Simultaneous high speed optical and impedance analysis of single particles with a microfluidic cytometer
David
Barat†
a,
Daniel
Spencer†
a,
Giuseppe
Benazzi
a,
Matthew Charles
Mowlem
b and
Hywel
Morgan
*a
aSchool of Electronics and Computer Science, University of Southampton, Highfield, Southampton, SO17 1BJ, UK. E-mail: hm@ecs.soton.ac.uk
bNational Oceanography Centre, University of Southampton Waterfront Campus, European Way, Southampton, S014 3ZH, UK
First published on 3rd November 2011
Abstract
We describe a microfluidic cytometer that performs simultaneous optical and electrical characterisation of particles. The microfluidic chip measures side scattered light, signal extinction and fluorescence using integrated optical fibres coupled to photomultiplier tubes. The channel is 80 μm high and 200 μm wide, and made from SU-8 patterned and sandwiched between glass substrates. Particles were focused into the analysis region using 1-D hydrodynamic focusing and typical particle velocities were 0.1 ms−1. Excitation light is coupled into the detection channel with an optical fibre and focused into the channel using an integrated compound air lens. The electrical impedance of particles is measured at 1 MHz using micro-electrodes fabricated on the channel top and bottom. This data is used to accurately size the particles. The system is characterised using a range of different sized polystyrene beads (fluorescent and non-fluorescent). Single and mixed populations of beads were measured and the data compared with a conventional flow cytometer.
Introduction
Flow cytometry is a well known technique that is used to count and analyse single particles suspended in fluid at high speed. The technology has many applications in clinical diagnostics, biochemistry, biology and environmental sensing. In commercial devices, particles flow at high speed through an optical detection region where they are illuminated by one or more laser beams providing scatter and fluorescent signals.1,2 Small angle forward scattered light (FSC) is used to size particles, whilst internal properties, (granularity, viability) are determined from larger angle side scattered light (SSC).1,2 Particles can also be sized according to their electric properties, as in the “Coulter counter”.1 In the Coulter counter, a small orifice divides two compartments and particles suspended in an electrolyte flow through this orifice. When an electric field is applied between the two compartments the current path through the orifice is perturbed by a particle. Because cells behave as insulators at low frequencies, the current is blocked and this change is used to count and size particles.Many commercial machines use a combination of both optical and electrical techniques to measure cells and other particles. These systems are complex and are capable of extremely high throughputs; up to 105cells per second.1 However, they are expensive and bulky, and are unsuitable for applications such as point of care diagnosis or in situ analysis. In an attempt to miniaturise the functionality of a flow cytometer, miniaturised Lab-on-Chip (LoC) systems have been developed; for recent reviews see.3–6 Chip-based devices have a number of advantages - they can process extremely small sample volumes (tens of μLs) containing low numbers of particles while maintaining high statistical accuracy. They offer the possibility of integrating sophisticated sample handling and detection modalities, within a single chip, allowing the development of novel assays and measurement systems. Miniature flow cytometers were first demonstrated by Kamentsky in the mid-1960s.7 Over the last decade a number of different microfabricated flow cytometers have been described, mostly optical. Early devices demonstrated the principle of miniaturisation, but the sensitivity and particle throughput was very poor. Miniaturisation and integration of optical components into LoC devices is an area of considerable activity,8 and important to the development of miniature flow cytometer systems. Efficient coupling and focusing of light into a microfluidic channel is a challenge and generally an optical fibre or waveguide is used; many different designs have been published.9–17
One of the first attempts at integration was by Kruger et al.,9 who implemented a leaky waveguide and an avalanche photodiode into a microfluidic channel. A holographic diffraction grating was used to collect light; they demonstrated fluorescence detection of beads. Wang et al.10 fabricated a device with integrated waveguides and a lens that focused the light into the centre of a channel. They used scattered light to count and discriminate beads, at speeds of up to 25 beads per second. Sheath flow was used to centre the sample stream in the optical detection region. This was one of the first papers to demonstrate integrated optical elements in a micro-flow cytometer. Tung et al.11 used solid-state lasers and PIN photodiodes with lock-in amplification. Using microgrooves for fibre alignment, they were able to guide incident light and collect fluorescence from a detection region. They demonstrated two-colour fluorescence detection (440 nm and 635 nm) and multi-angle detection (45°, 135° and 180°), measuring fluorescently labelled yeast cells at a rate of up to 500 particles per second.
Simple waveguides are generally lossy and a number of techniques have been developed to improve them. Using an SU-8 core and PDMS cladding, Mogensen et al.12 produced waveguides with a propagation loss of about 4.5 dB cm−1 and 1.4 dB cm−1 at 532 nm and 633 nm respectively. Bliss et al.13 launched light into a device using liquid-core/liquid-cladding waveguide (L2 waveguide). The incident light was launched through a fibre-to-waveguide coupler propagating into an L2 waveguide. Although these waveguides have low propagation losses, the overall losses are significant due to the number of interfaces. An improvement uses integrated wavelength-selective optical waveguides.14 Using a long length liquid-core waveguide (PDMS and glycerol) doped with dye molecules they demonstrated a detection waveguide which selectively absorbs the excitation light (532 nm), with propagation losses of 120 dB cm−1 at this wavelength, but only 4.4 dB cm−1 at 633nm. However this system is limited by the auto-fluorescence of the dyes, adding noise to the detection. Another technique is an antiresonant reflecting optical waveguide, ARROW. Bernini et al.15 demonstrated a micro flow cytometer using silicon integrated hollow core ARROW. The excitation light and sample fluid travel co-linearly through the detection region. Fluorescence is collected using two orthogonal fibres and by adding an L2 waveguide,16 they focused the incident light vertically and horizontally. However, the fluidic part was complex and no data on cell/particle analysis presented. Lee et al.17 described a micro flow cytometer with buried SU-8/SOG optical waveguides with propagation losses of 4 dB cm−1 at 633 nm. They used an etched single-mode optical fibre to couple the incident light into the waveguide. Integration of micro-lenses can improve focusing of incident light into the detection region. Compound micro-lenses collect and focus light without optical aberration but these lenses must be precisely fabricated and aligned in order to minimise optical aberration and achieve precise focusing along the optical axis. Multimode fibres have also been used to collect light in miniature cytometers. These fibres have a larger diameter core than single mode fibres so that they collect more light; device fabrication is somewhat simpler too. Examples of the use of such fibres for miniature micro-machined cytometers can be found in the work of Ligler et al.,18–20
Various impedance cytometers have also been developed and these show promise for high-speed analysis of particles based upon size and dielectric properties - for a review see.21 With single frequency excitation, micro-cytometers can count and size particles, but multi frequency measurements have been used for discrimination of cells.22,23 In conventional cytometry, particle diameter is measured from the FSC signal, but this parameter is difficult to implement in a miniaturised system. Impedance analysis offers an attractive alternative for particle volume determination. The technology is somewhat simpler to miniaturise than optical systems since the device only requires pairs of aligned microelectrodes fabricated within a microfluidic channel.
To maximise the functionality of a miniature particle analysis system, we have developed a micro-cytometer that can simultaneously measure the optical and electrical properties of single particles. Incident light is launched through an integrated compound lens.24 The system measures side scattered light using optical fibres inserted in grooves, and fluorescence light with fibres placed orthogonal to the excitation light. Micro-electrodes simultaneously measure the low-frequency electrical impedance (volume) of the particles.22,25 The complete system is characterised by measuring a range of different sized beads (fluorescent and non-fluorescent) and the data compared with a conventional flow cytometer.
System overview
Fig. 1(a) is a schematic diagram of the micro-cytometer chip, showing 1-D hydrodynamic sample focusing, the sample detection region, and the layout of the optics. Also shown are the impedance micro electrodes. Particles are hydrodynamically focused into the middle of the channel, passing through the detection region where their electrical and optical properties are measured.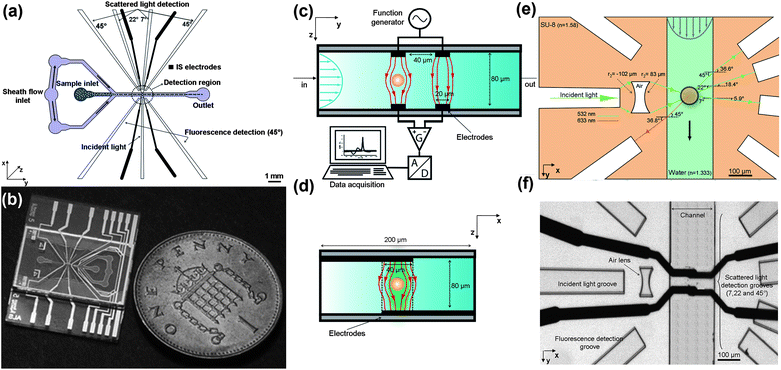 | ||
| Fig. 1 Overview of the cytometer chip and details of the optical and electronic setup. (a): Schematic diagram showing 1-D hydrodynamic focusing, micro-electrodes for impedance spectroscopy and the different grooves for holding the fibres (incident light, scattered light and fluorescence). (b): Photograph of a chip fabricated from glass and SU-8. (c): Cross section of the detection area showing the microelectrodes and the setup for impedance spectroscopy. (d): View of the electrodes as seen along the microfluidic channel demonstrating how 1-D hydrodynamic focusing centres the particles in the electric field. (e): Details of the optical design, showing the compound air lens, the light collection fibres and the optical paths of the detection region, (the refractive index of SU-8 and water are at 532 nm). (f): Photograph of the final microfabricated structure. The two black lines crossing the channels are the microelectrodes which are slightly misaligned. | ||
The microfluidic chips were fabricated using photolithography and full wafer thermal bonding; the fabrication process has been described elsewhere.26,27 Metal electrodes consisting of 200 nm thick platinum with a 20 nm Ti seed layer were fabricated on 100 mm diameter glass wafers by photolithography. Electrodes were 20 μm wide with 40 μm gap. SU-8 resist was used to define the fluidic channel, grooves for the fibres and the air lens. The microfluidic channel is 80 μm high and 200 μm wide in the detection region. SU-8 negative photoresist (Microchem) was spin coated onto the surface of Pyrex wafers over the patterned electrodes, followed by a soft bake. The two SU-8 layers (one on each wafer) were each 40 μm thick, giving a total channel depth of 80 μm, which enabled insertion of the optical fibres. Pairs of wafers were aligned and bonded using a thermo-compression technique to form sealed microfluidic structures. The wafers were designed with alignment marks on each chip to aid accurate alignment, so that the electrodes and channels could be aligned to within 5 μm. Individual chips were released from the bonded wafer pair by dicing, and holes for fluids made using a mechanical drill. Fig. 1(b) shows a photograph of a chip, which is approximately 25 mm wide.
Measurement principle
Fig. 1(c) shows the impedance spectroscopy measurement region, which has been described previously.28 Two pairs of electrodes are fabricated on the top and bottom of the channel, forming a differential measurement system. As shown in Fig. 1(d), the electrodes span the width of the channel (200 μm in the x-direction) and overlap in the centre by 40 μm. This overlap region defines the sample measurement volume, which is 40 μm × 20 μm × 80 μm (high). AC voltages are applied to the upper two electrodes; when a cell passes between the electrodes the current is disturbed and this signal is measured using a lock-in amplifier, and processed to give the impedance. Analysis and discrimination of cells requires two or more frequencies, but in this work, only a single frequency (1 MHz) is used for particle counting and sizing. The impedance data is analysed using custom written software.Details of the optics are shown in Fig. 1(e). A groove in the SU-8 holds a fibre which launches incident light perpendicular to the channel. This light is focused into a sheet across the width of the channel using an air compound lens (r1 = −102 μm, r2 = 83 μm) - for further details see.24 Fluorescence emission is collected with fibres placed in two grooves on the same side as the incident light (at 135°). A 7° fibre was used to measure the optical extinction (EX) signal - light loss due to absorption or scatter out of the field of view of the detector when a particle passes through an incident beam.2 The chip was designed for two more collection fibres to be placed at 22° and 45°, to measure side scattered light (SSC). The data from these two collection fibres was qualitatively similar (for the beads) and therefore only light from the 45° fibre was collected and analysed. Fig. 1(f) is a photograph of the detection region showing the lens, fibre grooves and the two pairs of electrodes (with slight offset due to fabrication).
Materials and methods
Beads/microfluidic chip
Fluidic connections were made by clamping the chip within a block machined from PEEK. The block uses a gasket to seal to the glass chip and has three holes for input, output and focusing fluids delivered by tubes. Tubes with inner diameters of 0.3, 0.5 and 0.8 mm for sample, focusing and output fluids respectively were used. The connector block was mounted on a PCB with impedance detection circuitry and the entire assembly mounted on an x–y–z stage on an optical bench above a bespoke confocal microscope.26 The following beads were used: fluorescent 7.32 μm beads (Bangs Laboratories), non-fluorescent 10 μm beads and fluorescent 15 μm beads (Invitrogen); fluorescent 10 μm beads and non-fluorescent 20 μm and 25 μm beads (Polysciences) and fluorescent 31 μm beads (Duke Scientific Corp). For the single population experiments, 15 μm and 31 μm fluorescent beads were used, with excitation wavelength of 532 nm and emission at 580 nm. The concentration of each sample was approximately 120 beads per μL. Beads were suspended in PBS with 11% sucrose (for neutral density), and 0.1% food dye to visualise the sample stream. The volumetric flow rate was typically 50 μL min−1. The sheath flow and sample flow were withdrawn through the channel using a syringe pump connected to the output port. The ratio of sample to flow stream was controlled by adjusting the relative heights of the sample and sheath reservoirs to produce a sample width ratio of approximately 1 to 10.Optical system
Light from a 532 nm, 20 mW solid-state laser was launched into the incident light fibre using free space optics. All the fibres were multi-mode fibres (Polymicro Technologies), held in the SU-8 grooves using refractive index matching optical glue (NOA73, Norland). The detection fibres were coupled to photomultipliers (Hamamatsu Photonics Ltd, UK) with band-pass filters for fluorescence light (HQ575/50M, Chroma Technologies) and a notch filter to remove incident light (NF01-532U-25, Semrock). The photomultiplier signals were sampled at 120 kHz with a 16-bit A–D card (NI6034E, National Instruments) and the data captured and analysed with software written in Matlab and LabVIEWImpedance system
A sinusoidal voltage of 1 Vpp at 1 MHz was applied to both pairs of electrodes and the current measured using custom built electronics and a lock-in amplifier (SR844, Stanford Research Instruments). The output from the lock-in consists of the real component (in phase) and imaginary component (90° out of phase) of the impedance. The lock-in outputs were sampled as above, at the same data rate. The low frequency in-phase impedance signal was used to trigger acquisition of the data.Conventional flow cytometry
Conventional flow cytometric analysis of cells was performed using a FACSAria (Becton Dickson) equipped with two lasers: 488 nm solid state (20 mW) and 633 nm HeNe (20 mW). FACSFlow sheath fluid was used and samples flow was at a pressure of 20.3 psi through a 100 μm nozzle. The instrument was controlled by a PC running FACSDiVa software. FACS analysis was performed without the use of sucrose or food dye.Results and discussion
Integrated optics
The optical system was designed to provide collimated light in the channel, with a beam size approximately equal to the largest particle diameter, around 50 μm. Ideally, the light should have little divergence, minimising scatter and overspill into neighbouring collection fibres. The design of the chip was based on that of Wang et al.10 and was designed using the simulation software ZEMAX operating in non-sequential mode. Light from a laser, coupled into an optical fibre, was modelled as an elliptical source. The Gaussian profile of the emitted light was set (in ZEMAX, Gx = Gy = 21.69) to have a distribution matching the numerical aperture of the fibre (NA = 0.22). The ray tracing simulation, shown in Fig. 2(a), indicates that the beam width (FWHM) is around 70 μm as plotted in Fig. 2 (b). Fig. 2 (c) shows a photograph of the light passing through the microfluidic channel. The channel was filled with a solution of fluorescein and the fluorescent emission collected with a microscope. A cross-sectional profile of the light intensity taken in the centre of the channel is shown in Fig. 2(b), together with a Gaussian fit to this profile. The two sharp dips in intensity occur because the impedance electrodes obscure the light in these regions. The experimentally measured FWHM for the beam is approximately 140 μm, twice the simulated value. Further details can be found in.24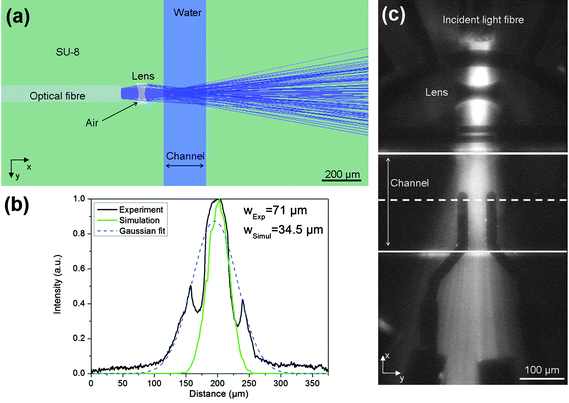 | ||
| Fig. 2 Diagram showing focusing of the incident light across the channel. (a): Ray tracing simulation of the optical system designed in ZEMAX with 100,000 analysis rays and an incident power of 1 W @ 532 nm. (b) Profile of the measured light intensity across the channel width at the midpoint (see image in (c)). Also shown are the simulated results which give a FWHM of 70 μm compared with a measured FWHM of 140 μm. The two sharp dips in measured intensity occur because the electrodes obscure the light in these regions. The detector viewer for the simulation is 400 × 400 μm2 with 150 pixels for X and Y axis. (c) Photographs of the light in the channel imaged using fluorescein solution. The lines indicate the boundaries of the channel and the dotted line is the centre of the channel. The dark areas are the electrodes. | ||
Microfluidic flow cytometry
Two sets of experiments were performed; one to analyse single populations of beads, ranging in size from 7.32 μm to 31 μm diameter. A second set of experiments analysed mixed populations. In each case, the samples were analysed by FACS. No signal could be obtained from the 7° FSC fibre for the smallest beads because the S/N ratio was too low, so this information was discarded.Single populations
Fig. 3 is an example of data for 15 μm diameter fluorescent beads, comparing the micro-cytometer with the FACS. Fig. 3(a) and 3(c) show scatter plots of fluorescence (at 580 nm) and 45° SSC plotted against impedance for the micro-cytometer. Histograms of impedance (a) and SSC (b) are also shown, inset. For comparison, FACS data are shown in Fig. 3(b) and 3(d). The forward scatter signal (FSC-Area) is on the x-axis; FSC histograms (b) and SSC (d) are inset. The FACS FSC-A data shows that the beads have a non-Gaussian size distribution, with a CV of 10%. The impedance signal measures electrical volume whilst FSC is proportional to particle diameter. The CV for the effective diameter calculated from the impedance signal is 11%, comparable to the FACS FSC. The fluorescence values are similar, with a CV of 12.7% from the FACS, and 17.1% from the micro-cytometer (see Table 1). The SSC data (Fig. 3(c) and 3(d)) shows a single approximately Gaussian distribution in both cases. The CV in SSC for the micro-cytometer was again similar at 12%, compared to 13.4% from the FACS. Similar data was obtained for all sizes of beads measured, ranging from 7 μm to 31 μm diameter. The data for impedance, 45°SSC and extinction for all these beads are summarised as histograms in Fig. 4. The extinction signal could not be measured for the smallest beads (7 μm and 10 μm) because of a low SNR. The CV for all these populations, together with flow rates are summarised in Table 1.| Bead diameter (μm) | Events measured | Sample flow rate (μL min−1) | Manufacture's CV (%) | CV size (%) | CV 45° SSC (%) | CV Fluorescence (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Micro-cytometer | FACS | Micro-cytometer | Micro-cytometer (|Z|) | FACS (FSC-A) | Micro-cytometer | FACS (SSC-A) | Micro-cytometer | FACS (PE-A) | ||
| 7 | 1036 | 1419 | 14.2 | 7.2 | 23.4 | 6.8 | 38.4 | 5.9 | ||
| 10 | 1622 | 1539 | 43.4 | 4.1 | 24.4 | 3.9 | 44.8 | 12.3 | ||
| 15 | 804 | 1415 | 29.4 | 6.5 | 10.8 | 10.3 | 12.0 | 13.4 | 17.1 | 12.7 |
| 20 | 1114 | 1652 | 32.8 | 2.4 | 9.4 | 13.3 | 26.7 | 13.5 | ||
| 25 | 3111 | 1195 | 30.6 | 5.9 | 4.4 | 1.7 | 18.8 | 4.9 | ||
| 31 | 822 | 994 | 24.5 | 11.0 | 11.9 | 7.7 | 20.1 | 13.0 | 23.8 | 15.8 |
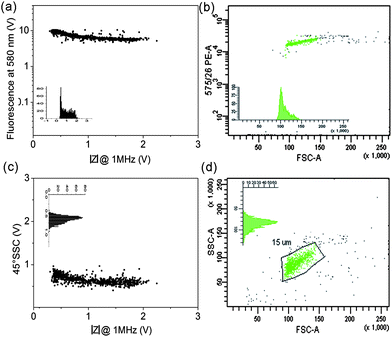 | ||
| Fig. 3 Scatter plots for fluorescent 15 μm beads measured using the micro-cytometer and the BD FACSAria. (a) 580 nm fluorescence vs. impedance (at 1 MHz), inset is a histogram of impedance. (c) 45° Side scatter signal vs. impedance at 1 MHz; inset shows histogram of the 45°SSC. Both data sets are from the micro-cytometer. (b) 575 nm fluorescence signal vs. forward scattered light, signal area (FSC-A) for the FACS, inset shows FSC histogram. (d) 45° side scattered signal vs. FSC-A from the FACS; inset shows SSC histogram. | ||
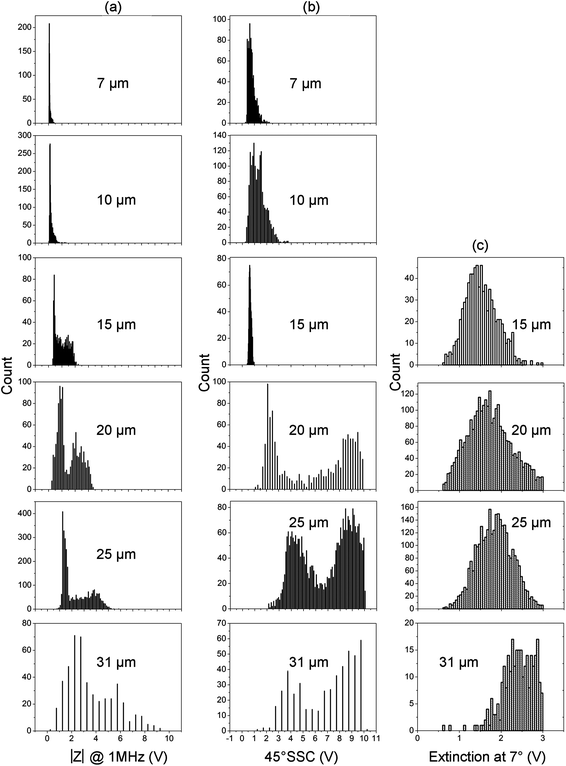 | ||
| Fig. 4 Summary of data obtained with the micro-cytometer for a wide size range of beads from 7 μm to 31 μm diameter. (a) Histograms of impedance, (b) Histograms of the 45° side scatter and (c) extinction at 7°. For particles of 15 μm and above, two distinct populations are observed in the impedance and SSC signals, owing to the onset of inertial focusing. | ||
At the flow rate used in these experiments, two populations were observed in impedance and SSC for beads greater than 15 μm diameter (Fig. 4). Di Carlo et al.29,30 has shown that when particles flow through microfluidic channels at intermediate Reynolds numbers, they experience a combination of counteracting inertial lift forces (wall lift) and shear gradient lift which focuses them into equilibrium positions near the channel walls. Our microfluidic chip has a rectangular cross section, which leads to focusing into two equilibrium positions.25,31,32
The impedance signal from our chip, which has two pairs of overlapping parallel electrodes, depends on the vertical position of the particle in the channel, but not on the horizontal position.25 Particles moving along the bottom of the channel close to the measurement electrodes (see Fig. 1(c)) have a higher impedance signal than those flowing in the top half of the channel (connected to the source). Particles flowing in the centre of the channel have the lowest impedance signal. The particle transit time can be obtained from the impedance signals and since the flow profile across the channel is parabolic, this transit time reflects the distance of the particle from the centre of the channel in the vertical or z-axis (Fig. 1). A density plot of transit time vs. impedance magnitude for 25 μm diameter beads is shown in Fig. 5(a). The data shows that there are two populations of particles, one moving in the top half of the channel (lobe L1) with a small distribution in impedance magnitude, and another in the bottom half of the channel with a much wider distribution (L2). As particles move off centre (along the vertical axis) the impedance signal increases in magnitude but by a different amount in each of the upper and lower halves of the channel. This leads to the tick-shaped distribution shown in Fig. 5(a,b). As particle move off centre, the shape of the impedance-time signal also becomes distorted from the simple double anti-symmetric Gaussian that occurs in the centre. The transit time is calculated using simple peak detection, but as the signal becomes more distorted this method introduces errors and the transit time becomes biased, see ref. 25 for further details. Therefore, although the particles flow with the same speed in the top and bottom halves (lobes L1 and L2), experimentally calculated transit times are skewed. The actual particle transit time is the mean of the two values shown in L1 and L2. The flow rate used in this experiment was 50 μL min−1, equivalent to a mean fluid velocity of 50 mm s−1 and a peak velocity of 120 mm s−1. This equates to a minimum particle transit time of 0.5 ms (60 μm electrode centre to centre spacing), equal to the minimum experimental value determined from the impedance signals - Fig. 5(a).
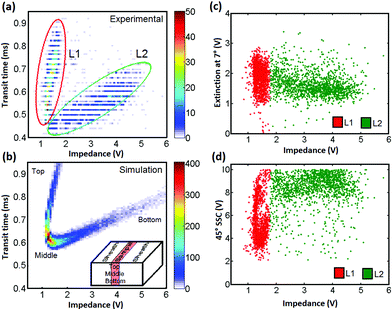 | ||
| Fig. 5 (left) Density plot of transit time vs. impedance magnitude for 25 μm diameter beads. (a) Experimental results, (b) Simulated data. Two populations of particles are observed in the experimental results, one in the top half of the channel (L1) and a second in the bottom half of the channel (L2). Right panel: (c) Extinction, and (d) SSC vs. impedance for the same data as in (a) Each event is colour coded according to its position within one of the two lobes in (a); red top, green bottom of the channel. The extinction data does not depend on position within the channel. The SSC data demonstrates clear top/bottom differentiation. | ||
The distribution in transit times and impedance seen in this figure shows the onset of inertial focusing where particles begin to move from the fastest flow region (channel centre) into the upper and lower halves. Observation of the 1-D hydrodynamically focused particle stream with a microscope indicated that the sample width was approximately 40 μm wide, and flowed in the centre of the channel. Quantitative analysis of the number density of particles in each of the two lobes in Fig. 5(a) shows that the distribution top to bottom is 50/50 with greatest numbers of particles in the centre of the channel, as observed for 1-D hydrodynamic focusing in a rectangular channel.
This data can be compared with simulation – Fig. 5(b). The electric field and impedance signal was simulated using COMSOL, the particle velocity determined from the analytical solution to the Navier Stokes equation33 and the impedance calculated as described previously.25 The simulation was performed assuming viscous flow and assuming a random distribution of particle position in the channel. The combination of a parabolic flow profile together with the random particle distribution means that most of the particles flow through the channel centre (with τ = 0.6 ms). Comparing simulations with data (Fig. 5(a)), demonstrates that at the intermediate Reynolds numbers used in these experiments (≈10) inertial lift forces are beginning to have an effect and the particles are focused away from the centre into two vertical equilibrium positions.
Fig. 5(c, d) shows SSC and extinction data, against impedance for the same beads (25 μm diameter). Each event has been colour coded according to its position within one of the two lobes in Fig. 5(a). The extinction data is independent of position within the channel; particles simply obscure the light path as they move, regardless of z-position. However, the SSC data demonstrates clear top/bottom differentiation. Particles that move along the bottom half of the channel and have a higher impedance also have a higher SSC signal than those with the lower impedance that move in the top half. There are two populations as seen in the histogram in Fig. 4(b), demonstrating that variations in the z-position of particles leads to large changes in the reflected optical signal strength. By contrast the extinction signal has a single wide distribution, and is less dependent on particle position.
Mixed populations
A mixture of beads (10 μm and 15 μm diameter fluorescent; 20 μm and 25 μm diameter plain) in approximately equal numbers was measured to test the capability of the system to distinguish different populations from a mix. The individual populations cannot be identified from the raw data because the positions of the particles in the channel give rise to pairs of distributions. Identification of the individual populations was performed from the impedance signal. The raw impedance signal approximates to two anti-symmetric Gaussians with a precise shape that depends on particle position in the channel. Particles flowing in the top half of the channel have a tight distribution in impedance, whilst those flowing close to the measurement electrodes (Fig. 5(c)) have a much wider distribution in signal magnitude. This information can be used to filter the data, removing events from particles travelling in the bottom half of the channel. This was done as follows: Each single impedance event was fitted to a double Gaussian to determine impedance magnitude and particle speed from the time between each peak.25 The shape of an arbitrary impedance signal can be characterised by a w parameter defined by w = dt/σ where σ is the standard deviation of a Gaussian.31 The w-parameter identifies the shape of the impedance signal and thus the vertical position of the particle within the channel. In the analysis, events with a small w-parameter are deleted, effectively removing beads travelling in the lower half of the channel, reducing the CV of any single population, but at the expense of halving the throughput. Although this mathematical operation discards 50% of the events, it demonstrates the utility of impedance for accurate particle volume measurement. This data processing could be substantially improved. For example, a full simulation of the impedance signals for particles at all positions would provide a template function for signal processing by convolution, which would eliminate the positional dependence and enable all the events to be used. However, such data processing is non-trivial and is beyond the scope of this paper. Alternatively, the electrodes could be moved closer to the inlet to minimise inertial effects, but this would cause other problems particularly correlating impedance and optical signals. The simplest solution would be to implement microfluidic 2-D focusing techniques18–20 to ensure that all particles flow through the centre of the channel.The simultaneously measured electrical (impedance) and optical (SSC and fluorescence) properties of the mixed population of beads after w-filtering is shown in Fig. 6. Approximately 2500 events are plotted; both SSC and fluorescence against impedance (trigger channel). Histograms for each parameter are also shown. The figure shows that the 4 different bead sizes can be distinguished from optical side scatter, but the impedance signal provide much better discrimination between the populations. The fluorescence signal from the 10 μm and 15 μm beads provides easy discrimination in this channel. For comparison, the same mixed population analysed with FACS (fluorescence vs. forward scatter) is also shown.
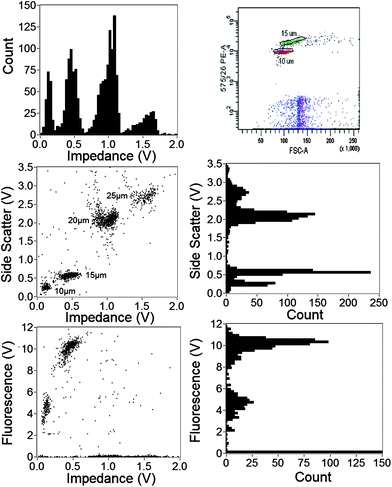 | ||
| Fig. 6 SSC, fluorescence and impedance data from a mixture of different beads (10, 15, 20 and 25 μm diameter) after w-filtering. Also shown is FACS fluorescence data for the same mixture. Particles can be discriminated from either SSC or impedance, as well as fluorescence intensity. | ||
Conclusion
We have demonstrated a microfluidic cytometer that simultaneously measures fluorescence, scattered light and electrical impedance of single particles. Incident light is focused into the chip using an optical fibre coupled to an integrated air lens, and microelectrodes are used to measure particle impedance to give particle volume. The system has been characterized with single and mixed populations of polystyrene beads (plain and fluorescent). The CV of beads measured using the microcytometer are comparable to the manufacturers' and also to values obtained with a BD FACSAria. The SSC was measured using a single fibre placed at 45°, but additional light collecting fibres placed at smaller angles (FSC) could be useful in providing further discrimination of particles, particularly cells. Analysis of the impedance and fluorescence of a mixed population of beads was performed by selective filtering of data, discarding particles travelling in the bottom half of the channel. This crude technique discards 50% of the events, but much more advanced fitting algorithms could be developed to account for and entirely eliminate this positional dependence. Alternatively a 2D hydrodynamic focusing scheme could be incorporated to flow the sample through the centre of the channel. Flowing particles at higher flow rates would also reduce the CV due to inertial focusing effects, but would increase the complexity of fabrication. In summary, we have demonstrated simultaneous impedance, fluorescence and SSC analysis in a microfluidic cytometer with integrated micro-optics. The system clearly differentiates 4 different sized beads (10, 15, 20 and 25 μm) based on either SSC and/or impedance, and identifies fluorescent beads according to their intensities.Acknowledgements
This work is supported by the RMST (Ruggedised MicroSystem Technology for marine measurement) project (EP/E016774/1). We thank Philips Research Cambridge for assistance with the impedance analysis software.References
- H. M. Shapiro, Practical Flow Cytometry, 4th edn, Wiley, 2005 Search PubMed.
- R. C. Sobti and A. Krishan Edts, Advanced flow cytometry: applications in biological research, Springer, 2003 Search PubMed.
- F. S. Ligler, The microflow cytometer, Pan Stanford, Singapore, 2010 Search PubMed.
- S. H. Cho, J. M. Godin, C. H. Chen, W. Qiao, H. Lee and Y. H. Lo, Recent advancements in optofluidic flow cytometer, Biomicrofluidics, 2010, 4, 043001 CrossRef.
- D. A. Ateya, J. S. Erickson, P. B. Howell Jr, L. R. Hilliard, J. P. Golden and F. S. Ligler, The good, the bad, and the tiny: a review of microflow cytometry, Anal. Bioanal. Chem., 2008, 391, 1485–1498 CrossRef CAS.
- D. Huh, W. Gu, Y. Kamotani, J. B. Grotberg and S. Takayama, Microfluidics for flow cytometric analysis of cells and particles, Physiol. Meas., 2005, 26, R73–R98 CrossRef.
- L. A. Kamentsky, M. R. Melamed and H. Derman, Spectrophotometer: new instrument for ultrarapid cell analysis, Science, 1965, 150, 630–631 CAS.
- J. Godin, C. H. Chen, S. H. Cho, W. Qiao, F. Tsai and Y. H. Lo, Microfluidics and photonics for bio-System-on-a-Chip: a review of advancements in technology towards a microfluidic flow cytometry chip, J. Biophotonics, 2008, 1, 355–376 CrossRef CAS.
- J. Krüger, K. Singh, A. O'Neill, C. Jackson, A. Morrison and P. O'Brien, Development of a microfluidic device for fluorescence activated cell sorting, J. Micromech. Microeng., 2002, 12, 486–494 CrossRef.
- Z. Wang, J. El-Ali, M. Engelund, T. Gotsæd, I. R. Perch-Nielsen, K. B. Mogensen, D. Snakenborg, J. P. Kutter and A. Wolff, Measurements of scattered light on a microchip flow cytometer with integrated polymer based optical elements, Lab Chip, 2004, 4, 372–377 RSC.
- Y. C. Tung, M. Zhang, C. T. Lin, K. Kurabayashi and S. J. Skerlos, PDMS-based optofluidic micro flow cytometer with two-color, multi-angle fluorescence detection capability using PIN photodiodes, Sens. Actuators, B, 2004, 98, 356–367 CrossRef.
- K. B. Mogensen, J. El-Ali, A. Wolff and J. P. Kutter, Integration of polymer waveguides for optical detection in microfabricated chemical analysis systems, Appl. Opt., 2003, 42, 4072–4079 CrossRef CAS.
- C. L. Bliss, J. N. McMullin and C. J. Backhouse, Rapid fabrication of a microfluidic device with integrated optical waveguides for DNA fragment analysis, Lab Chip, 2007, 7, 1280–1287 RSC.
- C. L. Bliss, J. N. McMullin and C. J. Backhouse, Integrated wavelength-selective optical waveguides for microfluidic-based laser-induced fluorescence detection, Lab Chip, 2008, 8, 143–151 RSC.
- R. Bernini, E. De Nuccio, F. Brescia, A. Minardo, L. Zeni, P. M. Sarro, R. Palumbo and M. R. Scarfi, Development and characterization of an integrated silicon micro flow cytometer, Anal. Bioanal. Chem., 2006, 386, 1267–1272 CrossRef CAS.
- R. Bernini, E. De Nuccio, A. Minardo, L. Zeni and P. M. Sarro, Liquid-core/liquid-cladding integrated silicon arrow waveguides, Opt. Commun., 2008, 281, 2062–2066 CrossRef CAS.
- G.-B. Lee, C.-H. Lin and G.-L. Chang, Micro Flow Cytometers Integrated with Buried SU-8/SOG Optical Waveguides, Sens. Actuators, A, 2003, 103, 165–170 CrossRef.
- J. P. Golden, J. S. Kim, J. S. Erickson, L. R. Hillard, P. B. Howell, G. P. Anderson, M. Nasir and F. S. Ligler, Multi-wavelength microflow cytometer using groove-generated sheath flow, Lab Chip, 2009, 9(13), 1942–1950 RSC.
- J. S. Kim, G. P. Anderson, J. S. Erickson, J. P. Golden, M. Nasir and F. S. Ligler, Multiplexed detection of bacteria and toxins using a microflow cytometer, Anal. Chem., 2009, 81(13), 5426–5432 CrossRef CAS.
- A. L. Thangawng, J. S. Kim, J. P. Golden, G. P. Anderson, K. L. Robertson, V. Low and F. S. Ligler, A hard microflow cytometer using groove-generated sheath flow for multiplexed bead and cell assays, Anal. Bioanal. Chem., 2010, 398, 1871–1881 CrossRef CAS.
- T. Sun and H. Morgan, Single cell microfluidic impedance cytometry: a review, Microfluid. Nanofluid., 2010, 8, 423–443 CrossRef CAS.
- D. Holmes, D. Pettigrew, C. H. Reccius, J. D. Gwyer, C. van Berkel, J. Holloway, D. E. Davies and H. Morgan, Leukocyte analysis and differentiation using high speed microfluidic single cell impedance cytometry, Lab Chip, 2009, 9, 2881–2889 RSC.
- G. Mernier, W. Hasenkamp, N. Piacentini and P. Renaud, Multiple-frequency impedance measurements in continuous flow for automated evaluation of yeast cell lysis, Sens. Actuators, B, 2010 DOI:10.1016/j.snb.2010.10.050.
- D. Barat, G. Benazzi, M. C. Mowlem, J. M. Ruano and H. Morgan, Design, simulation and characterisation of integrated optics for a microfabricated flow cytometer, Opt. Commun., 2010, 233, 1987–1992 CrossRef.
- D. Spencer and H. Morgan, Positional dependence of particles in microfludic impedance cytometry, Lab Chip, 2011, 11, 1234–1239 RSC.
- D. Holmes, J. K. She, P. L. Roach and H. Morgan, Bead-based immunoassays using a micro-chip flow cytometer, Lab Chip, 2007, 7, 1048–1056 RSC.
- J. M. Ruano-Lopez, M. Aguirregabiria, M. Tijero, M. T. Arroyo, J. Elizalde, J. Berganzo, I. Aranburu, F. J. Blanco and K. Mayora, A new SU-8 process to integrate buried waveguides and sealed microchannels for a Lab-on-a-Chip, Sens. Actuators, B, 2006, 114, 542–551 CrossRef.
- C. Bernabini, D. Holmes and H. Morgan, Micro-impedance cytometry for detection and analysis of micron-sized particles and bacteria, Lab Chip, 2011, 11, 407–412 RSC.
- D. Di Carlo, J. F. Edd, K. J. Humphry, H. A. Stone and M. Toner, Particle segregation and dynamics in confined flows, Phys. Rev. Lett., 2009, 102, 094503 CrossRef.
- D. Di Carlo, Inertial microfluidics, Lab Chip, 2009, 9, 3038–3046 RSC.
- R. M. Pugo, S. C. Deane, C. Glasse, M. R. Burcher, H. Morgan and C. H. Reccius, Flow speed particle focusing in microfluidic impedance measurements, Microtass conference, Groningen, October 2–9 (2010) Search PubMed.
- S. C. Hur, H. T. K. Tse and D. Di Carlo, Sheathless inertial cell ordering for extreme throughput flow cytometry, Lab Chip, 2010, 10, 274–280 RSC.
- R. Thomas, H. Morgan and N. Green, Negative DEP traps for single cell immobilisation, Lab Chip, 2009, 9, 1534–1540 RSC.
Footnote |
| † These authors contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2012 |
