Study of lead accumulation in bones of Wistar rats by X-ray fluorescence analysis: aging effect
Diana
Guimarães
*a,
Maria Luísa
Carvalho
b,
Vera
Geraldes
c,
Isabel
Rocha
c and
José Paulo
Santos
a
aCentro de Física Atómica CFA(FC/UL), Departamento de Física, Faculdade de Ciências e Tecnologia, FCT, Universidade Nova de Lisboa, 2829-516 Monte da Caparica, Portugal. E-mail: dianafcg@gmail.com; Tel: +351-212948576
bCentro de Física Atómica CFA(FC/UL), Departamento de Física, Faculdade de Ciências, Universidade de Lisboa, Av. Prof. Gama Pinto 2, 1649-003, Lisboa, Portugal. Tel: +351-217904751
cInstituto de Fisiologia, Faculdade de Medicina de Lisboa and Instituto de Medicina Molecular, Lisboa, Av. Prof. Egas Moniz, 1649-028 Lisboa, Portugal. Tel: +351-217999411
First published on 31st October 2011
Abstract
The accumulation of lead in several bones of Wistar rats with time was determined and compared for the different types of bones. Two groups were studied: a control group (n = 20), not exposed to lead and a contaminated group (n = 30), exposed to lead from birth, first indirectly through mother's milk, and then directly through a diet containing lead acetate in drinking water (0.2%). Rats age ranged from 1 to 11 months, with approximately 1 month intervals and each of the collections had 3 contaminated rats and 2 control rats. Iliac, femur, tibia–fibula and skull have been analysed by Energy Dispersive X-ray Fluorescence Technique (EDXRF). Samples of formaldehyde used to preserve the bone tissues were also analysed by Electrothermal Atomic Absorption (ETAAS), showing that there was no significant loss of lead from the tissue to the preservative. The bones mean lead concentration of exposed rats range from 100 to 300 μg g−1 while control rats never exceeded 10 μg g−1. Mean bone lead concentrations were compared and the concentrations were higher in iliac, femur and tibia–fibula and after that skull. However, of all the concentrations in the different collections, only those in the skull were statistically significantly different (p < 0.05) from the other types of bones. Analysis of a radar chart also allowed us to say that these differences tend to diminish with age. The Spearman correlation test applied to mean lead concentrations showed strong and very strong positive correlations between all different types of bones. This test also showed that mean lead concentrations in bones are negatively correlated with the age of the animals. This correlation is strong in iliac and femur and very strong in tibia–fibula and skull. It was also shown that the decrease of lead accumulation with age is made by three plateaus of accumulation, which coincide, in all analysed bones, between 2nd–3rd and 9th–10th months.
Introduction
Lead pollution has nowadays its origin in the industrial societies, mainly in countries with non-existent or lenient industrial regulations. Besides the efforts being made, human beings are still exposed to lead levels 100- to 1000-fold higher than pre-industrial humans.1 There is much concern about the adverse effects of this exposure in the population, because lead serves no useful biological role in the human body.Lead ingested, inhaled or absorbed through the skin is initially distributed to the several tissues by the erythrocyte–plasma compartment, particularly into bones where lead competes with Ca for the formation of the primary crystalline matrix of bone, hydroxyapatite.2 A great percentage of total body lead (about 90% in adults and 70% in children) is found in bones,3 with a half-life in the order of years to decades.4 Once lead is slowly eliminated from bones, bone lead concentration may serve as a long-term exposure biomarker, and, consequently, a better predictor of some health effects than blood lead (approximately 30 days half-life).5
Nevertheless, the skeleton cannot be considered as an inert repository for lead, since it slowly releases the lead into the bloodstream. The gradual release from the bone serves as a persistent source of toxicity even after the end of external exposure. This mobilization can be increased by several factors like age, sex, nutritional status, and by some special conditions associated with bone turn-over, such as pregnancy, lactation, menopause, osteoporosis, immobilization, hyperthyroidism and bone fractures. During pregnancy, lead accumulated in bones is transferred to the foetus during the resorption of maternal bone for the production of the foetal skeleton.6 About 80% of cord blood appears to be from maternal bone lead stores.7 The continuous remodelling process of the bone makes this tissue not only a target of lead contamination from the environment (referred to as external dose) but also a source of lead contamination due to the mobilization of lead stores (described as internal dose) to soft tissues, resulting in toxic manifestations.
The distribution of lead in bones, however, is not homogeneous and depends on the type of bone: trabecular (or spongy) or cortical (or compact). In childhood lead accumulation will occur predominantly in trabecular bones, and during adulthood in both cortical and trabecular bones.8 Nevertheless, the cortical bone may be a better indicator of long-term cumulative exposure to lead than the trabecular bone, since the latter exchanges lead more actively with blood.5,9
Bone lead has been associated with several health problems, namely elevations in blood pressure and hypertension,10 increased risk of Parkinson's disease11 and poor cognitive test performances.12 It is also known that bone lead accumulation may impair bone growth and remodelling with occurrence of decreased bone density and increased bone resorption activity.13
Although there is no defined limit for the lead concentration in bones and health outcomes, recent studies provide evidence for adverse effects of lead concentrations above 10 μg g−1.14
Despite the studies done until now, there is still a lack of information about the correlation of lead in several types of bones during a long period of time. In this work, we studied lead accumulation with age in iliac bone, femur, tibia–fibula (analysed as one) and skull of several Wistar rats, to provide a better understanding of the effect of lead in bone retention. Measurements were made using the Energy Dispersive X-ray Spectrometry (EDXRF) technique. Analysis of formaldehyde used to preserve the bone samples was performed using the Electro-Thermal Atomic Absorption Spectrometry (ETAAS) technique to confirm if there was any loss of lead from the samples to the preservative.
Materials and methods
Animals
This study used Wistar rats (a strain of albino rats that belongs to the species Rattus Norvegicus), aged from 1 to 11 months. The rats were maintained under standard animal facility, in accordance with EU legislation on animal experimentation. Animals were placed in cages with commercial pellet food (Panlab A04) and waterad libitum, kept at 18 ± 1 °C and a relative humidity of 60 ± 10%, on a 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 h dark–light cycle.
12 h dark–light cycle.
The experimental chronogram was designed to show the accumulation of lead within the bones of animals during different stages of their development, from newborns to adults in a later stage of life. In order to achieve this purpose, a monthly evaluation of lead exposure was performed in animals belonging to both the control and exposed groups. Each of the 10 age groups included 2 control and 3 lead exposed rats, in a total of 50 Wistar rats. The exposed animals received a supplement of 0.2% of lead acetate in drinking water since the foetal period whilst the control animals got normal drinking water. The lead exposure regimen was based on the previous validated study of Bielarczyk et al.,15 and prepared by dissolving lead acetate (Panreac Quimica SA-Lead (II) Acetate 3-hydrate DIDATIC) in distilled water.
At the end of each month of exposure, the animals were sacrificed by an overdose of sodium pentobarbitone (100 mg mL−1 per kg body weight).
Four different bones were collected, namely, iliac bone, femur, tibia–fibula, and skull (frontal, parietal and occipital bones). The samples were stored in plastic containers, different for each rat, containing a solution of 10% formaldehyde with 7.4 pH (37–38% w/w, Panreac) and stored at 2 °C to avoid deterioration. The skull samples were stored in separate containers from the rest of the bones, because of their small size.
Sample preparation
Analysis of lead in samples
The detection limit of this spectrometer was obtained by using the standard reference pattern of Bone Ash 1400 from National Bureau of Standards (NBS), and calculated according to Custódio et al.17 To test the accuracy of the measurements, the reference materials NYS Caprine and Bovine from the Wadsworth Center, namely Bovine Bone 05-02 and Caprine Bone 05-04, were analysed.
The calibration was performed with the lead stock standard solution (1000 mg L−1Fluka Analytical) using calibration curves (peak area measurements) ranging from 0 to 30 μg L−1. The detection and quantification limits (LOD, LOQ) were calculated according to Miller and Miller,18 and the values 0.7 μg L−1 for LOD and 2.1 μg L−1 for LOQ were obtained.
Formaldehyde samples were spiked in order to test the accuracy of the spectrometer in this type of matrix. Known amounts of lead up to 6 μg L−1, 15 μg L−1 and 24 μg L−1 were spiked and the samples analyzed. The recoveries obtained were 98 ± 7%, 102 ± 4% and 101 ± 2% respectively (X ± RSD-Relative Standard Deviation, n = 3). Three injections of each sample were made and an RSD less than 10% was obtained.
Results and discussion
Bone lead measurements
The limit of detection was calculated to be 5 μg g−1. The reference materials were also measured and have reference values of (16.1 ± 0.3) μg g−1 and (31.5 ± 0.7) μg g−1, respectively, and the measured values (n = 3) were (17 ± 2) μg g−1 and (29 ± 3) μg g−1, respectively. RSD less than 10% was obtained for all the samples measured.In Table 1 are presented the lead concentrations measured in the bones of Wistar rats non-exposed and exposed since the foetal period.
| Bone lead concentration/μg g−1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Iliac | Femur | Tibia–fibula | Skull | ||||||
| Exposed | Control | Exposed | Control | Exposed | Control | Exposed | Control | ||
| Age/months | 1 | 298 ± 91 | 7 ± 1 | 298 ± 97 | 7 ± 1 | 311 ± 123 | 8 ± 1 | 236 ± 85 | 7 ± 1 |
| 2 | 271 ± 32 | 7 ± 1 | 250 ± 37 | 8 ± 2 | 242 ± 31 | 6 ± 1 | 182 ± 20 | 7 ± 1 | |
| 3 | 199 ± 19 | 8 ± 1 | 182 ± 18 | 8 ± 1 | 189 ± 19 | 8 ± 1 | 144 ± 14 | 8 ± 1 | |
| 4 | 199 ± 19 | 8 ± 1 | 193 ± 19 | 8 ± 1 | 182 ± 28 | 7 ± 1 | 149 ± 24 | 7 ± 1 | |
| 5 | 209 ± 20 | 8 ± 1 | 207 ± 36 | 8 ± 1 | 201 ± 20 | 7 ± 2 | 167 ± 17 | 7 ± 1 | |
| 6 | 209 ± 22 | 7 ± 1 | 194 ± 19 | 8 ± 1 | 185 ± 18 | 8 ± 1 | 162 ± 23 | 8 ± 3 | |
| 7.5 | 209 ± 34 | BDL | 188 ± 38 | BDL | 176 ± 45 | BDL | 147 ± 42 | BDL | |
| 9 | 182 ± 18 | BDL | 183 ± 18 | BDL | 184 ± 18 | BDL | 137 ± 15 | BDL | |
| 10 | 101 ± 17 | BDL | 112 ± 20 | BDL | 105 ± 21 | BDL | 101 ± 19 | BDL | |
| 11 | 102 ± 26 | BDL | 108 ± 34 | BDL | 106 ± 32 | BDL | 98 ± 26 | BDL | |
For the exposed rats, the values are very similar between iliac, femur and tibia–fibula, ranging from approximately 300 μg g−1 to near 100 μg g−1. The skull also has a lower limit of about 100 μg g−1, but an upper limit of about 250 μg g−1. The highest average lead concentration in all types of bones occurs in the 1 month old collection, and the lowest is found in the two last collections.
Other similar works omit crucial information, such as the exposure doses and time, the diversity of bones (in many cases comparing only similar types of bones), and if the published data refer to either dry, wet or ash weight. Due to this lack of information, it is difficult to establish a precise comparison. Nevertheless, we may conclude that our results are about the same order of magnitude or one order higher than the values found in other works, for example, the work of Denton et al.,2a Mahaffey et al.19 and Rader et al.20 However, in these works the exposure regimen was lower than the one used in our work and rats were not exposed since the foetal period.
In the control rats, along all age groups, values are lower than the reference value reported by ATSDR,14 10 μg g−1, above which there are descriptions of adverse health effects. Values similar to the ones measured in our work have also been reported in femur of control rats.20
Using the Mann–Whitney U test to compare exposed and non-exposed rats of the same age, significant differences (p < 0.001) were revealed for the lead concentration in the four types of bone samples. Assuming, conservatively, the detection limit value for the cases with BDL values, the measured lead concentration values in all bones of control rats are about 18 to 43 times less than the respective contaminated group.
Despite the 1st month collection, with coefficients of variation ranging from 40 to 31% for the several bones, the measured lead concentrations don't show a high standard deviation, however, the coefficient of variation is never inferior to 10%.
The mean lead concentration values between the different types of bones are displayed in the radar chart in Fig. 1. As can be seen, the concentration of lead in iliac bone shows a tendency to be higher than femur, tibia–fibula and skull. While the concentrations of lead in femur and tibia–fibula are similar, skull presents a considerable tendency to have a lower mean lead concentration. These tendencies, however, tend to disappear in all types of bones as the rats become older. This result corroborates the suggestion of Aufderheide and Wittmers8 that accumulation takes place predominantly in trabecular bone during childhood and in both cortical and trabecular bones in adulthood. This happens because in childhood the bone calcification is most dynamic in the trabecular bone, whilst in adulthood, calcification takes place at sites of remodelling in both cortical and trabecular bones.
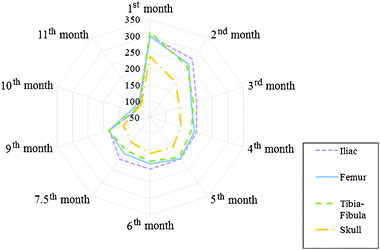 | ||
| Fig. 1 Distribution of mean lead concentrations (μg g−1) in iliac bone, femur, tibia–fibula and skull as a function of age (months). | ||
To assess whether these differences are statistically significant, we applied a Wilcoxon test between the different types of bones during the time of exposure. The test showed p values of p < 0.05 only when comparing the skull with the other types of bones. The differences between lead accumulation in skull and other bones were also reported in other works. Denton et al.2a results showed a higher lead concentration in femur than in skull, independent of the amount of lead ingested. Holtzman21 and Smith et al.22 also found the same tendency, while works made in humans, for e.g. Barry and Mossman,3a Khandekar and Anand,23 and Drasch et al.,24 found the highest lead concentration in skull. The difference between works carried out in humans and rats may be explained by the greater amount of compact bones in rat femurs than in skulls.2a
Whilst femur, tibia and fibula (long bones) are composed of trabecular bone tissue covered by compact bones, skull is primarily composed of dense compact tissue, and iliac bone is composed mainly of trabecular bone. Since the spongy bones are more vascular than the compact bones, it is expected that the iliac bone presents a higher mean lead concentration. Due to the higher blood affluence, we expect iliac bones to have the higher mean lead concentrations followed by femur and tibia–fibula and then skull, as it was observed in this work. However, in some works such as Drasch et al.,24 pelvic bone showed lower lead concentration than femur.
To study the correlation between elemental concentrations and their dependence on age, a Spearman correlation matrix was carried out (Table 2). A very strong statistically significant positive correlation between the lead concentrations in femur and iliac, skull and iliac and skull and femur was found. Strong positive correlations between the other types of bones can also be observed. According to this test, there is a strong negative correlation with age for all bone lead concentrations. This correlation tends to be very strong in tibia–fibula and skull.
Both Table 1 and Fig. 1, along with the Spearman test, show that there is a decrease by steps in mean lead concentration accumulated in all bones with age. To test this hypothesis, the Kruskal–Wallis test was applied to the different bones between all the collections. Results show that there are three different plateaus of accumulation (Table 3) for all the bones: plateau 1 (P1) containing the youngest rats of the 1st and 2nd collections; plateau 2 (P2) containing rats aged from 3 to 9 months; and plateau 3 (P3) containing the two older collections. In Table 3 are listed the mean values of each plateau, as well as the p values obtained with the Kruskal–Wallis test between the collections that form each plateau. These results show that they are not statistically different. Subsequent application of the Mann–Whitney test between the different plateaus for each bone, with p values of p ≤ 0.001 for all cases also corroborates the statistical difference and individuality of each plateau.
| P1 | P2 | P3 | |
|---|---|---|---|
| a Values are in μg g−1. | |||
| Iliac bone | 284 ± 62a | 202 ± 20a | 101 ± 21a |
| p = 0.749 | p = 0.257 | p = 0.873 | |
| Femur | 274 ± 68a | 191 ± 21a | 110 ± 27a |
| p = 0.337 | p = 0.362 | p = 0.749 | |
| Tibia–fibula | 277 ± 85a | 186 ± 20a | 105 ± 26a |
| p = 0.624 | p = 0.542 | p = 0.337 | |
| Skull | 211 ± 59a | 149 ± 19a | 99 ± 22a |
| p = 0.394 | p = 0.214 | p = 0.749 |
The decrease of mean lead concentration in bones over time, and the youngest high skeletal retention, has also been observed in other studies.20,24,25 The fast formation of bones in young animals and inclusion of lead into the new bone contribute to this increased retention.20,25,26 Important age dependent factors also contribute to the lead concentration decrease with age, namely the gastrointestinal absorption,27 the skeletal uptake,28 and the lead intake.27b,29 All these factors are considerably higher in younger animals than in adults. However, other studies made in human bones showed that there was an increase in the lead concentration with age, due to the decrease in the bone turnover rate.3b,30 Our work demonstrates that lead concentrations in bone decrease with age. Considering the statements of the above referred works, this might be indicative that in spite of the decrease of the bone turnover rate with age, the decreases in ingestion, absorption and retention of lead with age are the predominant factors.
The high lead concentrations found in the first month could be explained by the fact that the rats were delivered from mothers already contaminated with lead. As stated by Ronchetti et al.31 and Gardella,32 when pregnancy culminates, an identity exists between the blood lead concentrations in the mother and the child and, consequently, between both bone lead concentrations.
Formaldehyde lead measurements
To confirm that lead did not migrate from the bone tissues to the preservative, 5 samples of formaldehyde containing contaminated bones (iliac bone, femur and tibia–fibula) and 5 samples of formaldehyde containing contaminated skull were analysed. The formaldehyde measured values and the respective bone tissue concentrations are listed in Table 4.| Samples of contaminated rats | Tissue/μg g−1n = 2 | Formaldehyde/mg L−1n = 3 | |
|---|---|---|---|
| Lowest lead concentration bone tissue | 10 month old-rat 1 | 106 ± 20 | BDL |
| 6 month old-rat 2 | 166 ± 16 | BQL | |
| 4 month old-rat 3 | 197 ± 19 | 0.0037 ± 0.0003 | |
| 2 month old-rat 2 | 268 ± 26 | 0.0084 ± 0.0002 | |
| 1 month old-rat 3 | 248 ± 24 | BDL | |
| Skull | 6 month old-rat 1 | 180 ± 17 | BDL |
| 5 month old-rat 1 | 186 ± 19 | BDL | |
| 4 month old-rat 2 | 129 ± 13 | BDL | |
| 3 month old-rat 2 | 135 ± 14 | BDL | |
| 1 month old-rat 1 | 331 ± 33 | BDL | |
The results show that the concentration of lead in formaldehyde is, at least, about 5 orders of magnitude lower than the concentration of lead in the tissues. This allows us to conclude that the lead diffusion may be neglected in the present work.
Conclusions
The results of this work have demonstrated that there is a significant negative strong correlation between bone mean lead concentrations and age. This decrease in lead accumulation is made by steady plateaus of accumulation. One of the possible explanations for this decrease is the predominance of some age dependent behaviours such as decrease of lead intake, decrease of skeletal uptake and decrease of lead retention. It was noticed that the highest mean lead concentration values occur in rats of 1 month-old, which may be explained by the prenatal exposure.The relationship between mean lead concentrations in all types of bones was also studied and strong positive correlations were found. Differences between the values of lead concentrations in all bones were statistically tested. Besides iliac bone present higher lead concentrations than the other bone tissues, the differences were not statistically significant, except for skull that presented the lower mean lead concentration. These differences, however, tend to be attenuated with age.
The knowledge of these results provides a better understanding of the accumulation of this heavy element on bone tissues and especially its dependency on age.
Acknowledgements
This research was supported in part by FCT––Fundação para a Ciência e a Tecnologia (Portugal) Project No. PEstOE/FIS/UI0303/2011, financed by the European Community Fund FEDER through the COMPETE–––Competitiveness Factors Operational Programme. D. Guimarães acknowledges Fundação para a Ciência e Tecnologia (FCT) for the Ph.D. Grant (SFRH/BD/38788/2007). The authors acknowledge Professor José-Luís Capelo and REQUIMTE for the technical support in the ETAAS measurements.Notes and references
- B. Lanphear, D. Burgoon, S. Rust, S. Eberly and W. Galke, Environmental exposures to lead and urban children's blood lead levels, Environ. Res., 1998, 76, 120–130 CrossRef CAS.
- (a) J. Denton, G. Potter and J. Santolucito, A comparison of skull and femur lead levels in adult rats, Environ. Res., 1980, 23, 264–269 CrossRef CAS; (b) R. Lloyd, C. Mays, D. Atherton and F. Bruenger, PB-210 Studies in beagles, Health Phys., 1975, 28, 575–583 CrossRef CAS.
- (a) P. S. I. Barry and D. B. Mossman, Lead concentrations in human tissues, Br. J. Ind. Med., 1970, 27, 339–351 CAS; (b) P. S. I. Barry, Comparison of concentrations of lead in human tissues, Br. J. Ind. Med., 1975, 32, 119–139 CAS; (c) P. S. I. Barry, in The biogeochemistry of lead in the environment, ed. J. O. Niagru. Elsevier/North Holland Biomedical Press, Amsterdam, 1978, vol. Part B, pp. 97–150 Search PubMed.
- (a) H. Hu, M. Rabinowitz and D. Smith, Bone lead as a biological marker in epidemiologic studies of chronic toxicity: Conceptual paradigms, Environ. Health Perspect., 1998, 106, 1–8 CrossRef CAS; (b) J. McGowan, Bone: Target and source of environmental pollutant exposure, Otolaryngol.–Head. Neck Surg., 1996, 114, 220–223 CrossRef CAS.
- A. Schutz, S. Skerfving, J. Ranstam and J. Christoffersson, Kinetics of lead in blood after the end of occupational exposure, Scand. J. Work, Environ. Health, 1987, 13, 221–231 CAS.
- (a) C. Franklin, M. Inskip, C. Baccanale, C. Edwards, W. Manton, E. Edwards and E. OFlaherty, Use of sequentially administered stable lead isotopes to investigate changes in blood lead during pregnancy in a nonhuman primate (Macaca fascicularis), Fundam. Appl. Toxicol., 1997, 39, 109–119 CrossRef CAS; (b) B. Gulson, C. Jameson, K. Mahaffey, K. Mizon, M. Korsch and G. Vimpani, Pregnancy increases mobilization of lead from maternal skeleton, J. Lab. Clin. Med., 1997, 130, 51–62 CrossRef CAS; (c) B. Gulson, J. Pounds, P. Mushak, B. Thomas, B. Gray and M. Korsch, Estimation of cumulative lead releases (lead flux) from the maternal skeleton during pregnancy and lactation, J. Lab. Clin. Med., 1999, 134, 631–640 CrossRef CAS.
- B. Gulson, K. Mizon, M. Korsch, J. Palmer and J. Donnelly, Mobilization of lead from human bone tissue during pregnancy and lactation––a summary of long-term research, Sci. Total Environ., 2003, 303, 79–104 CrossRef CAS.
- A. Aufderheide and L. Wittmers, Selected aspects of the spatial-distribution of lead in bone, Neurotoxicology, 1992, 13, 809–819 CAS.
- (a) J. Borjesson, L. Gerhardsson, A. Schutz, S. Mattsson, S. Skerfving and K. Osterberg, In vivo measurements of lead in fingerbone in active and retired lead smelters, Int. Arch. Occup. Environ. Health, 1997, 69, 97–105 CAS; (b) U. Nilsson, R. Attewell, J. Christoffersson, A. Schutz, L. Ahlgren, S. Skerfving and S. Mattsson, Kinetics of lead in bone and blood after end of occupational exposure, Pharmacol. Toxicol. (Copenhagen), 1991, 68, 477–484 CAS.
- (a) F. Gerr, R. Letz, L. Stokes, D. Chettle, F. McNeill and W. Kaye, Association between bone lead concentration and blood pressure among young adults, Am. J. Ind. Med., 2002, 42, 98–106, DOI:10.1002/ajim.10096; (b) N. Vaziri, Mechanisms of lead-induced hypertension and cardiovascular disease, Am. J. Physiol.: Heart Circ. Physiol., 2008, 295, H454–H465, DOI:10.1152/ajpheart.00158.2008; (c) S. Korrick, D. Hunter, A. Rotnitzky, H. Hu and F. Speizer, Lead and hypertension in a sample of middle-aged women, Am. J. Public Health, 1999, 89, 330–335 CrossRef CAS.
- M. Weisskopf, J. Weuve, H. Nie, M. Saint-Hilaire, L. Sudarsky, D. Simon, B. Hersh, J. Schwartz, R. Wright and H. Hu, Association of Cumulative Lead Exposure with Parkinson's Disease, Environ. Health Perspect., 2010, 118, 1609–1613, DOI:10.1289/ehp.1002339.
- (a) M. Weisskopf, H. Hu, R. Mulkern, R. White, A. Aro, S. Oliveira and R. Wright, Cognitive deficits and magnetic resonance spectroscopy in adult monozygotic twins with lead poisoning, Environ. Health Perspect., 2004, 112, 620–625 CrossRef CAS; (b) R. Wright, S. Tsaih, J. Schwartz, A. Spiro, K. McDonald, S. Weiss and H. Hu, Lead exposure biomarkers and mini-mental status exam scores in older men, Epidemiology, 2003, 14, 713–718, DOI:10.1097/01.EDE0000081988.85964.db.
- (a) A. Escribano, M. Revilla, E. Hernandez, C. Seco, J. GonzalezRiola, L. Villa and H. Rico, Effect of lead on bone development and bone mass: A morphometric, densitometric, and histomorphometric study in growing rats, Calcif. Tissue Int., 1997, 60, 200–203 CrossRef CAS; (b) H. Gruber, H. Gonick, F. KhalilManesh, T. Sanchez, S. Motsinger, M. Meyer and C. Sharp, Osteopenia induced by long-term, low- and high-level exposure of the adult rat to lead, Miner. Electrolyte Metab., 1997, 23, 65–73 CAS; (c) M. Ronis, J. Aronson, G. Gao, W. Hogue, R. Skinner, T. Badger and C. Lumpkin, Skeletal effects of developmental lead exposure in rats, Toxicol. Sci., 2001, 62, 321–329 CrossRef CAS; (d) J. Hamilton and E. O'Flaherty, Influence of lead on mineralization during bone-growth, Fundam. Appl. Toxicol., 1995, 26, 265–271 CrossRef CAS.
- ATSDR, Toxicological Profile for Lead, Atlanta, Georgia, 2005.
- H. Bielarczyk, X. Tian and J. Suszkiw, Cholinergic denervation-like changes in rat hippocampus following developmental lead exposure, Brain Res., 1996, 708, 108–115 CrossRef CAS.
- (a) A. Rindby, Software for Energy-Dispersive X-Ray Fluorescence, X-Ray Spectrom., 1989, 18, 113–118 CrossRef CAS; (b) J. Boman and J. Isakson, Comparison between 2 X-ray-analysis software packages, X-Ray Spectrom., 1991, 20, 305–314 CrossRef CAS.
- P. Custodio, M. Carvalho, F. Nunes, S. Pedroso and A. Campos, Direct analysis of human blood (mothers and newborns) by energy dispersive X-ray fluorescence, J. Trace Elem. Med. Biol., 2005, 19, 151–158, DOI:10.1016/j.jtemb.2005.09.002.
- J. C. Miller and J. N. Miller, Statistics and chemometrics for analytical chemistry, Prentice Hall, Harlow, 4th edn, 2000, p. xvi, 271 p Search PubMed.
- K. Mahaffey, J. Rader, J. Schaefer and S. Kramer, Comparative toxicity to rats of lead acetate from food or water, Bull. Environ. Contam. Toxicol., 1980, 25, 541–546 CrossRef CAS.
- J. Rader, J. Peeler and K. Mahaffey, Comparative toxicity and tissue distribution of lead acetate in weanling and adult-rats, Environ. Health Perspect., 1981, 42, 187–195 CrossRef CAS.
- R. B. Holtzman, Measurement of the natural contents of RaD (Pb210) and RaF (Po210) in human bone--estimates of whole-body burdens, Health Phys., 1963, 9, 385–400 CrossRef CAS.
- R. G. Smith, J. Szajnar and L. Hecker, Study of lead levels in experimental animals, Environ. Sci. Technol., 1970, 4, 333–338 CrossRef CAS.
- R. N. Khandekar and S. J. Anand, Measurement of Pb-210 (RaD) in human bone at Bombay, India, Health Phys., 1971, 20, 83–85 CAS.
- G. A. Drasch, J. Bohm and C. Baur, Lead in human bones. Investigations on an occupationally non-exposed population in southern Bavaria (F.R.G) I. Adults, Sci. Total Environ., 1987, 64, 303–315 CrossRef CAS.
- S. Han, X. Qiao, F. Kemp and J. Bogden, Lead exposure at an early age substantially increases lead retention in the rat, Environ. Health Perspect., 1997, 412–417 CrossRef CAS.
- B. Momcilovic and K. Kostial, Kinetics of lead retention and distribution in suckling and adult rats, Environ. Res., 1974, 8, 214–220 CrossRef CAS.
- (a) E. McCabe, Age and sensitivity to lead toxicity––Review, Environ. Health Perspect., 1979, 29, 29–33 CrossRef CAS; (b) K. Mahaffey, Biotoxicity of lead-influence of various factors, Fed. Proc., 1983, 42, 1730–1734 CAS; (c) J. I. Rader, E. M. Celesk, J. T. Peeler and K. R. Mahaffey, Retention of lead acetate in weanling and adult rats, Toxicol. Appl. Pharmacol., 1983, 67, 100–109 CrossRef CAS.
- R. W. Leggett, An age-specific kinetic model of lead metabolism in humans, Environ. Health Perspect., 1993, 101, 598–616 CrossRef CAS.
- (a) J. Quarterman and E. Morrison, Effect of age on absorption and excretion of lead, Environ. Res., 1978, 17, 78–83 CrossRef CAS; (b) D. Smith, H. Mielke and J. Heneghan, Subchronic lead feeding study in male rats, Arch. Environ. Contam. Toxicol., 2008, 55, 518–528 CrossRef CAS.
- (a) H. Schroeder and I. Tipton, Human body burden of lead, Arch. Environ. Health, 1968, 17, 965–978 CAS; (b) S. B. Gross, E. A. Pfitzer, D. W. Yeager and R. A. Kehoe, Lead in human tissues, Toxicol. Appl. Pharmacol., 1975, 32, 638–651 CrossRef CAS.
- R. Ronchetti, P. Van den Hazel, G. Schoeters, W. Hanke, Z. Rennezova, M. Barreto and M. P. Villa, Lead neurotoxicity in children: Is prenatal exposure more important than postnatal exposure?, Acta Paediatr. (Oxford, U. K.), 2006, 95, 45–49 Search PubMed.
- C. Gardella, Lead exposure in pregnancy: A review of the literature and argument for routine prenatal screening, Obstet. Gynecol. Surv., 2001, 56, 231–238 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
