Synthesis of chiral α-amino acid-derived 1H-1,2,4-triazoles and 1,2,4-triazines†
Ebrahim H.
Ghazvini Zadeh
a,
Bahaa El-Dien M.
El-Gendy
ab,
Alex G.
Pop
a and
Alan R.
Katritzky
*ac
aCenter for Heterocyclic Compounds, Department of Chemistry, University of Florida, Gainesville, FL 32611-7200. E-mail: katritzky@chem.ufl.edu.Address; Fax: +352-392-919; Tel: +352-392-0554
bDepartment of Chemistry, Faculty of Science, Benha University, Benha, Egypt 13511
cDepartment of Chemistry, King Abdulaziz University, Jeddah, 21589, Saudi Arabia
First published on 14th November 2011
Abstract
Enantiopure N-(Cbz, Fmoc, Boc, or Ac)-1H-1,2,4-triazole- (8a–p, and 8f′) and previously unknown N-Cbz-1,2,4-triazine-derived α-amino acids (11a–d, 11d′, 13a,b, and 13a′) were synthesized using microwave irradiation. Reaction conditions led unexpectedly to simultaneous cyclization, deprotection and acetylation of N-Boc-aminoacylamidrazones 7m,n to afford N-acetyl-1H-1,2,4-triazoles 8o,p.
Introduction
In order to develop peptides as therapeutic agents, it is often necessary to introduce structural motifs which improve bioavailability and slow down biodegradation.1–3 Thus, diverse 1,2,4-triazoles have been designed and synthesized as amide bond isosteres4–6 or mimics7,8 to achieve these two important goals. For example, 1,2,4-triazole derivatives of L-tryptophan 1, developed as ghrelin receptor (GHS-R1a) ligands,9 which were shown to include potent agonists, partial agonists, and antagonists for the GHS-1a receptor. In addition, lysine scaffolds bearing the 1,2,4-triazolyl moiety 2 have been reported recently as histone deacetylase (HDAC) inhibitors10 showing high metabolic stability and potency in enzyme and cell-based assays. Moreover, dipeptido-1,2,4-triazole derivatives 3 exhibit high levels of CNS activity similar to the well known pharmaceutical insomnia sedative Triazolam 4 (Fig. 1).11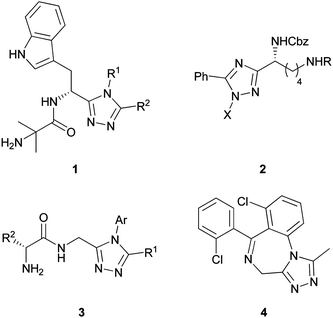 | ||
| Fig. 1 Selected nonproteinogenic α-amino acid-derived 1,2,4-triazoles. | ||
The most notable protocol used to access such 1,2,4-triazoles is the one devised by Borg and co-workers but this protocol suffers from relatively harsh conditions (180–200 °C), limited versatility and moderate yields.12 We now introduce an efficient microwave-assisted two-step protocol for the synthesis of a variety of chiral N-(Cbz, Fmoc, or Boc)-3-(α-aminoacyl)-1H-1,2,4-triazoles (8a–n, and 8f′) in yields of 54–72% over two steps.‡
In addition, we now disclose a new series of 1,2,4-triazines as new α-amino acid derivatives (11a–d, 11d′, 13a,b, and 13a′) of potentially high proteolytic stability.13–15 Such novel amino acid derivatives may mimic 1,2,4-triazines currently used for treatment of hypertension, inhibition of platelets,16antimalarials,17anticonvulsants,18 antibacterials19 and antidepressants.20
Results and discussion
The synthesis of N-(Pg)-α-amino acyl conjugates of 1H-1,2,4- triazole was envisaged to involve coupling of N-(Pg)-α-amino acylbenzotriazoles 5 with 2-pyridylamidrazone 6 to afford the corresponding N-(Pg)-α-aminoacylamidrazones 7, followed by cyclization of 7 to 1H-1,2,4-triazoles 8 (Scheme 1).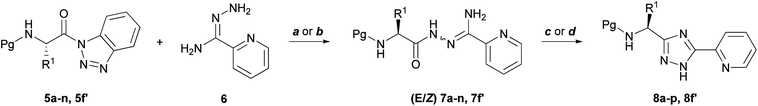 | ||
| Scheme 1 Synthetic approach toward the synthesis of N-(Cbz, Fmoc, Boc or Ac)-3-(α-aminoacyl)-1H-1,2,4-triazoles (8a–p, and 8f′). Reagents and conditions: (a) DIPEA (1 equiv) CH3CN, rt., 5 h; (b) CH3CN, reflux, 2 h; (c) HOAc, 130 °C, 1 h; (d) HOAc, microwave (50 W), 140 °C (5 min). | ||
The reaction of 5 with amidrazone 6 was carried out in dry acetonitrile either at room temperature or with heating to reflux, and yielded 7 as white solids. DIPEA enhanced the rate of the coupling reaction and preserved the base-labile Fmoc group (Table 1).
| Entry | Acyl Amidrazone 7 | R1 | Pg | % | 1H-1,2,4-triazole 8 | Pg | % |
|---|---|---|---|---|---|---|---|
| a HOAc, Δ (1 h). b microwave (50 W), 130 °C (5 min). c HOAc (2%) in ethanol, Δ (2 h). d microwave in HOAc (100 W), 180 °C (10 min). | |||||||
| 1 | a | CH(CH3)2 | Cbz | 73 | a | Cbz | 90a |
| 2 | b | CH2C6H5 | Cbz | 72 | b | Cbz | 89a |
| 3 | c | H | Cbz | 85 | c | Cbz | 80b |
| 4 | d | CH(CH3)CH2CH3 | Cbz | 75 | d | Cbz | 84a |
| 5 | e | methylene-3-indole | Cbz | 69 | e | Cbz | 90b |
| 6 | f | CH3 | Cbz | 80 | f | Cbz | 75b |
| 7 | f′ | CH3 (D,L) | Cbz | 74 | f′ | Cbz | 90a |
| 8 | g | CH(CH3)2 | Fmoc | 79 | g | Fmoc | 90a |
| 9 | h | H | Fmoc | 75 | h | Fmoc | 89a |
| 10 | i | CH2CH(CH3)2 | Fmoc | 71 | i | Fmoc | 80b |
| 11 | j | CH2OtBu | Fmoc | 77 | j | Fmoc | 84a |
| 12 | k | CH2Ph | Fmoc | 76 | k | Fmoc | 90b |
| 13 | l | methylene-3-indole | Fmoc | 72 | l | Fmoc | 75b |
| 14 | m | H | Boc | 91 | m | Boc | 79c |
| 15 | n | CH3 | Boc | 71 | n | Boc | 83c |
| 16 | m | H | Boc | 91 | o | Ac | 90d |
| 17 | n | CH3 | Boc | 71 | p | Ac | 89d |
The presence of two sets of chemical shifts in the 1H and 13C NMR spectra of acylamidrazones 7 in DMSO-d6 demonstrates the existence of two isomers 7(Z) and 7(E). The free energy of activation (ΔG≠) for 7f was calculated from variable temperature NMR to be 18.2 Kcal/mol (Fig. 2).
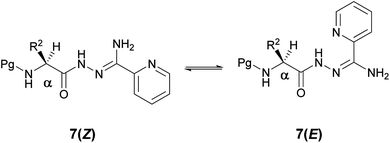 | ||
| Fig. 2 Isomeric forms 7(Z) and 7(E). | ||
Cyclization of 7 into 1H-1,2,4-triazole 8 was examined following different literature procedures.12 Low yields (<10%) of 8 were obtained when 7a–c were heated at their melting points for 20 min,12 or when stirred in conc. H2SO4 for 1 h.12 However, heating 7 at 130 °C in HOAc for 1 h successfully led to 1H-1,2,4- triazoles 8 in 84–90%. Alternatively, microwave irradiation (5 min, 50 W at 140 °C) of 7 in acetic acid (0.3 g/1 mL) accelerated the reaction rate, providing yields of 75–90%.
Cyclization of N-Boc-aminoacylamidrazones 7m,n in boiling acetic acid for 1 h afforded a number of products by TLC. Control over the reaction results was achieved when 7m,n and cat. HOAc were stirred in boiling EtOH, which yielded exclusively N-Boc-3-(α-aminoacyl)-1H-1,2,4-triazoles 8m,n. On the other hand, when 7m,n were subjected to microwave irradiation of 100 W at 180 °C, N-acetyl-1H-1,2,4-triazole-derived amino acids 8o,p were isolated in 89–90% yields, perhaps through simultaneous cyclization, deprotection and acetylation of 7m,n (Scheme 1). HPLC on a Chiralcel-OD column (hexanes: iPrOH, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) confirmed the enantiomeric purity of 8f (Table 1, entry 6); the L-enantiomer 8f showed a single peak with retention time of 11.59 min, whereas the racemic mixture 8f′ showed two peaks with retention times of 11.60 and 12.54 min (See page 22 in the Supplementary information†).
3) confirmed the enantiomeric purity of 8f (Table 1, entry 6); the L-enantiomer 8f showed a single peak with retention time of 11.59 min, whereas the racemic mixture 8f′ showed two peaks with retention times of 11.60 and 12.54 min (See page 22 in the Supplementary information†).
The synthesis of N-Cbz-α-amino acid-derived 1,2,4-triazines 11a–d, and 11d′ was inspired by the work of Saraswathi.21,22Amino acid hydrazides are important synthetic intermediates and have been used recently for the preparation of azadipeptide nitriles as highly potent and proteolytically stable inhibitors of papain-like cysteine proteases.23 We have used amino acid hydrazides 9a–g,a′,f′ to prepare N-Cbz-α-amino acid-derived 1,2,4-triazines 11a–d,a′ and 13a,b,a′ by reaction with 2,4′-dibromoacetophenone 10 and acenaphthenequinone 12 respectively (Schemes 2 and 3). Firstly, amino acid hydrazides 9 and 2,4′-dibromoacetophenone 10 were heated under reflux in EtOH/HOAc and the reaction was catalyzed by KOAc to afford 1,2,4-triazines 11c,d and 11d′ in 28–43% yields (Scheme 2). The use of microwave irradiation (50 W, 95 °C, 1 h) resulted in cleaner reactions and increased the yield in the cases of 11a,b. HPLC on a Chirobiotic T column (4.6 × 250 mm, flow rate of 0.4 mL/min, MeOH:H2O with 0.1% TFA), confirmed the enantiomeric purity of 11d; the L-enantiomer 11d showed a single peak with retention time of 6.05 min, whereas the racemic mixture 11d′ showed two peaks with retention times of 6.42 and 7.0 min.
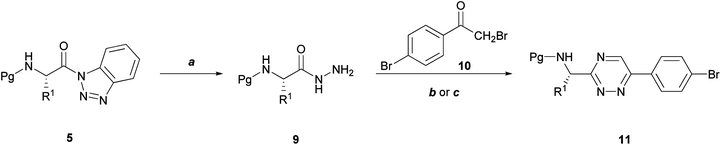 | ||
| Scheme 2 Route towards the synthesis of N-Cbz-1,2,4-triazine-derived α-amino acids (11a–d, 11d′). Reagents and conditions: (a) NH2NH2.H2O, THF, 15 min, rt; (b) 9 (2 equiv.), KOAc, HOAc, EtOH, reflux, 7 h; (c) 9 (2 equiv.) KOAc, HOAc, EtOH, microwave (50 W, 95 °C, 1 h). | ||
![Synthesis of [1,2-e][1,2,4]triazines 13.](/image/article/2012/MD/c1md00177a/c1md00177a-s3.gif) | ||
| Scheme 3 Synthesis of [1,2-e][1,2,4]triazines 13. | ||
Zhao and his co-workers reported the synthesis of tri-substituted 1,2,4-triazines using arylhydrazide and 1,2-diones in the presence of excess NH4OAc at 180 °C.24 This method was successful when N-Cbz-amino acid hydrazides 9 and 1,2-acenaphthenedione 12 were heated under microwave irradiation in the presence of 2 equiv. of NH4OAc to prepare N-Cbz-aminoacyl-derived 3,5,6-trisubstituted 1,2,4-triazines 13a,b and 13a′ in 61–68% yields (Scheme 3, Table 3).
| Entry | 5 | Pg | R1 | 9 | mp (°C) | Yield % |
|---|---|---|---|---|---|---|
| 1 | a | Cbz | CH(CH3)2 (L) | a | 163.0–165.025 | 86 |
| 2 | o | Cbz | CH(CH3)2 (D,L) | a′ | 126.0–128.0 | 90 |
| 3 | b | Cbz | CH2C6H5 | b | 153.0–155.023 | 94 |
| 4 | c | Cbz | H | c | 93.0–95.026 | 70 |
| 5 | d | Cbz | CH(CH3)CH2CH3 | d | 159.0–161.0 | 87 |
| 6 | e | Cbz | methylene-3-indole | e | 163.0–165.0 | 90 |
| 7 | f | Cbz | CH3 (L) | f | 111.0–113.026 | 78 |
| 8 | f′ | Cbz | CH3 (D,L) | f′ | 116.0–118.0 | 80 |
| 9 | i | Fmoc | CH2CH(CH3)2 | g | 165.0–167.0 | 75 |
Conclusions
α-Amino acid-derived N-(Cbz, Fmoc, Boc, or Ac)-1H-1,2,4-triazoles (8a-p, and 8f′) and previously unknown N-Cbz-1,2,4-triazines (11a–d, 11d′, 13a,b, and 13a′) were synthesized in moderate to high yields with retention of chirality in the case of 8 and 11. We were not able to achieve good separation in the case of 13 using the HPLC columns we have in our laboratory. The devised synthetic protocol for 1H-1,2,4-triazole- (8a–p, and 8f′) is general, scalable, and the reaction conditions are mild, reproducible and compatible with different protecting groups.Notes and references
- B. Groner, Peptides as Drugs: Discovery and DevelopmentWILEY VCH Verlag GmbH & Co. KGaA, Weinheim, 2009, p. 1 Search PubMed.
- N. Narendra, H. S. Lalithamba and V. V. Sureshbabu, Tetrahedron Lett., 2010, 51, 6169–6173 CrossRef CAS.
- K. A. Witt, T. J. Gillespie, J. D. Huber, R. D. Egleton and T. P. Davis, Peptides, 2001, 22, 2329–2343 CrossRef CAS.
- H. J. Wadsworth, S. M. Jenkins, B. S. Orlek, F. Cassidy, M. S. Clark, F. Brown, G. J. Riley, D. Graves, J. Hawlins and C. B. Naylor, J. Med. Chem., 1992, 35, 1280–1290 CrossRef CAS.
- S. M. Jenkins, H. J. Wadsworth, S. Bromidge, B. S. Orlek, P. A. Wyman, G. J. Riley and J. Hawkins, J. Med. Chem., 1992, 35, 2392–2406 CrossRef CAS.
- G. Burrell, J. M. Evans, M. S. Hadley, F. Hicks and G. Stemp, Bioorg. Med. Chem. Lett., 1994, 4, 1285–1290 CrossRef CAS.
- W. R. Tully, C. R. Gardner, R. J. Gillepsie and R. Westwood, J. Med. Chem., 1991, 34, 2060–2067 CrossRef CAS.
- S. K. Thompson, A. M. Eppley, J. S. Frazee, M. G. Darcy, R. T. Lum, T. A. Tomaszeck, L. A. Ivanoff, J. F. Morris, E. J. Sternberg, D. M. Lambert, A. V. Fernandez, S. R. Petteway, T. D. Meek, B. W. Metclaff and J. G. Gleason, Bioorg. Med. Chem. Lett., 1994, 4, 2441–2446 CrossRef CAS.
- L. Demange, D. Boeglin, A. Moulin, D. Mousseaux, J. Ryan, G. Berge, D. Gagne, A. Heitz, D. Perrissoud, V. Locatelli, A. Torsello, J.-C. Galleyrand, J.-A. Fehrentz and J. Martinez, J. Med. Chem., 2007, 50, 1939–1957 CrossRef CAS.
- S. Manku, M. Allan, N. Nguyen, A. Ajamian, J. Rodrigue, E. Therrien, J. Wang, T. Guo, J. Rahil, A. J. Petschner, A. Nicolescu, S. Lefebvre, Z. Li, M. Fournel, J. M. Besterman, R. Déziel and A. Wahhab, Bioorg. Med. Chem. Lett., 2009, 19, 1866–1870 CrossRef CAS.
- K. Hirai, T. Fujishita, T. Ishiba, H. Sugimoto, S. Matsutani, Y. Tsukinoki and K. Hirose, J. Med. Chem., 1982, 25, 1466–1473 CrossRef CAS.
- S. Borg, G. Estenne-Bouhtou, K. Luthman, I. Csöregh, W. Hemelink and U. Hacksell, J. Org. Chem., 1995, 60, 3112–3120 CrossRef CAS; V. N. Nuriev, N. V. Zyk and S. Z. Vatsadze, ARKIVOC, 2005, iv, 208–224 Search PubMed; O. Pintilie, L. Profire, V. Sunel, M. Popa and A. Pui, Molecules, 2007, 12, 103–113 CrossRef.
- A. R. Katritzky, B.-E. M. El-Gendy, E. Todadze and A. A. A. Abdel-Fattah, J. Org. Chem., 2008, 73, 5442–5445 CrossRef CAS.
- A. R. Katritzky, C. El-Nachef, K. Bajaj, J. Kubik and D. N. Haase, J. Org. Chem., 2010, 75, 6009–6011 CrossRef CAS.
- A. R. Katritzky, A. A. Shestopalov and K. Suzuki, ARKIVOC, 2005, vii, 36–55 Search PubMed.
- A. Monge, J. Palop, C. Ramizrez and F. A. E. Font, Eur. J. Med. Chem., 1991, 26, 179–188 CrossRef CAS.
- L. C. March, G. S. Bajwa, J. Lee, K. Wasti and M. M. Joullie, J. Med. Chem., 1976, 19, 845–848 CrossRef CAS.
- D. L. Trepanier, E. R. Wagner, G. Harris and D. Allan, J. Med. Chem., 1966, 9, 881–885 CrossRef CAS.
- K. Srinivas, U. Srinivas, R. Jayathirtha, K. Bhanuprakash, K. Harakishore and U. S. Murthy, Bioorg. Med. Chem. Lett., 2005, 15, 1121–1123 CrossRef CAS.
- C. Rubat, P. Coudert, S. Marvel, J. Fialip and J. Couquelet, J. Pharm. Pharmacol., 1997, 49, 1019–1024 CrossRef CAS.
- T. V. Saraswathi and V. R. Srinivasan, Tetrahedron, 1977, 33, 1043–1051 CrossRef CAS.
- M. Ernd, M. Heuschmann and H. Zipse, Helv. Chim. Acta, 2005, 88, 1491–1518 CrossRef CAS.
- R. Löser, M. Frizler, K. Schilling and M. Gütschow, Angew. Chem., Int. Ed., 2008, 47, 4331–4334 CrossRef.
- Z. Zhao, W. H. Leister, K. A. Strauss, D. D. Wisnoski and C. W. Lindsley, Tetrahedron Lett., 2003, 44, 1123–1127 CrossRef CAS.
- G. Verardo, P. Geatti and B. Lesa, Synthesis, 2005, 559–564 CrossRef CAS.
- B. F. Erlanger and E. Brand, J. Am. Chem. Soc., 1951, 73, 3508–3510 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental data for compounds. See DOI: 10.1039/c1md00177a |
| ‡ Example: Synthesis of 8f according to method A: (S)-Benzyl 1-(3-(pyridin-2-yl)-1H-1,2,4-triazol-5-yl)ethylcarbamate: A solution of (S)-benzyl (1-(2-(amino(pyridin-2-yl)methylene)hydrazinyl)-1-oxopropan-2-yl)carbamate (0.68 g, 2.0 mmol) in glacial acetic acid (1.00 mL) was heated to reflux for 1 h. The solution was poured over cold brine (20 mL) and the precipitate was filtered and washed with water. The resulting white solid was suspended in methanol (5 mL) and was filtered and dried in vacuo to afford the title product as white microcrystals (0.55 g, 1.7 mmol, 86%). mp 156.0–158.0 °C. 1H NMR (DMSO-d6, 300 MHz) δ 14.53 (br s, 1H), 8.68 (d, J = 4.2 Hz, 1H), 8.04 (d, J = 7.8 Hz, 1H), 7.96 (t, J = 7.5 Hz, 1H), 7.82 (br s, 1H), 7.49 (t, J = 5.7 Hz, 1H), 7.42–7.22 (m, 5H), 5.04 (s, 2H), 4.85 (quin, J = 7.2 Hz, 1H), 1.47 (d, J = 7.2 Hz, 3H). 13C NMR (DMSO-d6, 75 MHz) δ 155.6, 149.5, 137.6, 137.1, 128.3, 127.7, 124.6, 121.2, 65.3, 44.8, 20.1. Anal. Calcd. for C17H17N5O2 (323.36): C, 63.15; H, 5.30; N, 21.66. Found: C, 63.10; H, 5.32; N, 21.82. |
| This journal is © The Royal Society of Chemistry 2012 |
