Location of disorder in coiled coil proteins is influenced by its biological role and subcellular localization: a GO-based study on human proteome†‡
Meenakshi
Anurag
a,
Gajinder Pal
Singh
b and
Debasis
Dash
*a
aG.N.R. Knowledge Center for Genome Informatics, Institute of Genomics and Integrative Biology (CSIR-IGIB), CSIR, Delhi, 110007, India. E-mail: ddash@igib.res.in
bInstitute of Biochemistry, Biological Research Centre, Szeged, Hungary
First published on 25th October 2011
Abstract
Intrinsic disorder in proteins has been explored to study lack of structure–function aspects of many proteins. The current study focuses on coiled coils which are often linked to intrinsic disorder. We present a sequence level analysis of human coiled coils to find out if this is universally true for all coiled coils. When annotated coiled-coil regions were collected from UniProt and investigated with disorder prediction tools namely—IUPred and DISpro, three patterns were commonly observed—disordered coiled coils (DisCCs), ordered coiled coils (OCCs) and the last one having a disordered region outside the coiled-coil region (DOCCs). Differential enrichment in the gene ontology was seen in these three categories. We found that OCCs are enriched in structural components of the extracellular space including the fibrinogen complex and laminin complex. On the contrary, DisCCs were found to be exclusively over-represented in proteins involved in actin filament, lamellipodium, cell junction, macromolecule complexes, ciliary rootlet and nucleolus. DOCCs are found to be associated with many regulatory and adaptor functions including positive regulation of calcium ion transport via store-operated calcium channel activity, cytoskeletal adaptor activity etc. Other than the GO-based analysis, sequence level analysis showed that disordered coiled-coil regions bear a high proportion of low-complexity regions as compared to ordered coiled coils. The former also has a higher probability of forming a dimer as compared to the ordered counterpart. Our study shows that the in silico approach of mapping of disorder in or around coiled coils in other biological systems or organisms can be applied to understand and rationalize the mode of action of these dynamic motifs.
Introduction
Coiled coils have been of particular interest to molecular biologists because of their ideal architecture for protein folding and designing studies and have gained popularity because of their applicability to synthetic biology and nanotechnology.1,2 They bear the signature heptad repeats comprising of a seven-residue pattern denoted “abcdefg” with residues a, d forming the hydrophobic core and e, g usually charged or polar. There are two major hypotheses regarding the folding of the coiled coils: one states that the folding of the motif is because of the collision between the unstructured monomeric units3 whereas the other hypothesis states that dimerization is preceded by partial helix formation.4Coiled coils, apart from being structurally interesting, play important roles in transcription control, association and organization of complexes, chromosomal and cell cycle maintenance etc. They are also vital for the fusion of viral and cellular membrane and hence are being widely studied in HIV.5,6 Recently, Barth et al.7 used computational methods to design peptides that inhibited coiled coil formation between the metastable coiled-coil region of yeast septin—Cdc12—and its natural binding partner Cdc3. The study highlights designing of specific peptide inhibitors that can bind to the protein domain lacking intrinsic structural stability.
Coiled coils are often linked to intrinsic disorder at a sequence level because coiled coils are frequently disordered as monomers and become folded upon association and formation of quaternary structure.8 Studies of Stalk Domain of ncd Motor protein in Drosophila have shown two states of coiled coil—reversible and irreversible.9 The reversibility and irreversibility can be linked to the intrinsic disorder of the protein or segment. Intrinsically disordered proteins (IDPs) are known to remain disordered in their native state and a conformational transition can fold them into a more compact or rigid structure induced upon binding or by altered physiological conditions.
The current work primarily addresses two questions—first, how commonly coiled coils are disordered and second, how are they differentially enriched in terms of functions, cellular components and biological processes. In this study we categorized the coiled coil proteins into three sets—disordered coiled coils (DisCCs), ordered coiled coils (OCCs) and disorder outside coiled coil (DOCCs) and performed in silico analysis to find out the processes and cellular components that are significantly and differentially associated with the three categories. Studying the human coiled coils will help synthetic biologists and nanotechnologists to design novel inhibitors, switches and regulators.10 This study also highlights the sequence–structure relation of coiled coils.
Materials and methods
Data set
A set of 1968 human proteins annotated as bearing coiled-coil regions were retrieved from the UniProt database.11,12 The coiled coil domain length and their position information were extracted from the GFF file. There might be cases where proteins have been reported to bear a coiled-coil region but not mentioned in the UniProt database, these have not been considered in this study. The human variation and their associated disease information was retrieved from “Human polymorphisms and disease mutations” release 08-Feb-2011 (UniProt).Disorder prediction and GO analysis
Prediction for the presence of intrinsic disorder was run on 1968 coiled coil bearing proteins. Intrinsically disordered regions (score ≥ 0.5 and length of consecutive amino acid ≥ 30) were identified using IUPred13 and verified using DISPRO.14 The disorder in coiled coils was found by mapping the coordinates of the intrinsic disordered region (IUPred predicted) with coiled coils (obtained from UniProt). 883 proteins were verified to be disordered by both IUPred as well as DISPro (Fig. 1). The proteins which were predicted to be disordered only by one algorithm were separated out as an inconclusive set (648) and were not considered for further study. Mapping of disordered and coiled coil coordinates gave two sets—one which had 598 proteins harboring “disordered coiled coils” (DisCCs) and other with 285 proteins with “disorder outside coiled coil” (DOCCs). 437 proteins lacked potential IDRs, i.e. they belonged to the ordered coiled coil set (OCCs). This filtering approach is explained in Fig. 1, where a very stringent criterion of identifying the disordered region has been implemented and stretches less than 30 amino acids have not been considered in the categorization. Further, differential enrichment of cellular components, processes and molecular functions in the three sets was found by carrying out Gene Ontology (GO) analysis using GOEAST.15 GOEAST uses hyper-geometric test as a default method to identify statistically enriched or depleted ontologies. Multi-GOEAST functionality was used to compare the three sets of coiled coils. It is also claimed to be one of the most updated source of data.15 It was also used for the easy visualization of differentially represented GOs.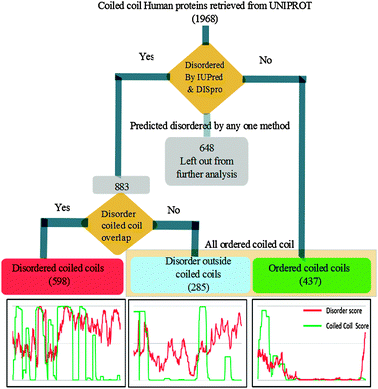 | ||
| Fig. 1 Workflow depicting the categorization of proteins as disordered coiled coils (DisCCs), ordered coiled coils (OCCs) and disorder outside coiled coil domains (DOCCs). | ||
Amino acid composition
Coiled-coil regions were extracted from protein sequences based on their UniProt coordinates. DisCC regions comprised of the coiled coils which were predicted to be disordered and the ordered coiled-coil region from DOCCs and OCCs were combined to form a set of regions called All Ordered Coiled coils (AOCCs). These sets of regions were used for amino acid composition.Amino acid composition was calculated for the coiled-coil regions of DisCCs and AOCCs. The feature was calculated using web server PROFEAT,16 which is freely accessible at http://jing.cz3.nus.edu.sg/cgi-bin/prof/prof.cgi. The mean value of the percentage composition for each amino acid was calculated.
Enrichment tests for oligomerization states and low complexity regions
To test enrichment of the two oligomerization states—dimer and trimer—in the coiled-coil regions of disordered coiled coil and ordered coiled coil, Multicoil17 was run on the two sets of regions—DisCC and AOCC (cumulative set of DOCCs and OCCs). The program predicts probability of oligomerization states for a coiled-coil region based on background data for dimeric and trimeric coiled coils.17Low complexity regions (LCRs) in the coiled-coil regions of DisCC and AOCCs sets were predicted using Seg (ftp://ftp.ncbi.nih.gov/pub/seg/seg) which implements the method of Wootton and Federhen18 which identifies compositionally biased regions in the amino acid sequence. LCRs were predicted using the default parameters of Seg. The total length of LCRs was calculated for each coiled-coil region of the two sets.
Finally, a Wilcoxon rank sum test is performed on the oligomerization state probability as well as proportion (percentage length) of LCRs in coiled coils. Two separate tests were performed—the first test assesses a p-value for the null hypothesis that there is no difference between dimeric and trimeric state probabilities in DisCCs and AOCCs, the second test was performed with the null hypothesis that there is no difference between the proportion of LCRs in coiled coils in DisCCs and AOCCs. The tests were performed using Wilcox.test function of R which implements Wilcoxon rank sum test on provided vectors.
Results
We analyzed a set of 1968 human proteins annotated as coiled coil for the presence of disordered regions. We used two methods of disorder prediction—namely IUPred and DISpro. IUPred predicts the disorder score based on an inter-residue interaction matrix. DISpro, on the other hand, is a neural network based method which works on a background set of curated disordered proteins. Disorder prediction and coordinate mapping of coiled coils and IDRs resulted (Fig. 1) in a set of 598 proteins harbouring disordered coiled coils (DisCCs) and 285 proteins having disordered regions outside coiled coils (DOCCs). We also found 437 coiled coil proteins which were devoid of the disordered region (OCCs).We enquired into the enrichment of certain functional classes in these three categories of coiled coils by performing gene ontology analysis on these protein sets for finding relevant biological function, process and localization. GOEAST is a powerful tool to study the enrichment patterns of gene ontology (GO) from a given set of genes/proteins. We used these three sets of proteins to get GO enrichment data. 488 out of 598 DisCCs, 375 out of 437 OCCs and 256 out of 285 DOCCs had associated gene ontology information in GOEAST and hence were used further for the enrichment analysis. The GO enrichment data obtained for the three categories were compared to find differentially enriched molecular functions, biological processes and cellular components. Important cellular components enriched in different sets of coiled coils have been depicted in Fig. 2 which has been generated using canvas utility of TinkerCell.19 Three different colours red, blue and green represent DisCC, DOCC and OCC respectively. The statistical significance of the enrichment of DisCCs, DOCCs and OCCs in various cellular components is provided as ESI† (Table S1).
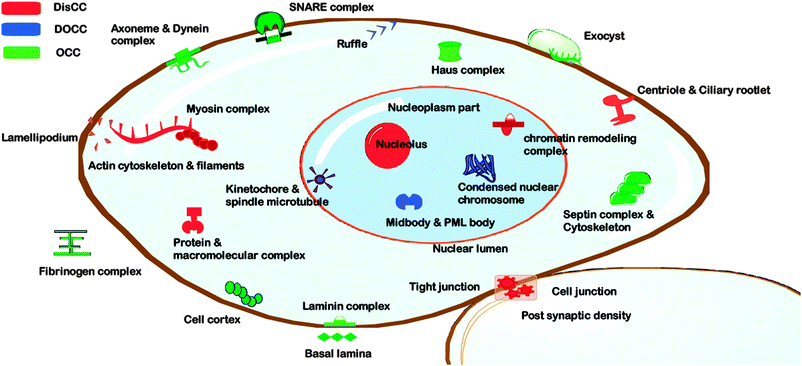 | ||
| Fig. 2 Enrichment of different categories of coiled coil proteins in various cellular components as found by gene ontology analysis. The disordered coiled coils (DisCCs) are shown in red, ordered coiled coils (OCCs) in green and disorder outside coiled coils (DOCCs) in blue. | ||
Performing the cellular component analysis using multiple set GO comparison utility of GOEAST, we found that OCCs are enriched in structural components of the extracellular space including the fibrinogen complex and laminin complex (Fig. 2). Membrane trafficking complexes like SNARE complexes and exocysts were also found to be enriched in OCCs.
We found DisCCs to be exclusively over-represented in proteins involved in actin filament, lamellipodium, cell junction, macromolecule complexes, ciliary rootlet, nucleolus etc. (Fig. 2) As evident most of the cellular components listed prior contribute to motility and mechanical integrity of the cell.
DOCCs were rich in cellular components including kinetochore–microtubule complexes, ruffle and midbody (Fig. 2). This subset of coiled coil proteins was observed to be associated with biological processes including regulation of calcium ion transport. They were found to be associated with many regulatory and adaptor functions including positive regulation of calcium ion transport via store-operated calcium channel activity, negative regulation of endocytosis, regulation of GTPase activity, cytoskeletal adaptor activity etc.
Amino acid enrichment
The study shows that DisCCs are particularly enriched in charged residues including glutamic acid (E) and arginine (R), hydrophilic residues viz. glycine (G) and aromatic residues like phenylalanine (F) and tyrosine (Y) (Fig. 3). On the contrary, AOCCs were found to be enriched in hydrophobic residues like alanine (A), leucine (L) and methionine (M).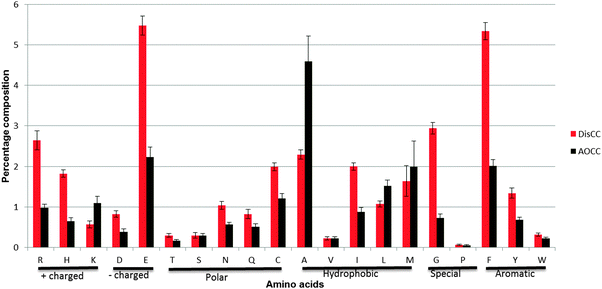 | ||
| Fig. 3 Amino acid composition of DisCCs and AOCCs. The X-axis shows the 20 amino acids and the Y-axis shows the percentage composition of the amino acids in the coiled-coil region. The error bar depicts the standard error of mean (±SEM). | ||
Oligomerization states of coiled coils
Multicoil was used to predict dimeric and trimeric states of the coiled-coil region for DisCCs and AOCCs separately. The difference in the distribution probability scores of dimerization between the two sets was found to be significant with a p-value ≪ 1 × 10−10 as compared to the less significant difference in probability of the trimeric state p-value = 0.01345 (Wilcoxon test, see Materials and methods). The median of probability of dimer forming coiled coil in DisCC was higher (0.95) than AOCC (0.78). An overview of the distribution of probabilities for dimeric and trimeric states of oligomerization for the two sets of coiled-coil regions is shown in the form of a boxplot (Fig. S2, ESI†).Low complexity regions in DisCCs and AOCCs
Intrinsically disordered proteins are known to harbor low-complexity regions. LCRs were identified in coiled-coil regions from DisCC and AOCC sets. It was observed that LCRs are present in significantly high proportion in DisCCs as compared to AOCCs. The Wilcoxon test was applied which rejected the null hypothesis that DisCCs and AOCCs bear a similar proportion of LCRs with a p-value of 0.005. The median of proportion of LCRs was calculated to be 34.09% for DisCCs and 29.19% for AOCCs.Disordered coiled coils and associated diseases
Based on disease related variation data and literature survey we identified few DisCCs that are associated with diseases or important in host–pathogen interactions. Designing of artificial coiled coils with a desired function is currently an active research field20–22 and getting insight into the role of coiled coils and their flexibility in host–pathogen interaction can be important. Disease associated variation data showed that there are twenty-nine DisCC proteins which are associated with different diseases (for details see Table S3, ESI†) and further analysis revealed that there were five proteins which harbor mutations in the DisCC region that are associated with diseases. The proteins along with their respective mutation and associated diseases are shown in Table 1.| UniProt-id | Gene | Var-id | Mutation | Site | Disease | MIM-id |
|---|---|---|---|---|---|---|
| B1AK53 | ESPN | VAR_043455 | R774Q | 774 | Deafness autosomal dominant without vestibular involvement (DFNAWVI) | [MIM:606351] |
| P02671 | FIBA | VAR_010731 | E545V | 545 | Amyloidosis type 8 (AMYL8) | [MIM:105200] |
| P02671 | FIBA | VAR_010732 | R573L | 573 | Amyloidosis type 8 (AMYL8) | [MIM:105200] |
| Q3SXY8 | ARL13B | VAR_054372 | R200C | 200 | Joubert syndrome type 8 (JBTS8) | [MIM:612291] |
| Q7Z4S6 | KIF21A | VAR_019400 | M947R | 947 | Congenital fibrosis of extraocular muscles type 1 (CFEOM1) | [MIM:135700] |
| Q7Z4S6 | KIF21A | VAR_019401 | M947V | 947 | Congenital fibrosis of extraocular muscles type 1 (CFEOM1) | [MIM:135700] |
| Q7Z4S6 | KIF21A | VAR_027021 | M947T | 947 | Congenital fibrosis of extraocular muscles type 1 (CFEOM1) | [MIM:135700] |
| Q7Z4S6 | KIF21A | VAR_019402 | R954Q | 954 | Congenital fibrosis of extraocular muscles type 1 (CFEOM1) | [MIM:135700] |
| Q7Z4S6 | KIF21A | VAR_019403 | R954W | 954 | Congenital fibrosis of extraocular muscles type 1 (CFEOM1) | [MIM:135700] |
| Q9ULD2 | MTUS1 | VAR_035184 | Q1201R | 1201 | Hepatocellular carcinoma (HCC) | [MIM:114550] |
Discussion
Sequence level enrichments and their possible impact on oligomerization
Romero et al.23 used entropy and alphabet size as parameters to study the complexity of a sequence and showed that coiled coils have lower complexity than globular proteins. In the current study we further explored the difference in sequence based complexity by identifying the LCRs in the two categories of coiled coils. The abundance of LCRs in DisCCs was higher than those in AOCCs justifying the role of the disordered region in the flexibility of coiled coils.The heptad repeats have prefered positions for hydrophobic (a and d) and charged (e and g) amino acids. The current observation suggests that amongst the hydrophobic residues isoleucine (I) is enriched in DisCCs and alanine (A) and leucine (L) are favored by AOCCs (Fig. 3). Since leucine and isoleucine have the same composition and size, the packing geometry plays a major role in oligomerization of coiled coils and studies have shown that presence of isoleucine at both a and d positions is preferred in a trimeric oligomeric state.23,24 Our observed marginal high probability of DisCCS to form trimers over AOCCs can be because of the high frequency of isoleucines in DisCCs (p-value of the Wilcoxon test = 0.01345).
The charged amino acids occupy e and g positions of the heptad repeats. In a study conducted by Kohn et al.25 it was observed that increasing the frequency of glutamic acid (E) destabilizes the helical conformation of the dimeric coiled coils pushing the conformation towards a random coiled coil state. In another report26 the group also showed that the protonation of glutamic acid increases the stability of the coiled coils and that as the number of glutamic acid residues that are protonated increases the dimer shifts from a less stable to a more stable form. Since DisCCs were found to be enriched in glutamic acids, the dimer formation of such a coiled coil might be triggered by protonation of the residue which in turn is caused by the change in the pH (low).
Coiled coils are known to be highly versatile motifs despite their simple architecture. It was first discovered as a structural feature of alpha-keratin.27 Coiled coils are found particularly enriched in “skeletal proteins” and “motor proteins”.
Skeletal proteins
Skeletal proteins are the mechanical basis of the cytoskeleton. In eukaryotic cells the filamentous cytoskeleton is comprised of microtubules, actin containing microfilaments and intermediate filaments. These are often associated with motility and cell division.The actin cytoskeleton and lamellipodium were found to be significantly enriched in DisCCs. Actin cytoskeletal proteins are known to play an integral role in cell shape determination, motility, cytokinesis and cell–cell or cell–matrix interactions. Along with myosins the actin cytoskeletal disordered set consists of angiomotin, aniline, coronin-1A, espin and other proteins involved in mechanical integrity of cell, adhesion and motility. Similarly, lamellipodium is essential for motility, membrane domain organization, substrate adhesion and phagocytosis.28 These processes involve a high level of regulation, recruitment and organization with one protein interacting with multiple partners which is a forte of IDRs.
Our analysis shows that cellular component kinetochore–microtubules comprising CLASP proteins have IDRs which lie outside the coiled coil motif (DOCC). These proteins have two homologs in humans—CLASP1 and CLASP2—which interact with clip proteins and few others involved in stabilization of the microtubules.29 They are associated with microtubule plus-end binding and regulate the affinity of kinetochore–microtubule attachments by decreasing the frictional drag.30 We found that these CLASP proteins are highly disordered except for the C-terminal coiled coil domain. This region in CLASP1 is involved in localization of kinetochore and in CLASP2 it is required for cortical localization. These coiled-coil regions are well conserved in the two homologs and hence might contribute to functionality of the complex.
In contrast, coiled coils associated with the laminin and fibrinogen complex were found to be enriched in OCCs. The proteins represented in these subsets are laminin subunit alpha 1, 2, 3 and beta 3 and fibrinogen like protein 1 and fibroleukin. These are primarily extracellular components which require the proteins to be soluble. A recent review supports the argument that IDRs are more represented in intracellular proteins as compared to the extracellular ones.31
Motor proteins
Cytoskeletal motor proteins are comprised of three major classes—kinesin, myosin and dynein.Kinesins are known as the courier machinery which is responsible for transport of a variety of cargos including intermediate filaments, mRNA, signaling molecules, membranous organelles etc.32 They are functional as heterodimers which have a globular head connected by a neck-linker which in turn is connected to the coiled coil domain followed by the cargo binding domain. There are two models proposed for the mechanism of kinesin walking. One is the inchworm model and the other is the hand-over-hand model. The former postulates that the stalk does not rotate during the step and one head always leads to the other33 while in the latter model the neck-linker region transits its conformation in such a way that the two heads alternate in the lead. This difference in the proposed mechanism can be due to the influence of position and degree of disorder in the neck-linker and coiled-coil region on the walking of the complex.
Another class of motor proteins—myosins—were found to be enriched in DisCCs. Most of these proteins are involved in microfilament motor activities and regulation. Myosins play a key role in muscular contraction which is itself a highly dynamic process and hence depends on flexible coiled coils. The myosins present in this set bear ATP, nucleotide and calmodulin binding domains. Non-muscle myosin II which were present in the disordered coiled coil set are associated with cell migration and cytokinesis and function by converting chemical energy to force using conformational switching techniques,34 hence we speculate that the coiled coils involved in these dynamic processes tend to favor disorder.
Contrary to kinesins and myosins, dynein complexes were predicted to lack significant length of IDRs and hence were categorized as ordered coiled coil proteins. Dynein is structurally composed of an ATPase domain, the microtubule-binding domain and separating the two is the anti-parallel coiled coil stalk domain.35 The stalk region is highly conserved and only an optimal length is found across the family. It has been reported that stalks that are too long or short hamper the proper functioning of dynein by hindering the packing of heads close to each other.36,37 ATP hydrolysis is known to bring about a conformational change in the head domain and the coiled coil domain acts as a rigid lever arm.2 A recent study suggests that ATP-induced motion produces a sliding motion in coiled coils which in turn helps the complex move along the cytoskeletal track.38 Our study supports this observation by providing insight into the lack of ability of the dynein coiled coils to bring about large conformational changes and use a relatively subtle sliding motion.
Association of disordered coiled coils with diseases and host–pathogen interactions
Coiled coils are crucial for host–pathogen interactions. Mycobacterium tuberculosis is known to survive and replicate in macrophages dodging the innate immune system of the host. Coronin 1 (also known as p57 or TACO), which is one of the proteins of the DisCC set, is known to mediate survival of mycobacteria in the host cell39 and is associated with phagosomes containing live bacterium. These proteins tend to aid survival of the pathogen as mycobacteria fails to survive in Kupffer cells where coronin is not expressed.40,41 But despite aiding the pathogen in surviving in the macrophages, it has been retained by the cells possibly because the protein is involved in multifunctional regulation of cytoskeleton, membrane trafficking and calcium signaling. This protein utilizes the coiled-coil region to form a stable trimer which is essential for its functionality. The proteins bearing a disordered region are well capable of interacting with multiple partners. We would suggest that experimentally studying point mutations in the disordered region of the coiled coil (424–439) in coronin 1A (UniProt id P31146) may provide insight into dynamic functioning of this coiled coil protein.Similarly, DisCCs bearing mutations were found to be associated with diseases like amyloidosis type 8, Joubert syndrome type 8, congenital fibrosis of extraocular muscles type 1, hepatocellular carcinoma etc. (Table 1). Intrinsically disordered proteins are known to be associated with amyloid related disorders.42 We found that mutations in fibrinogen α protein (FIBA) are associated with amyloidosis type 8. The mutations reported are in residue numbers 545 and 574 and exhibit substitution of disorder favoring (Glu and Arg)43 to order favoring residues (Val and Leu respectively).43 This suggests that a decrease in the flexibility of the coiled coil may result in improper functioning of the protein and hence might be associated with the diseased state. The molecular basis of this disease is still under exploration and the current finding can help in getting new insight into the mechanism of action of this protein in normal and diseased states.
Conclusion
Coiled coils are reported to favor intrinsic disorder but our study suggests this is not universally true. We have categorized coiled coils into three categories—one bearing the disordered coiled coils, another bearing the ordered coiled coils and the last one bearing a disordered region not overlapping with the coiled-coil region. The gene ontology analysis of the three categories highlighted significantly enriched classes of cellular components, functions and biological processes. It was found that DisCCs are enriched in nuclear and cytoplasmic components while OCCs are enriched in extracellular and membrane associated complexes. The sequence level analysis showed that disordered coiled-coil regions bear a high proportion of low-complexity regions as compared to ordered coiled coils. The former also has a higher probability of forming a dimer as compared to the ordered counterpart. Our study suggests that mapping of disorder in skeletal and motor proteins can be used to explain the mode of functionality of several proteins like kinesins, extracellular proteins like laminin subunits, fibroleukins, espin, myosins etc. Coiled coils are not only important for the cellular integrity and function but are also crucial in host innate response and survival of pathogens. We suggest a mutational experiment of coronin 1A protein in the disordered coiled-coil region ranging from residues 424–439 to study its effect on functionality of the protein and calcium signaling. We propose that the impact of these mutations on the flexibility of the coiled-coil region can play an important role in understanding the dysfunction of proteins associated with diseases viz. amyloidosis type 8, Joubert syndrome type 8, congenital fibrosis of extraocular muscles type 1, hepatocellular carcinoma etc.Abbreviations
| IDP | Intrinsically disordered proteins |
| DisCC | Disordered coiled coil |
| DOCC | Disorder outside coiled coil |
| OCC | Ordered coiled coil |
| AOCC | All ordered coiled coil |
| LCR | Low-complexity region |
Acknowledgements
M.A. would like to acknowledge Indian Council of Medical Research (ICMR) for the funding support. The authors would also like to sincerely thank Dr G. P. S. Raghava for his valuable comments and discussion in structuring the manuscript. D.D. would like to thank Prof. Keith Dunker for his input.References
- E. H. Bromley, K. Channon, E. Moutevelis and D. N. Woolfson, Peptide and protein building blocks for synthetic biology: from programming biomolecules to self-organized biomolecular systems, ACS Chem. Biol., 2008, 3(1), 38–50 CrossRef CAS.
- A. Lupas, Coiled coils: new structures and new functions, Trends Biochem. Sci., 1996, 21(10), 375–382 CAS.
- H. R. Bosshard, E. Durr, T. Hitz and I. Jelesarov, Energetics of coiled coil folding: the nature of the transition states, Biochemistry, 2001, 40(12), 3544–3552 CrossRef CAS.
- J. K. Myers and T. G. Oas, Reinterpretation of GCN4-p1 folding kinetics: partial helix formation precedes dimerization in coiled coil folding, J. Mol. Biol., 1999, 289(2), 205–209 CrossRef CAS.
- W. Weissenhorn, A. Dessen, S. C. Harrison, J. J. Skehel and D. C. Wiley, Atomic structure of the ectodomain from HIV-1 gp41, Nature, 1997, 387(6631), 426–430 CrossRef CAS.
- J. J. Skehel and D. C. Wiley, Coiled coils in both intracellular vesicle and viral membrane fusion, Cell (Cambridge, Mass.), 1998, 95(7), 871–874 CAS.
- P. Barth, A. Schoeffler and T. Alber, Targeting metastable coiled-coil domains by computational design, J. Am. Chem. Soc., 2008, 130(36), 12038–12044 CrossRef CAS.
- V. N. Uversky, J. R. Gillespie and A. L. Fink, Why are “natively unfolded” proteins unstructured under physiologic conditions?, Proteins, 2000, 41(3), 415–427 CrossRef CAS.
- T. Makino, H. Morii, T. Shimizu, F. Arisaka, Y. Kato and K. Nagata, et al. Reversible and irreversible coiled coils in the stalk domain of ncd motor protein, Biochemistry, 2007, 46(33), 9523–9532 CrossRef CAS.
- M. Larzabal, E. C. Mercado, D. A. Vilte, H. Salazar-Gonzalez, A. Cataldi and F. Navarro-Garcia, Designed coiled-coil peptides inhibit the type three secretion system of enteropathogenic Escherichia coli, PLoS One, 2010, 5(2), e9046 Search PubMed.
- The Universal Protein Resource (UniProt) in 2010, Nucleic Acids Res., 2010, 38(Database issue), D142–D148.
- R. Apweiler, A. Bairoch, C. H. Wu, W. C. Barker, B. Boeckmann and S. Ferro, et al. UniProt: the Universal Protein knowledgebase, Nucleic Acids Res., 2004, 32(Database issue), D115–D119 CrossRef CAS.
- Z. Dosztanyi, V. Csizmok, P. Tompa and I. Simon, IUPred: web server for the prediction of intrinsically unstructured regions of proteins based on estimated energy content, Bioinformatics, 2005, 21(16), 3433–3434 CrossRef CAS.
- J. Cheng, M. Sweredoski and P. Baldi, Accurate Prediction of Protein Disordered Regions by Mining Protein Structure Data, Data Min. Knowl. Discovery, 2005, 11(3), 213–222 CrossRef.
- Q. Zheng and X. J. Wang, GOEAST: a web-based software toolkit for Gene Ontology enrichment analysis, Nucleic Acids Res., 2008, 36(Web Server issue), W358–W363 CrossRef CAS.
- H. B. Rao, F. Zhu, G. B. Yang, Z. R. Li and Y. Z. Chen, Update of PROFEAT: a web server for computing structural and physicochemical features of proteins and peptides from amino acid sequence, Nucleic Acids Res., 2011, 39(Web Server issue), W385–W390 CrossRef CAS.
- E. Wolf, P. S. Kim and B. Berger, MultiCoil: a program for predicting two- and three-stranded coiled coils, Protein Sci., 1997, 6(6), 1179–1189 CrossRef CAS.
- J. C. Wootton and S. Federhen, Analysis of compositionally biased regions in sequence databases, Methods Enzymol., 1996, 266, 554–571 CAS.
- D. Chandran, F. T. Bergmann and H. M. Sauro, TinkerCell: modular CAD tool for synthetic biology, J. Biol. Eng., 2009, 3, 19 CrossRef.
- A. W. Reinke, R. A. Grant and A. E. Keating, A Synthetic Coiled-Coil Interactome Provides Heterospecific Modules for Molecular Engineering, J. Am. Chem. Soc., 2010, 132(17), 6025–6031 CrossRef CAS.
- A. Yoshizumi, J. M. Fletcher, Z. Yu, A. Persikov, G. J. Bartlett and A. L. Boyle, et al. Designed coiled coils promote folding of a recombinant bacterial collagen, J. Biol. Chem., 2011, 286, 17512–17520 CrossRef CAS.
- M. H. Robson and A. Kros, Self-assembly of coiled coils in synthetic biology: inspiration and progress, Angew. Chem., Int. Ed., 2010, 49(17), 2988–3005 CrossRef.
- P. Romero, Z. Obradovic and A. K. Dunker, Folding minimal sequences: the lower bound for sequence complexity of globular proteins, FEBS Lett., 1999, 462(3), 363–367 CrossRef CAS.
- P. B. Harbury, T. Zhang, P. S. Kim and T. Alber, A switch between two-, three-, and four-stranded coiled coils in GCN4 leucine zipper mutants, Science, 1993, 262(5138), 1401–1407 CAS.
- W. D. Kohn, C. M. Kay and R. S. Hodges, Protein destabilization by electrostatic repulsions in the two-stranded alpha-helical coiled-coil/leucine zipper, Protein Sci., 1995, 4(2), 237–250 CrossRef CAS.
- W. D. Kohn, O. D. Monera, C. M. Kay and R. S. Hodges, The effects of interhelical electrostatic repulsions between glutamic acid residues in controlling the dimerization and stability of two-stranded alpha-helical coiled-coils, J. Biol. Chem., 1995, 270(43), 25495–25506 CrossRef CAS.
- I. MacArthur, STRUCTURE OF alpha-KERATIN, Nature, 1943, 152, 38–41 CrossRef CAS.
- J. V. Small, T. Stradal, E. Vignal and K. Rottner, The lamellipodium: where motility begins, Trends Cell Biol., 2002, 12(3), 112–120 CrossRef CAS.
- H. Maiato, E. A. Fairley, C. L. Rieder, J. R. Swedlow, C. E. Sunkel and W. C. Earnshaw, Human CLASP1 is an outer kinetochore component that regulates spindle microtubule dynamics, Cell (Cambridge, Mass.), 2003, 113(7), 891–904 CAS.
- I. Matos, A. J. Pereira, M. Lince-Faria, L. A. Cameron, E. D. Salmon and H. Maiato, Synchronizing chromosome segregation by flux-dependent force equalization at kinetochores, J. Cell Biol., 2009, 186(1), 11–26 CrossRef CAS.
- K. Nishikawa, Natively unfolded proteins: An overview, Biophysics, 2009, 5, 53–58 CrossRef CAS.
- L. S. Goldstein and A. V. Philp, The road less traveled: emerging principles of kinesin motor utilization, Annu. Rev. Cell Dev. Biol., 1999, 15, 141–183 CrossRef CAS.
- W. Hua, J. Chung and J. Gelles, Distinguishing inchworm and hand-over-hand processive kinesin movement by neck rotation measurements, Science, 2002, 295(5556), 844–848 CrossRef CAS.
- O. Pylypenko and A. M. Houdusse, Essential “ankle” in the myosin lever arm, Proc. Natl. Acad. Sci. U. S. A., 2011, 108(1), 5–6 CrossRef CAS.
- M. A. Gee, J. E. Heuser and R. B. Vallee, An extended microtubule-binding structure within the dynein motor domain, Nature, 1997, 390(6660), 636–639 CrossRef CAS.
- M. Samso, M. Radermacher, J. Frank and M. P. Koonce, Structural characterization of a dynein motor domain, J. Mol. Biol., 1998, 276(5), 927–937 CrossRef CAS.
- R. B. Vallee and M. A. Gee, Make room for dynein, Trends Cell Biol., 1998, 8(12), 490–494 CrossRef CAS.
- T. Kon, K. Imamula, A. J. Roberts, R. Ohkura, P. J. Knight and I. R. Gibbons, et al. Helix sliding in the stalk coiled coil of dynein couples ATPase and microtubule binding, Nat. Struct. Mol. Biol., 2009, 16(3), 325–333 CAS.
- R. Jayachandran, V. Sundaramurthy, B. Combaluzier, P. Mueller, H. Korf and K. Huygen, et al. Survival of Mycobacteria in Macrophages Is Mediated by Coronin 1-Dependent Activation of Calcineurin [Abstract], Cell (Cambridge, Mass.), 2007, 130(1), 37–50 CAS.
- V. Sundaramurthy and J. Pieters, Interactions of pathogenic mycobacteria with host macrophages, Microbes Infect., 2007, 9(14–15), 1671–1679 CrossRef CAS.
- J. M. Solomon, G. S. Leung and R. R. Isberg, Intracellular replication of Mycobacterium marinum within Dictyostelium discoideum: efficient replication in the absence of host coronin, Infect. Immun., 2003, 71(6), 3578–3586 CrossRef CAS.
- V. N. Uversky, C. J. Oldfield and A. K. Dunker, Intrinsically disordered proteins in human diseases: introducing the D2 concept, Annu. Rev. Biophys., 2008, 37, 215–246 CrossRef CAS.
- R. M. Williams, Z. Obradovi, V. Mathura, W. Braun, E. C. Garner and J. Young, et al. The protein non-folding problem: amino acid determinants of intrinsic order and disorder, Pac. Symp. Biocomput., 2001, 89–100 CAS.
Footnotes |
| † Published as part of a Molecular BioSystems themed issue on Intrinsically Disordered Proteins: Guest Editor M. Madan Babu. |
| ‡ Electronic supplementary information (ESI) available. See DOI: 10.1039/c1mb05210a |
| This journal is © The Royal Society of Chemistry 2012 |
