Compact and autonomous multiwavelength microanalyzer for in-line and in situ colorimetric determinations
Zaira M.
da Rocha
a,
Cynthia S.
Martinez-Cisneros
*b,
Antonio C.
Seabra
a,
Francisco
Valdés
c,
Mario R.
Gongora-Rubio
d and
Julian
Alonso-Chamarro
b
aLaboratório de Sistemas Integráveis, Universidade de São Paulo, São Paulo, Brazil
bGrup de Sensors i Biosensors, Universitat Autònoma de Barcelona, Bellaterra, Spain. E-mail: cynthia.martinez@uab.es; Fax: +34 935812379; Tel: +34 935811836
cInstituto Tecnológico de la Laguna, Torreón, Mexico
dInstituto de Pesquisas Tecnológicas de São Paulo, São Paulo, Brazil
First published on 3rd November 2011
Abstract
Nowadays, the attainment of microsystems that integrate most of the stages involved in an analytical process has raised an enormous interest in several research fields. This approach provides experimental set-ups of increased robustness and reliability, which simplify their application to in-line and continuous biomedical and environmental monitoring. In this work, a novel, compact and autonomous microanalyzer aimed at multiwavelength colorimetric determinations is presented. It integrates the microfluidics (a three-dimensional mixer and a 25 mm length “Z-shape” optical flow-cell), a highly versatile multiwavelength optical detection system and the associated electronics for signal processing and drive, all in the same device. The flexibility provided by its design allows the microanalyzer to be operated either in single fixed mode to provide a dedicated photometer or in multiple wavelength mode to obtain discrete pseudospectra. To increase its reliability, automate its operation and allow it to work under unattended conditions, a multicommutation sub-system was developed and integrated with the experimental set-up. The device was initially evaluated in the absence of chemical reactions using four acidochromic dyes and later applied to determine some key environmental parameters such as phenol index, chromium(VI) and nitrite ions. Results were comparable with those obtained with commercial instrumentation and allowed to demonstrate the versatility of the proposed microanalyzer as an autonomous and portable device able to be applied to other analytical methodologies based on colorimetric determinations.
Introduction
Nowadays, the increasing need of highly integrated analyzers able to provide in situ and in-time information has promoted the research interest lately observed in the field of Micro Total Analysis Systems (μTAS). Several miniaturized, robust and high performance analyzers for in situ and continuous monitoring of key chemical parameters in environmental and biomedical applications have been reported.1–5 In this sense, simple, inexpensive and miniaturized devices able to produce rapid and reproducible analysis are frequently obtained by the combination of flow injection analysis techniques and spectrophotometric-based methodologies (i.e. colorimetric techniques). Nevertheless, the inherent sensitivity reduction of the optical pathway and the complexity involved for the integration of the different platforms associated with miniaturized colorimetric analyzers have provoked a modular design for this type of devices. Modular colorimetric analyzers most frequently found in the literature operate as dedicated photometers using light emitting diodes (LEDs) as emitters and low-cost detectors such as photodiodes,1 phototransistors2 or reverse-biased LEDs.3Analyzers based on LEDs and photodetectors are compact, robust and permit the use of several wavelengths. Indeed, the simultaneous application of several LEDs yields more versatile detection systems that allow the elimination of some relevant interference during measurements such as turbidity and Schlieren effects, among others. However, the integration of multiple light emitters/detectors increases complexity and, consequently, the final cost of the devices. Fonseca and Raimundo4 proposed a photometer based on an array of eight LEDs that was successfully applied to the simultaneous determination of Zn(II) and Cu(II) in pharmaceutical and metal alloy samples. However, since the device was in a modular configuration, its dimensions and complex experimental set-up rendered it unfit for in-field applications.
Analytical instruments designed for in-field applications not only require high versatility and the possibility of measuring several parameters either simultaneously or sequentially, but also require high reliability, portability, robustness, stability and the capability to work autonomously during long periods of time under unattended conditions.5 The miniaturization of continuous flow analyzers, using the proper technology, permits the integration of several stages of the analytical process in a single microfluidic device. These stages might include sampling, pretreatment, separation, reaction and detection, among others. This approach provides several advantages including low reagent and power consumption, reduced reaction time, portability for in situ applications, low-cost, self-calibration capabilities (i.e. multicommutation-based systems), versatile designs and a great potential for automation.6
Until now, numerous wavelength colorimetric microanalyzers based on different materials and fabrication technologies have been proposed. Analytical devices from the micrometre to the centimetre scale are usually fabricated using substrate materials such as silicon, glass and polymers with their associated fabrication technologies.7,8 In spite of the well-established fabrication processes, high precision and reliability offered by these technologies, highly integrated devices based on them frequently imply high costs and complex and lengthy fabrication processes.
Recently, low-temperature co-fired ceramics (LTCC), commonly used for electronic purposes, have been applied as novel substrates to fabricate microsystems. Unlike silicon, glass and polymers, this technology requires no clean rooms, allows the easy development of complex three-dimensional structures with perfect sealing between layers and involves reduced prototyping times. Regarding analytical applications, its chemical and physical properties allow us to obtain robust and reusable devices provided with long lifetimes. Moreover, since this technology is perfectly compatible with screen-printing techniques, electronics can be easily integrated with microfluidic platforms in the same substrate, allowing the procurement of highly integrated devices.8
Some applications of the LTCC technology regarding the fabrication of modular colorimetric microanalyzers have been presented. Most of them involve the integration of a glass window onto the microfluidic device for its later coupling to an optical detection system usually based on a LED and a photodetector. Modular microanalyzers with these features include devices that have been used to determine chromium(VI)9 and cobalt(II)10 in water and urea in milk.11 Other modular colorimetric microanalyzers use optical fibers as an intermediate stage to the optical detection system.12,13 Most of these devices include the electronics associated with the optical detection system control and signal processing in a separate module. In spite of the reasonable integration level achieved for some of them, there is not a compact multiwavelength microanalyzer reported in the literature that integrates all the platforms required for high performance measurements based on colorimetric techniques. The use of modular microanalyzers reduces the robustness of the experimental set-up and the reproducibility of the analysis since the coupling between modules can introduce instability (frayed cables, false contacts, loose connectors, etc.). Therefore, a compact configuration would be preferred.
In the present work, a highly integrated continuous flow multiwavelength microanalyzer for colorimetric determinations is presented. It includes the microfluidics (a three-dimensional mixer and a “Z-shape” flow cell with a length of 25 mm), a multiwavelength optical detection system (a LED array and a photodetector) and the associated electronics for signal processing and drive, all in the same device. The flexibility provided by its design allows the microanalyzer to be operated either in single fixed mode to provide a dedicated photometer or in multiple wavelength mode to obtain discrete pseudospectra. To increase its reliability, a multicommutation sub-system was developed and integrated with the experimental set-up to automate the microanalyzer operation, making it able to work under unattended conditions. Using this approach, the microanalyzer was able to calibrate itself by the automatic in-line dilution of a single stock solution containing the analyte. Additionally, sensitivity could be easily adjusted by software through the modification of the sample volume. To demonstrate its capabilities, the multiwavelength and autonomous colorimetric microanalyzer was initially characterized without chemical reactions involved using four acidochromic dyes (methyl orange, methylene blue, bromocresol green and phenol red). Once evaluated in procedures not involving chemical reactions, colorimetric procedures associated with the determination of key environmental parameters such as phenol index, chromium(VI) and nitrite ions were also tested.
Experimental
Materials and equipment
DuPont 951 green tapes were used as a substrate for the fabrication of the compact multiwavelength microanalyzer, including electronics and fluidics. Electrical conductors were screen-printed using DuPont 6146 for solderable tracks, DuPont 6142D for internal tracks and DuPont 6141 for via filling (DuPont, UK).All the electronic components were carefully selected to improve the system response and reduce noise effects in signals. The electronic platform was based on a PIC18F4550 microcontroller (Microchip Inc., USA). Surface Mount Devices (SMD) were used to reduce the dimensions of the electronics.
The detection system consisted of a LED array of seven ultrabright LED dies (0.25 × 0.25 × 0.25 mm, Cromatek, Brazil) emitting at different wavelengths (420, 480, 515, 565, 592, 630 and 700 nm) and a TCS230 photodetector (TAOS, USA). Results provided by the optical detection system in the proposed microanalyzer were compared with those provided by an Ocean Optics USB4000 spectrophotometer (Ocean Optics, USA).
The continuous flow system set-up consisted of a peristaltic pump (Minipuls 3, Gilson, USA) equipped with flexible Tygon™ pumping tubes connected to the microfluidic inlets through 0.8 mm internal diameter polyethylene tubing. The multicommutation sub-system was based on a set of three-way solenoid valves (161T031, NResearch, Switzerland) controlled by a specially developed software. The user interface for data acquisition and configuration of the microanalyzer resided in a virtual instrument developed in Labview 8.2 (National Instruments, Brazil) running on a personal computer.
As a first approach, the microanalyzer was characterized using different acidochromic dye solutions. For this purpose, methyl orange (MO) 1 mM, methylene blue (MB) 1 mM, bromocresol green (BCG) 1 mM and phenol red (PR) 0.1 mM were prepared as stock solutions. During calibration, solutions at different concentrations were prepared in-line by the automatic serial dilution of the stock solutions using the multicommutation sub-system. These dyes were chosen as test solutions due to the close correspondence between their maximum absorption wavelength and the maximum emission wavelength of some of the LEDs in the optical detection system (MO: 507 nm; MB: 660 nm; BCG: 400 nm; PR: 557 nm).14
To evaluate the capabilities of the proposed microanalyzer in procedures involving chemical reactions, it was applied to determine some key environmental parameters such as phenol index, chromium(VI) and nitrite ions. For this purpose, standard solutions at different concentrations were prepared in-line and automatically from the corresponding analyte stock solution by its automatic serial dilution using the multicommutation sub-system. Colorimetric reagents prepared to determine phenol index, chromium(VI) and nitrite ions were those defined in their corresponding standard methods.15–17
Results and discussion
Microanalyzer fabrication
The general fabrication process for LTCC devices is described in detail elsewhere.8Fig. 1 presents the compact colorimetric multiwavelength microanalyzer developed in this work. The microfluidics were placed on one face (see Fig. 1A) of the microanalyzer and electronics on the opposite face (see Fig. 1B) to prevent any liquid leakage in the microfluidic platform from reaching the electronics. The device included three liquid inlets to increase its versatility, since some complex analyses require the use of several reagents. The three inlets converged in a point downstream before getting into the three-dimensional mixer (see Fig. 1E). The basic geometry of this mixer consisted of an “L-shape” section that turns the fluid through 90° on the same plane.18 The fluid is later guided to an upper/lower plane by means of a 90° rotation angle. The flow cell, placed after the three-dimensional mixer, was designed using a “Z-shape” configuration to define a horizontal channel that allowed obtaining a longer optical pathway when compared with conventional miniaturized analyzers based on colorimetric determinations. Once burnt out, the final flow-cell channel dimensions were 2 mm width, 1.8 mm high and 25.3 mm length (total volume: 91 μL).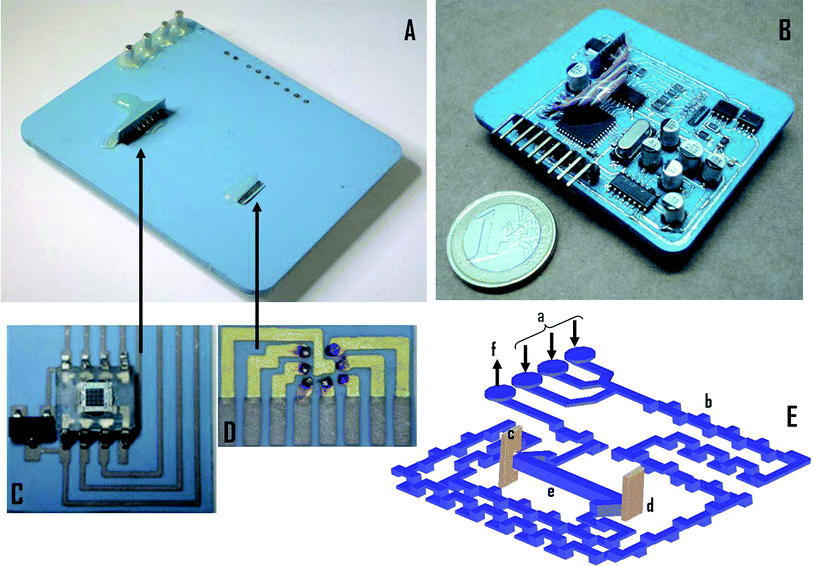 | ||
| Fig. 1 Continuous flow multiwavelength colorimetric microanalyzer (43 × 56 × 3 mm). (A) Top view of the microanalyzer with the microfluidic platform. (B) Bottom view of the microanalyzer with the electronic platform. (C) Photodetector module (13 × 14 × 0.4 mm). (D) LED array module (7 × 4 × 0.4 mm). (E) Internal three dimensional view of the microanalyzer; a: inlets; b: tridimensional mixer; c: photodetector module orthogonally integrated; d: LED array orthogonally integrated; e: flow cell (25 mm length, volume: 91 μl); f: outlet. | ||
The electronic board was designed as two stacked layers connected by electrical vias and considering the ceramics shrinkage of about 12.7% in the x–y plane and 15% in the z axis. All the electronic SMD elements were iron soldered once the device was burnt out. The electronic platform was covered with a polymeric layer of polydimethylsiloxane (PDMS Sylgard® 184, DACHS-KONDEL International S.A., Spain) to protect it from any liquid leakage and other harsh environments. To increase the reliability of the optical detection system, the LED array and the photodetector were mounted in separate modules, using LTCC materials, and later assembled to the body of the microanalyzer (see Fig. 1C and D). Using this approach, they can be replaced if needed without having to rebuild the whole microanalyzer.
The seven ultrabright LEDs were attached to a LTCC substrate that included the corresponding pads (see Fig. 1D) using an adhesive die attach epoxy. The bonding pads were electrically connected to the predefined pads on the LTCC substrate by wire bonding (Wedge Bond 7400, West Bond, USA). Once bonded, the LED array was encapsulated using epoxy resin (Araldite® CY 248 BR Ren HY 956, Maxiepoxi, Brazil). The photodetector was also integrated on a separate module that included a diode for overcurrent protection (see Fig. 1C). Both the photodetector and the LED assemblies were orthogonally tied to two predefined cavities in the body of the microanalyzer as depicted in Fig. 1. This integration was carefully performed to obtain the optimum optical alignment between elements, maximizing light measurements.
Electronics of the microanalyzer
The electronic platform, based on the PIC18F4550,19 was designed for data acquisition, signal processing and control of the optical detection system (LEDs and photodetector). The microcontroller was in continuous communication with a personal computer sending and receiving data through its serial port. The on-board program for setting up and controlling the device was developed in C++ language and later loaded to the microcontroller. This program enabled the user to interact with the microanalyzer through a virtual instrument developed as interface. The interface was designed to be simple so it could be operated by any inexperienced user. On the computer, the user can define the emission wavelength (420, 480, 515, 565, 592, 630 and 700 nm), the filter applied by the photodetector, the output-scaling factor and the sampling frequency, whenever needed. In addition, the electronic configuration allows defining a pulsed or continuous operation of the LEDs, depending on the application. The on-board reprogramming possibility of the microanalyzer increases its versatility and autonomy, since its operation can be modified to fit different analytical applications based on colorimetric determinations at different wavelengths (i.e.phosphate, nitrite, chromium, zinc, copper and chloride among others).4,20,21During measuring, the user can follow the system response on the screen and store the data generated in text files for later processing if required. Absorbance is calculated by the microcontroller using the following equation:22
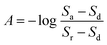 | (1) |
As previously mentioned, the optical detection system is based on a TCS230 photodetector and a LED array of seven elements. The photodetector consists of a programmable color light-to-frequency converter that integrates clear, red, green, and blue (RGB) filters in the same die. It provides a 12-bit resolution and a configurable output-scaling factor (100%, 20%, 2% or power-down).23 In spite of the great advantages provided by the photodetector, it presents some temperature dependence, which could produce considerable variations on the baseline with a drift inversely proportional to temperature changes. To determine the temperature influence on the photodetector, a preliminary test was performed. It was attached to an aluminium support and warmed up from 15 to 40 °C in increments of 0.5 °C per minute. A commercial LED was used as light source to illuminate the photodetector using air as medium. The signal presented variations in the order of 0.7% per °C. In addition, a decrease of the signal to noise ratio was also observed while temperature increased. To overcome this issue and taking advantage of the high thermal conductivity of LTCC materials, a temperature sensor (LM35, National Semiconductor, USA) was included in the photodetector module to offset temperature effects on it through software. Regarding the light emission array, since the seven LEDs emitted at different wavelengths and had different current–voltage characteristics, a normalization procedure was applied using the USB4000 spectrophotometer. This procedure consisted in selecting the optimum resistive value to be serial connected to each LED in order to normalize their light intensity.
Considering all the elements in the optical detection system, up to twenty-eight emission/filter wavelength combinations can be configured. The optimum match allows reducing sample interferences during measurements and increases the microanalyzer versatility to fit specific chemical reactions.
Multicommutation sub-system
According to current needs, applications regarding unattended in-line monitoring of environmental parameters in both static and mobile automatic stations require autonomous microanalyzers with self-calibrating capabilities. To provide the multiwavelength microanalyzer with the aforementioned features, a multicommutation sub-system is proposed and validated. One of the main potentialities of multicommutation is the establishment of a tandem stream where a number of different miscible solutions are introduced into the manifold by rapidly and sequentially switching the commutators (computer controlled valves). This unique stream can be seen as a set of neighboring solution slugs that undergo fast mixing while being transported through the analytical path.24 Following this approach, the multicommutation sub-system coupled to the microanalyzer was configured to prepare the standard solutions required for the calibration processes automatically and in-line from a single stock solution containing the analyte. Once calibrated, the microanalyzer was able to perform the automatic analysis of external samples without user interaction. The highest commutation speed available for the solenoid valves used in this work was found to be 100 ms. Multicommuted systems have been also proposed to implement controlled dilutions in order to extend the working concentration range (sensitivity), avoiding outlier samples. This feature may be considerably interesting when real samples at high concentrations require to be diluted in order to be analyzed in field applications, for example.Moreover, the use of multicommutation-based manifolds allows implementing sequential/simultaneous determinations, sample stopping for slow reactions, titrations, in-line analyte concentration/separation, etc.25 The use of multicommutation to automate analytical devices yields more reliable, flexible, accurate and reproducible analytical information with minimum user interaction, since some of the most important experimental parameters can be electronically controlled by software.
This approach significantly simplifies analytical methodologies where unstable samples/reagents are involved in order to obtain reliable results. Several applications focused on the advantages and potentialities offered by the application of multicommutation systems have been widely exposed in the literature.24,25
Experimental set-up
Taking advantage of the versatility provided by the microcontroller, the microanalyzer can be configured to operate either in single or multiple wavelength modes. The operation mode is determined by the software loaded to the microcontroller. In single fixed wavelength mode, the microanalyzer turns on a LED (in pulsed or continuous mode) and a photodetector filter (clear, RGB) through the virtual instrument. When the multiple wavelength mode is used, all LEDs in the array are automatically and sequentially turned on/off to generate a discrete pseudospectrum of the analyte under study. Due to the nature of the studies performed in this work, the photodetector was configured to work using its clear filter for all the experiments, since no significant difference was observed when other filters were applied. Nevertheless, the inclusion of such filters could be useful when discrimination processes among radiations at different wavelengths are required to avoid potential interferences.The microanalyzer was evaluated using the experimental set-up presented in Fig. 2. It was initially tested using acidochromic dyes and later evaluated in real applications involving colorimetric chemical reactions required to determine environmental parameters with significant relevance. In the later case, the baseline signal was obtained keeping both solenoid valves (V1 and V2) in their normally open position. In this way, MilliQ water flows through inlet 1 and mixes with the corresponding colorimetric reagents entering through inlets 2 and 3. For acidochromic dyes, inlets 2 and 3 were not used, since no additional colorimetric reagent was required. When valve V1 switched, a solution containing the sample/dye solution entered the microanalyzer during an electronically controlled prefixed time, defining an injection volume. This solution passed through the three-dimensional mixer (mixing with the colorimetric reagents, in the case of chemical reaction) and reached the flow cell, generating a transient signal that was registered by the optical detection system.
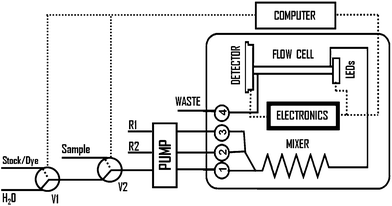 | ||
| Fig. 2 Block diagram of the experimental set-up used for the multiwavelength microanalyzer evaluation; V: solenoid valve, R: reagents. | ||
The multicommutation sub-system was programmed to perform controlled in-line dilutions to automate the calibration process using a single stock solution containing the analyte from which all the standard solutions were prepared. This approach simplifies the final experimental manifold and avoids the user interaction, which increases the system reproducibility and accuracy. For this purpose, the solenoid valve V1 was configured to switch between water and dye/stock solution. Diluent aliquots (MilliQ water) were interspersed in tandem with the dye/stock plugs. Each sample/diluent volumetric ratio corresponded to a desired concentration. Therefore, the calibration procedure was performed, in all cases, by the automatic in-line preparation of solutions containing different dye/analyte concentrations. The evaluation of the microanalyzer when applied to procedures involving chemical reactions was performed for three key environmental parameters: phenol index, chromium(VI) and nitrite ions. To evaluate the performance of the microanalyzer working in the multiple wavelength mode, the analysis of single and mixed acidochromic dyes was performed. When dye mixtures were tested, both dyes at different concentrations were simultaneously propelled through inlets 2 and 3; inlet 1 was not used during this study.
Microfluidic hydrodynamic parameters optimization
Different hydrodynamic parameters affect the analytical response of the microanalyzer. Some of them such as those including its passive microfluidic structures (length and configuration of both mixer and flow cell) were predefined during its fabrication and cannot be modified unless rebuilt. Nevertheless, other parameters such as injection volume and flow rate can be adjusted to optimize the microanalyzer performance.The optimization procedure was performed using acidochromic dyes as a first approach. The injection volume was studied in the range from 300 to 900 μL, keeping the flow rate at 1.5 mL min−1 in all cases and using a 5 μM methylene blue solution as sample. After optimization, a 500 μL injection volume was chosen since it provided a compromise between signal intensity and sample consumption. The flow rate was evaluated in the range from 0.5 to 2.0 mL min−1. Although higher flow rates reduce analysis time, repeatability increases at lower values since more data are acquired at the same sampling frequency in the electronic system and this in turn provides more precision to the final absorbance measurement. Moreover, the use of high flow rates increases reagent consumption, which is an undesirable condition when continuous in-line monitoring applications are required. After evaluation, the flow rate was fixed at 1 mL min−1. However, if a more drastic reduction of reagents consumption and maintenance costs is required, lower flow rates may be used without involving the loss of analytical features (i.e. in-field applications).
Similar procedures were performed to determine the optimum hydrodynamic parameters to measure phenol index, chromium(VI), and nitrite ions.
Microanalyzer evaluation in procedures not involving chemical reactions
The intrinsic response of the microanalyzer working in single fixed wavelength mode was initially evaluated in procedures where chemical reactions were not involved. For this purpose, four acidochromic dye solutions whose maximum absorption wavelengths closely matched with those emitted by the LEDs were used: methylene blue (MB), methyl orange (MO), bromocresol green (BCG) and phenol red (PR).In all cases, flow rate and injection volume were fixed at 1.0 mL min−1 and 500 μL, respectively. For each acidochromic dye, a calibration procedure was performed using standard solutions automatically prepared by the in-line dilution of a stock solution using the multicommutation sub-system. Table 1 summarizes results obtained after each calibration procedure. Even though the light sources used in the LED array of the microanalyzer are not monochromatic, results obtained presented quasi-linear responses with a correlation coefficient close to the unity in all cases. This responds to the proximity between the maximum absorbance wavelengths of the acidochromic dyes selected and the corresponding emission wavelengths of the LED used for each evaluation. Moreover, the flow-cell configuration allowed the microanalyzer to achieve a low limit of detection for all dyes, since a longer optical pathway was obtained (25 mm) when compared with conventional microsystems.
| Dye/μM | Calibration equation | % RSD | LED emission λ/nm |
|---|---|---|---|
| MB [1–10] | A = 0.0246(±0.0008)[MB] + 0.0017(±0.0014); r2 = 0.998 | 1.74% [10 μM] | 630 |
| MO [1–10] | A = 0.0341(±0.0001)[MO] + 0.0260(±0.0007); r2 = 0.984 | 0.85% [10 μM] | 490 |
| BCG [5–25] | A = 0.0117(±0.0001)[BCG] + 0.0271(±0.0007); r2 = 0.996 | 1.38% [10 μM] | 590 |
| PR [0.5–2.5] | A = 0.0700(±0.0003)[PR] + 0.0038(±0.0006); r2 = 0.996 | 1.96% [2.5 μM] | 565 |
To demonstrate the microanalyzer versatility, it was configured to operate in multiple wavelength mode to generate discrete pseudospectra. In this operational mode, the device automatically turns on/off all the LEDs sequentially and measures absorbance values at the wavelengths provided by each one. Before any discrete spectrum is obtained, the microanalyzer is calibrated. During calibration, it collects intensity values corresponding to each LED, using water as sample, and uses them as reference (Sr). Then, the measurement procedure begins by obtaining the Sa values from the sample in the flow cell. The absorbance values are calculated using eqn (1). Since the microanalyzer and the USB4000 spectrophotometer used different flow-cell lengths, 25 mm and 10 mm, respectively, values obtained with the microanalyzer were normalized for comparison purposes.
To demonstrate its capability to provide discrete pseudospectra of single analytes, the microanalyzer was applied to measure MB, MO, PR and BCG at different concentrations using all the wavelengths available in the LED array. Fig. 3 shows signals obtained using the microanalyzer (discrete points) and the USB4000 (continuous line) for MO determination. The agreement observed between signals obtained using both approaches demonstrates the microanalyzer capability to provide estimated pseudospectra very approximate to those provided by commercial instrumentation.
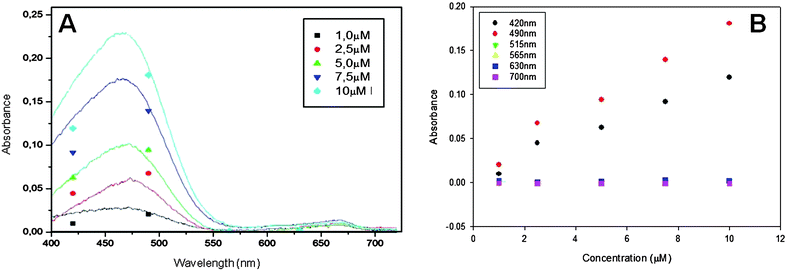 | ||
| Fig. 3 Microanalyzer response to different concentrations of MO. (A) Discrete spectra obtained with the microanalyzer (single points) and USB4000 (continuous lines). (B) Calibration plots obtained using the seven elements of the LED array. | ||
The possibility to make multiparametric determinations with the proposed microanalyzer in samples containing analytes with absorbance peaks sufficiently apart was also explored. For this purpose, it was used in multiple wavelength mode to analyze mixtures of MB and MO at different concentrations. In this case, each dye was propelled at the same flow rate through inlets 2 and 3, respectively, inlet 1 was not used. They were mixed in the mixer and the resulting solution was analyzed in the detection flow cell. Results obtained using the proposed microanalyzer were similar to those provided by the USB4000 spectrophotometer.
Signals corresponding to the characteristic absorption bands of each colorant were discriminated properly in mixed solutions containing both dyes at different concentration levels. However, better correspondence would have been attained if more LEDs were incorporated in the array. In the worst experimental situation, according to the concentration ratio between dyes in the mixed solution, the maximum error was found to be 15%.
Exploiting the possibility to measure sequentially at two or more wavelengths, the microanalyzer proposed could eliminate interference problems caused by turbidity and/or by refraction index variations in complex sample matrices. This feature would be useful when applied to real samples in field applications. Flow injection analyses are characterized by the formation of concentration gradients, caused by the dispersion of the sample in the carrier stream.26 When reagents and samples present clearly differentiated refraction index or viscosity, spurious signals produced by the denominated Schlieren effect may appear, affecting the analytical signals.27,28 An instrumental procedure to compensate Schlieren effects consists in measuring the sample at two wavelengths.27,29 One of them matches the working wavelength and the other can be performed at a wavelength where no analyte absorption occurs. In this procedure, we assume that both measurements are equally affected by the Schlieren effect and the molecular absorption process must occur only for one of them.
Microanalyzer evaluation for in-line environmental monitoring
The response of the proposed multiwavelength microanalyzer, operating in a single fixed wavelength mode, was also evaluated in procedures involving chemical reactions. To demonstrate its potential, it was applied to the automatic determination of several parameters with significant environmental relevance. For this purpose, the hydrodynamic parameters, the optical detection system and the multicommutation sub-system were optimized and configured to fit each measurement methodology.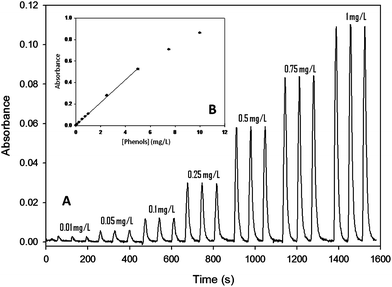 | ||
| Fig. 4 (A) Microanalyzer response for a concentration range from 0.01 to 1 mg L−1 of phenols. (B) Calibration plot for a range from 0.01 to 10 mg L−1. | ||
The system response for this analyte can be observed in Fig. 5A. A linear range was obtained from 0.01 to 5 mg L−1 (see Fig. 5B). The equation that describes the linear range is A = (0.339 ± 0.007)[Cr6+] + 0.012(±0.006); r2 = 0.998. For a concentration of 0.5 mg L−1, the RSD calculated was 2.07% (n = 17; 95% confidence). To demonstrate the reproducibility of the microanalyzer, signals used to calculate the RSD% are graphically presented in Fig. 5C. Similar graphs were obtained for phenols and nitrite ions.
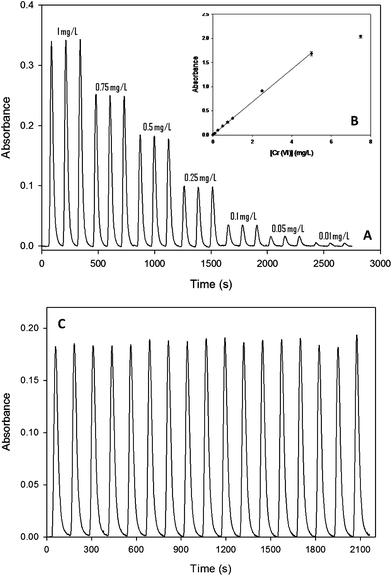 | ||
| Fig. 5 (A) Microanalyzer response when applied to chromium(VI) ion determination in the range from 0.01 to 1 mg L−1. (B) Calibration plot covering the whole range tested (0.01–7.5 mg L−1). (C) Reproducibility of the microanalyzer response (0.5 mg L−1). | ||
Under these operational conditions a sampling throughput of about 30 samples per hour and a reagent consumption of 2 mL·sample−1 were obtained.
Flow rates for reagents/carrier/sample solutions and sampling time were set at 1 mL min−1 and 18 s (300 μL), respectively. The experimental set-up was similar to the one previously described for phenols and chromium(VI). Samples ranging from 0.05 to 10 mg L−1 were automatically prepared in-line and analyzed. Fig. 6 presents the microanalyzer response. A linear range was obtained from 0.05 to 7.5 mg L−1. The equation that describes the linear range for this analyte is A = 0.160(±0.001)[NO2−] + (0.014 ± 0.002); r2 = 0.998. For a concentration of 0.1 mg L−1, the RSD obtained was 4.1% (n = 13; 95% confidence). In this case, a sampling throughput of about 24 samples per hour and a reagent consumption of 2.5 mL·sample−1 were obtained.
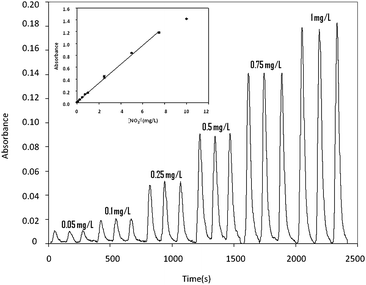 | ||
| Fig. 6 (A) Microanalyzer response for a nitrite ion concentration range from 0.05 to 1 mg L−1. (B) Calibration plot obtained for a range from 0.05 to 10 mg L−1. | ||
Lower or higher working ranges can be obtained using the same microanalyzer by simply adjusting the sample time in the multicommutation sub-system, which is easily performed by software. For nitrite determination, for example, a linear response in a range between 0.01 and 0.1 mg L−1 with enhanced sensitivity was obtained increasing the sampling time from 18 s to 24 s in the multicommutation sub-system. The equation that described the linear range in this case was: A = 0.029(±0.002)[NO2−] + (0.0005 ± 0.0001); r2 = 0.996.
Conclusions
A novel compact and autonomous multiwavelength microanalyzer aimed at colorimetric determinations was developed using the Low Temperature Co-fired Ceramics (LTCC) technology. Its autonomous, compact, low-cost and robust design allows its application to in situ and continuous determination of several key environmental parameters in both static and mobile automatic alert stations.The microanalyzer presented an integration and automation level not previously reported for devices developed for similar applications. Further work is under development to monolithically integrate sample pre-treatment stages that permit determining analytes that cannot be directly detected, at the present time, using colorimetric methodologies (i.e.nitrate ions).
Acknowledgements
The authors would like to acknowledge the Spanish, Brazilian and Mexican authorities and agencies for its financial support through projects CTQ2009-12128, CONSOLIDER INGENIO2010-CSD2006-12, PHB2010-0064-PC (MEC-CAPES) and P509AC0376 (CYTED). Z. M. da Rocha would like to acknowledge FAPESP (Project 2009/08486-4) for financial support. The authors would also like to acknowledge Met-Mex Peñoles for financial support.Notes and references
- F. R. P. Rocha and B. F. Reis, Anal. Chim. Acta, 2000, 409, 227–235 CrossRef CAS.
- M. O'Toole, K. T. Lau and D. Diamond, Talanta, 2005, 66, 1340–1344 CrossRef CAS.
- K. T. Lau, S. Baldwin, M. O'Toole, R. Shepherd, W. J. Yerazunis, S. Izuo, S. Ueyama and D. Diamond, Anal. Chim. Acta, 2006, 557, 111–116 CrossRef CAS.
- A. Fonseca and I. M. Raimundo, Jr, Anal. Chim. Acta, 2004, 522, 223–229 CrossRef CAS.
- D. Diamond, K. T. Lau, S. Brady and J. Cleary, Talanta, 2008, 75, 606–612 CrossRef CAS.
- C. S. Martínez-Cisneros, N. Ibáñez-García, F. Valdes and J. Alonso, Anal. Chem., 2007, 79, 8376–8380 CrossRef.
- K. Huikko, R. Kostiainen and T. Kotiaho, Eur. J. Pharm. Sci., 2003, 20, 149–171 CrossRef CAS.
- N. Ibáñez-García, C. S. Martínez-Cisneros, F. Valdes and J. Alonso, TrAC, Trends Anal. Chem., 2008, 27, 24–33 CrossRef.
- R. Alves-Segundo, N. Ibañez-Garcia, M. Baeza, M. Puyol and J. Alonso-Chamarro, Microchim. Acta, 2011, 172, 225–232 CrossRef CAS.
- N. Ibanez-Garcia, M. Puyol, C. M. Azevedo, C. S. Martinez-Cisneros, F. Villuendas, M. R. Gongora-Rubio, A. C. Seabra and J. Alonso, Anal. Chem., 2008, 80, 5320–5324 CrossRef CAS.
- W. T. Suarez, O. D. Pessoa-Neto, V. B. Dos Santos, A. R. D. Nogueira, R. C. Faria, O. Fatibello, M. Puyol and J. Alonso, Anal. Bioanal. Chem., 2010, 398, 1525–1533 CrossRef CAS.
- L. J. Golonka, H. Roguszczak, T. Zawanda, J. Radojewski, I. Grabowska, M. Chudy, A. Dybko, Z. Brzozka and D. Stadnik, Sens. Actuators, B, 2005, 111, 396–402 CrossRef.
- L. J. Golonka, T. Zawada, J. Radojewski, H. Roguszczak and M. Stefanow, Int. J. Appl. Ceram. Technol., 2006, 3, 150–156 CrossRef CAS.
- F. J. Green, The Sigma-Aldrich Handbook of Stains, Dyes and Indicators, 2nd Printing, M Sigma-Aldrich Corporation, USA, 1991 Search PubMed.
- APHA, Standard Methods for the Examination of Water and Wastewater, 5530-total phenolics, American Public Health Association, Washington, DC, 20th edn, 1998 Search PubMed.
- APHA, Standard Methods for the Examination of Water and Wastewater, 3500-Cr B, American Public Health Association, Washington, D.C, 20th edn, 2001 Search PubMed.
- APHA, Standard Methods for the Examination of Water and Wastewater, 4500-NO2− B Colorimetric Method, American Public Health Association, Washington, D.C, 21st edn, 2005 Search PubMed.
- N. Ibañez-Garcia, R. D. M. Gonçalves, Z. M. da Rocha, M. R. Gongora-Rubio, A. C. Seabra and J. Alonso-Chamarro, Sens. Actuators, B, 2006, 118, 67–72 CrossRef.
- PIC18F2455/2550/4455/4550 Data Sheet, Microchip Technology Inc., 2004 Search PubMed.
- C. K. Pires, B. F. Reis, A. Morales-Rubio and M. De La Guardia, Talanta, 2007, 72, 1370–1377 CrossRef CAS.
- A. Fonseca, I. M. Raimundo, J. J. R. Rokwedder and L. O. S. Ferreira, Anal. Chim. Acta, 2007, 603, 159–166 CrossRef CAS.
- T. S. Yeh and S. S. Tseng, J. Chin. Chem. Soc. (Taipei, Taiwan), 2006, 53, 1067–1072 CAS.
- Datasheet TCS230. Programmable Color Light to frequency Converter, TAOS Search PubMed.
- F. R. P. Rocha, B. F. Reis, E. A. G. Zagatto, J. L. F. C. Lima, R. A. S. Lapa and J. L. M. Santos, Anal. Chim. Acta, 2002, 468, 119–131 CrossRef CAS.
- M. A. Feres, P. R. Fortes, E. A. G. Zagatto, J. L. M. Santos and J. L. F. C. Lima, Anal. Chim. Acta, 2008, 618, 1–17 CrossRef CAS.
- F. R. P. Rocha and J. A. Nóbrega, J. Braz. Chem. Soc., 1997, 8, 625–629 CrossRef CAS.
- E. A. G. Zagatto, M. A. Z. Arruda, A. O. Jacintho and I. L. Matos, Anal. Chim. Acta, 1990, 234, 153–160 CrossRef CAS.
- F. R. P. Rocha and J. A. Nóbrega, Quim. Nova, 1996, 19, 636–640 CAS.
- E. A. G. Zagatto, B. F. Reis, M. Martinelli, F. J. Krug, F. H. Bergamin and M. F. Giné, Anal. Chim. Acta, 1987, 198, 153–163 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
