovych, D. Aulich, J. F. Wang, E. Bittrich, K. J. Eichhorn, P. Uhlmann, K. Hinrichs, M. Muller, M. Stamm and I. Luzinov, Adv. Funct. Mater., 2010, 20, 2240–2247 CrossRef.
- S. Santer, A. Kopyshev, J. Donges, H. K. Yang and J. Ruhe, Adv. Mater., 2006, 18, 2359–2362 CrossRef CAS.
- W. S. Choi, H. Y. Koo, J. Y. Kim and W. T. S. Huck, Adv. Mater., 2008, 20, 4504–4508 CrossRef CAS.
- L. Ionov, S. Sapra, A. Synytska, A. L. Rogach, M. Stamm and S. Diez, Adv. Mater., 2006, 18, 1453 CrossRef CAS.
- O. Tagit, N. Tomczak, E. M. Benetti, Y. Cesa, C. Blum, V. Subramaniam, J. L. Herek and G. J. Vancso, Nanotechnology, 2009, 20, 185501 CrossRef.
- I. Tokareva, S. Minko, J. H. Fendler and E. Hutter, J. Am. Chem. Soc., 2004, 126, 15950–15951 CrossRef CAS.
- I. Tokareva, I. Tokarev, S. Minko, E. Hutter and J. H. Fendler, Chem. Commun., 2006, 3343–3345 RSC.
- A. Moores and F. Goettmann, New J. Chem., 2006, 30, 1121–1132 RSC.
- S. K. Ghosh and T. Pal, Chem. Rev., 2007, 107, 4797–4862 CrossRef CAS.
- M. E. Stewart, C. R. Anderton, L. B. Thompson, J. Maria, S. K. Gray, J. A. Rogers and R. G. Nuzzo, Chem. Rev., 2008, 108, 494–521 CrossRef CAS.
- Y. Ofir, B. Samanta and V. M. Rotello, Chem. Soc. Rev., 2008, 37, 1814–1823 RSC.
- R. Wilson, Chem. Soc. Rev., 2008, 37, 2028–2045 RSC.
- A. J. Haes and R. P. Van Duyne, Anal. Bioanal. Chem., 2004, 379, 920–930 CrossRef CAS.
- K. A. Willets and R. P. Van Duyne, Annu. Rev. Phys. Chem., 2007, 58, 267–297 CrossRef CAS.
- X. H. Huang, S. Neretina and M. A. El-Sayed, Adv. Mater., 2009, 21, 4880–4910 CrossRef CAS.
- T. A. Bendikov, A. Rabinkov, T. Karakouz, A. Vaskevich and I. Rubinstein, Anal. Chem., 2008, 80, 7487–7498 CrossRef CAS.
- R. Lupitskyy, M. Motornov and S. Minko, Langmuir, 2008, 24, 8976–8980 CrossRef CAS.
- S. Gupta, P. Uhlmann, M. Agrawal, S. Chapuis, U. Oertel and M. Stamm, Macromolecules, 2008, 41, 2874–2879 CrossRef CAS.
- D. X. Li, Q. He, Y. Cui and J. B. Li, Chem. Mater., 2007, 19, 412–417 CrossRef CAS.
- V. Kozlovskaya, E. Kharlampieva, B. P. Khanal, P. Manna, E. R. Zubarev and V. V. Tsukruk, Chem. Mater., 2008, 20, 7474–7485 CrossRef CAS.
- O. Azzaroni, A. A. Brown, N. Cheng, A. Wei, A. M. Jonas and W. T. S. Huck, J. Mater. Chem., 2007, 17, 3433–3439 RSC.
- S. Lee and V. H. Perez-Luna, Langmuir, 2007, 23, 5097–5099 CrossRef CAS.
- T. Karakouz, A. Vaskevich and I. Rubinstein, J. Phys. Chem. B, 2008, 112, 14530–14538 CrossRef CAS.
- S. Gupta, M. Agrawal, P. Uhlmann, F. Simon, U. Oertel and M. Stamm, Macromolecules, 2008, 41, 8152–8158 CrossRef CAS.
- M. Mitsuishi, Y. Koishikawa, H. Tanaka, E. Sato, T. Makayama, J. Matsui and T. Miyashita, Langmuir, 2007, 23, 7472–7474 CrossRef CAS.
- S. Gupta, M. Agrawal, P. Uhlmann, F. Simon and M. Stamm, Chem. Mater., 2010, 22, 504–509 CrossRef CAS.
- S. Chakraborty, S. W. Bishnoi and V. H. Perez-Luna, J. Phys. Chem. C, 2010, 114, 5947–5955 CAS.
- X. Y. Liu, F. Cheng, Y. Liu, W. G. Li, Y. Chen, H. Pan and H. J. Liu, J. Mater. Chem., 2010, 20, 278–284 RSC.
- M. Karg and T. Hellweg, Curr. Opin. Colloid Interface Sci., 2009, 14, 438–450 CrossRef CAS.
- I. Tokarev, I. Tokareva, V. Gopishetty, E. Katz and S. Minko, Adv. Mater., 2010, 22, 1412 CrossRef CAS.
- S. Minko, Polym. Rev., 2006, 46, 397–420 CAS.
- I. Luzinov, S. Minko and V. V. Tsukruk, Soft Matter, 2008, 4, 714–725 RSC.
- M. Retsch, A. Walther, K. Loos and A. H. E. Muller, Langmuir, 2008, 24, 9421–9429 CrossRef CAS.
- R. Oren, Z. Q. Liang, J. S. Barnard, S. C. Warren, U. Wiesner and W. T. S. Huck, J. Am. Chem. Soc., 2009, 131, 1670 CrossRef CAS.
- Y. Lu, A. Wittemann and M. Ballauff, Macromol. Rapid Commun., 2009, 30, 806–815 CrossRef CAS.
- M. D. McConnell, A. W. Bassani, S. Yang and R. J. Composto, Langmuir, 2009, 25, 11014–11020 CrossRef CAS.
- S. Diamanti, S. Arifuzzaman, J. Genzer and R. A. Vaia, ACS Nano, 2009, 3, 807–818 CrossRef CAS.
- S. Gupta, M. Agrawal, M. Conrad, N. A. Hutter, P. Olk, F. Simon, L. M. Eng, M. Stamm and R. Jordan, Adv. Funct. Mater., 2010, 20, 1756–1761 CrossRef CAS.
- J. U. Kim and B. O'Shaughnessy, Macromolecules, 2006, 39, 413–425 CrossRef CAS.
- J. Yaneva, D. I. Dimitrov, A. Milchev and K. Binder, J. Colloid Interface Sci., 2009, 336, 51–58 CrossRef CAS.
- A. Halperin and E. B. Zhulina, Langmuir, 2010, 26, 8933–8940 CrossRef CAS.
- Y. Roiter and S. Minko, J. Am. Chem. Soc., 2005, 127, 15688–15689 CrossRef CAS.
- E. Kim, C. Kang, H. Baek, K. Hwang, D. Kwak, E. Lee, Y. Kang and E. L. Thomas, Adv. Funct. Mater., 2010, 20, 1728–1732 CrossRef CAS.
- K. S. Iyer, B. Zdyrko, H. Malz, J. Pionteck and I. Luzinov, Macromolecules, 2003, 36, 6519–6526 CrossRef CAS.
- E. Hutter, J. H. Fendler and D. Roy, J. Phys. Chem. B, 2001, 105, 11159–11168 CrossRef CAS.
- I. Horcas, R. Fernandez, J. M. Gomez-Rodriguez, J. Colchero, J. Gomez-Herrero and A. M. Baro, Rev. Sci. Instrum., 2007, 78, 013705 CrossRef CAS.
- Y. Roiter, M. Ornatska, A. R. Rammohan, J. Balakrishnan, D. R. Heine and S. Minko, Langmuir, 2009, 25, 6287–6299 CrossRef CAS.
- A. Nelson, J. Appl. Crystallogr., 2006, 39, 273–276 CrossRef CAS.
- B. Zdyrko, K. S. Iyer and I. Luzinov, Polymer, 2006, 47, 272–279 CrossRef CAS.
- P. Mulvaney, L. M. Liz-Marzan, M. Giersig and T. Ung, J. Mater. Chem., 2000, 10, 1259–1270 RSC.
- M. Lahav, A. N. Shipway and I. Willner, J. Chem. Soc., Perkin Trans. 1, 1999, 2, 1925–1931 Search PubMed.
- I. E. Sendroiu, S. F. L. Mertens and D. J. Schiffrin, Phys. Chem. Chem. Phys., 2006, 8, 1430–1436 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Ellipsometric, AFM, and spectroscopic data. See DOI: 10.1039/c1nr10932d |
| This journal is © The Royal Society of Chemistry 2012 |
