Altering surface energy of nanocrystalline diamond to control osteoblast responses
Lei
Yang
a,
Yawen
Li
c,
Brian W.
Sheldon
a and
Thomas J.
Webster
*ab
aSchool of Engineering, Brown University, Providence, RI 02912, USA. E-mail: yleibrown@gmail.com; Brian_Sheldon@brown.edu
bDepartment of Orthopaedics, Brown University, Providence, RI 02912, USA. E-mail: Thomas_Webster@brown.edu; Fax: +401-863-9107; Tel: +401-863-2318
cCollege of Engineering, Lawrence Technological University, Southfield, MI 48075, USA
First published on 12th October 2011
Abstract
The use of wear-resistant nanocrystalline diamond (NCD) for orthopedic and other medical device applications has received increasing interest as of late. This has led to the exploration of NCD surface modification methods to both resist and promote tissue-forming cell functions, and to further mechanistically understand such biological responses. This study represents one in a series of studies aimed to elucidate a facile and efficient method to alter NCD surface chemistry and surface free energy, and to further understand the correlation between osteoblast functions and these surface properties. Here, direct plasma treatment was used to modify NCD films with H2, O2 and NH3 gases. Surface functionalization of NCD films after the various plasma treatments were determined by X-ray photoelectron spectroscopy (XPS) and the NCD surface free energy generated by such chemical terminations were calculated by contact angle measurements. Results indicated that the plasma treatments effectively altered NCD surface chemistry and surface free energy, and a monotonic correlation between surface free energy and the hydrophilicity of the NCD films was established. Most importantly, osteoblast (bone forming cell) responses (including short-term attachment and long-term differentiation from non-calcium depositing to calcium depositing cells) on NCD of various surface free energies were evaluated, and a strong correlation between all osteoblast responses and the surface free energy of NCD was discovered for the first time. In particular, NCD which was treated with NH3 plasma had the highest free energy and exhibited the best osteoblast responses, whereas the worst osteoblast responses was found on the lowest surface free energy NCD surface which was treated by H2 plasma. A competitive mechanism of cell aggregate cohesivity and cell-substrate adhesivity is discussed to explain the surface free energy-dependent osteoblast functions on NCD. The results of this study provide an important understanding of osteoblast functions as mediated by NCD surface free energy which could be controlled by various chemical terminations. Eventually, this understanding can guide the design and fabrication of NCD for numerous biomedical applications.
Introduction
Due to its extremely high mechanical strength, wear resistance, chemical stability, biocompatibility properties and extraordinary electrical (field emission) properties, nanostructured diamond has rapidly emerged as a new biomaterial spanning from therapeutic, diagnostic to bioanalytical applications.1 Specifically, nanocrystalline diamond (NCD) thin films or coatings produced by inexpensive chemical-vapor-deposition (CVD) methods have been recently studied as robust bio-interfaces to bond bio-molecules with high selectivity and stability,2,3 promote bone tissue regeneration,4,5 induce and regulate neural stem cell differentiation,6 improve mesenchymal stem cell (MSC) adhesion and proliferation,7 and reduce thrombotic reactions.8Pivotal for these studies is to understand the interactions between material properties (mostly, NCD surface properties) and cells or bio-molecules as well as the mechanisms behind such interactions. For example, the unique topography and crystallinity of NCD have been proposed to have a significant impact on osteoblast (bone forming cell) adhesion, proliferation and differentiation.4,9NCD surface terminations have also revealed large effects on the wettability of NCD and, consequently, initial protein adsorption and bioactivity enhancing select cell adhesion.7,10–12
Importantly, CVD-grown NCD is usually hydrogen terminated (due to H2 used or a breakdown of hydrocarbon gases in the process), resulting in a hydrophobic surface (water contact angles usually >80–90°). Modifying NCD surfaces with fluorine terminations seldom changes NCD film wettability, while oxygen terminations can dramatically decrease water contact angles, increasing NCD hydrophilicity. To date, many studies have reported that hydrophilic oxygen-terminated NCD may improve select protein adsorption and/or cell responses compared to hydrophobic terminations, although the mechanisms behind these wettability-mediated responses have not been studied. However, in contrast to these results, studies have shown that hydrogen terminated NCD promotes MSC adhesion, proliferation, and osteoblastic differentiation over oxygen and fluorine terminated NCD.7,10 The discrepancy among these results suggests a high necessity to further investigate how NCD surface terminations affect cellular responses.
Moreover, in terms of techniques, certain surface modifications can be facile and effective to improve the biological performance of NCD films or coatings. Therefore, seeking appropriate surface terminations on NCD and understanding the relevant cellular responses are important for a wide range of biomedical applications of NCD. Specifically, for orthopaedic prosthetic applications, wear-resistant NCD can still be improved in terms of osseointegration properties, which are highly desirable for forming strong biological bonding between bone tissue and implants, ultimately, decreasing the chance of implant failure. For example, an in vitro study of as-received NCD revealed that only ∼10% of the originally seeded osteoblasts adhered to the diamond surface after 4 h; in comparison, ∼57% and ∼41% of the originally seeded osteoblasts adhered to nanostructured titanium and CoCrMo after 3 h, respectively.13
Although enhanced osteoblast or MSC osteogenic responses have been reported on oxygen terminated NCD compared to untreated NCD, even better cell responses as well as simple surface modification methods are desired. For example, a preliminary study on ammonia-hydrogen plasma treated NCD demonstrated increased 4-hour osteoblast adhesion compared to oxygen and hydrogen terminated NCD, indicating a strong potential for ammonia plasma treatment processes to promote even better osteoblast responses than other modification methods;14 however, this potential has not been systematically studied to date.
Thus, in the present study, surface chemical modification of NCD using a variety of plasmas (specifically, hydrogen, oxygen and ammonia plasmas, and resultant films were termed as NCDH, NCDO and NCDN, respectively) was developed and their impact on osteoblasts (i.e., attachment, morphology and differentiation from non-calcium depositing to calcium depositing cells) were studied in vitro. To create a focused study on NCD chemical modification alone and, thus, eliminate NCD topographical effects, NCD films with the same nanotopography (grain size 30–80 nm, root mean square roughness values ∼27nm) were carefully prepared and modified by the plasmas. The resultant wettability properties and surface energetics of the NCD films were characterized. Most importantly, the ability to alter the surface free energy of NCD by the various plasma treatments facilitated an effort to correlate osteoblast responses to the altered NCD surface free energy. As a result, a strong correlation between NCD surface free energy (resulting only from changes in surface functionalisation and not changes in roughness) and pertinent osteoblast responses was established here for the first time.
Results
Impact of plasma treatments on NCD surface morphology and chemistry
Plasma treatments using H2, O2 and NH3 revealed little effect on altering the surface topography of NCD. As shown in Fig. 1, SEM images of the various treated NCD revealed only slight changes in surface topography, except NCDN which had numerous bright fine dots on the surface. It is believed that this noticeable change on NCDN was due to electron charging artifacts resulting from changes in surface conductivity, because the AFM images did not reveal any significant changes in NCDN nanotopography compared to the other two samples (Fig. 1). In addition, the results of root mean square (RMS) roughness also confirmed little variation in NCD nanotopography (Table 1). A previous study also indicated that H2 plasma treatment did not alter NCD topography and surface roughness.5 Results of the present study confirmed this result and, moreover, it can be induced that H2, O2 or NH3 plasma treatments performed here also did not significantly alter NCD topography compared to untreated NCD. This is an important result for the study of the impact of surface energy on biological properties of NCD, since it is already known that differences in NCD topography affects osteoblast responses.4,5,9,15 Hence, by creating the same nanotopography on NCDH, NCDO and NCDN, there is a better chance to isolate the effects of surface chemistry (and associated surface free energy changes) on osteoblast responses.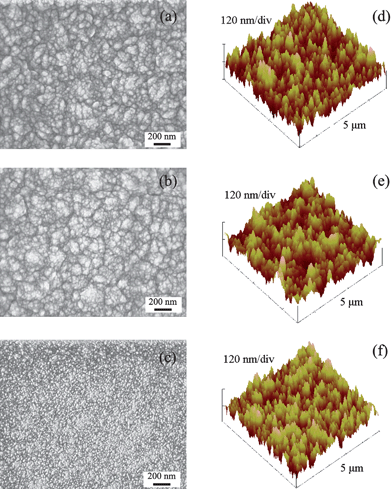 | ||
| Fig. 1 SEM and AFM images of NCDH (a and d), NCDO (b and e) and NCDN (c and f). | ||
| Substrate | RMS roughness (nm) | Contact angle measured by different liquids (°) | |||
|---|---|---|---|---|---|
| θ (H2O) | θ (Glycerol) | θ (Formamide) | θ (Tetradecane) | ||
| a Obtained from manufacturer. b Borosilicate glass (etched by 1 M NaOH). | |||||
| NCDH | 26.7 | 81 | 60 | 54 | ∼0 |
| NCDO | 27.9 | 66 | 52 | 31 | ∼0 |
| NCDN | 27.7 | 31 | 20 | 17 | ∼0 |
| Si | ∼0.5a | 54 | 64 | 59 | 17 |
| BG b | 5.5 | 61 | 68 | 36 | ∼0 |
The surface chemistry of the plasma-treated NCD films was analyzed by XPS and the spectra are shown in Fig. 2. Clear O 1s and N 1s peaks were found for NCDO and NCDN at binding energies of 531.5 eV and 397.5 eV, respectively. O 1s peaks were also observed in all the films, even the NCDH treated with pure H2 plasma; the same phenomenon has been reported in other studies which was attributed to the physiosorption of oxygen-containing molecules or surface oxidation.12,16,17 For the NCDO, most likely, the O 1s peak was attributed to C–O or C–OH groups on the diamond surface, according to numerous analyses from other studies on O2 plasma treated NCD.8,18 For the NCDN, according to Szunerits et al.,19 it is more likely to have –NH2groups bonded on NH3/H2 plasma treated diamond surfaces.
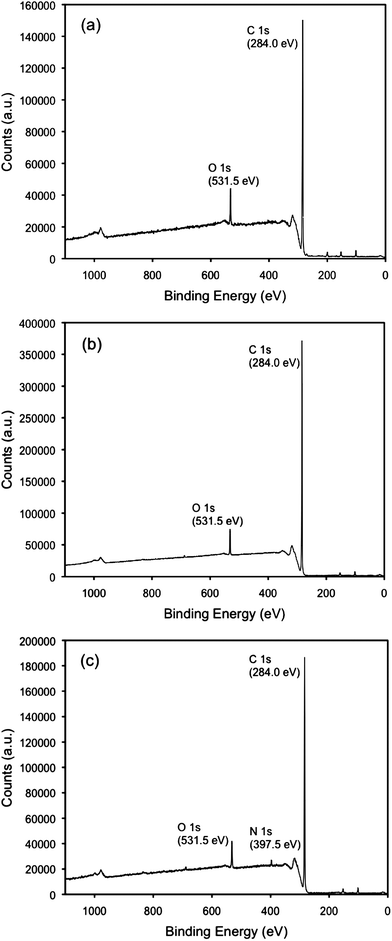 | ||
| Fig. 2 XPS spectra of NCDH (a), NCDO (b) and NCDN (c). | ||
Surface wettability and surface free energy of plasma treated NCD
Results from contact angle measurements using different test liquids are listed in Table 1. Water contact angle results indicated the following trend with hydrophilicity: NCDN > NCDO > NCDH. This hydrophilicity result agrees with the analysis of surface terminations on the various NCD films, since polar groups of –OH and –NH2 render the NCD surface more hydrophilic, while the –H terminated surface was more hydrophobic. The water contact angle of NCDO (θH2O = 66°) was higher than that of oxidized or O2 plasma treated NCD found in several other studies (θH2O = 7–30° were reported).8,12,18 This difference was due to the various treatment conditions (O2 concentrations, treatment time, etc.) used in these studies. The water contact angle of NCDO was found to be a function of time under the present conditions of plasma treatment and θH2O ∼ 20° was achieved when plasma treatment time extended from 15 min to 30 min (unpublished study). Klauser et al.12 also reported an elevated θH2O = 54° on NCD partially terminated with oxygen, which was close to the results in the present study. To reduce this complexity, the same plasma treatment time (i.e., 15 mins) was used here for all the NCD samples.The controls (Si and borosilicate glass (BG)) had similar water contact angles which were higher than that of NCDN. Contact angles of tetradecane on all the samples were close to zero except a contact angle of 17° was observed on Si. Since surface tension of tetradecane has only a dispersive component (polar component ∼ 0, see Experimental section Table 2), one can expect Si to have a much lower dispersive component in its surface free energy than that of tetradecane (i.e., 26.6 mN m−1). This expectation was confirmed by surface free energy calculation as shown in Fig. 3a, in which the dispersive component in surface free energy of Si was only 6.9 mN m−1.
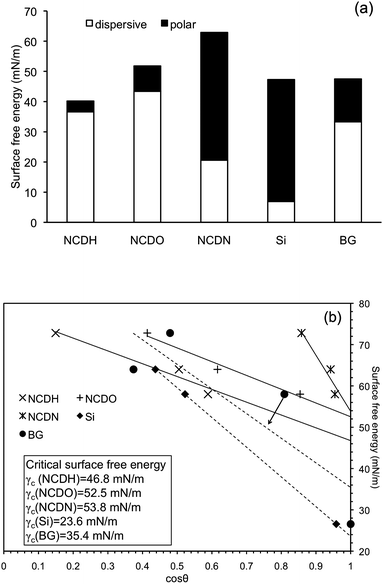 | ||
| Fig. 3 Calculated surface free energy (a) and Zisman plots to determine critical surface free energy (γc) (b) of NCDH, NCDO, NCDN and Si and borosilicate glass (BG) controls. The γc of each substrate was read where the trend line meets cosθ = 1. | ||
Surface free energy results of NCD films calculated from Eqn (1) are shown in Fig. 3a. Among all the NCD samples, NCDN exhibited the highest surface free energy (62.9 mN m−1), NCDO revealed the second highest (51.8 mN m−1), and NCDH had the lowest surface free energy (40.2 mN m−1). As expected, this trend matched the trend of hydrophilicity (water contact angle) measurements. Because the topographical effects on the hydrophilicity of NCD films were suppressed by creating samples with the same nanotopography, a monotonic relationship between surface free energy and hydrophilicity of NCD films can be established by the results from this study. This monotonic relationship was not valid for the Si and BG, which had similar surface free energies (47.3 and 47.5 mN m−1, respectively) but significantly different contact angles, probably because of the large differences in their surface roughness (topography) and bulk chemistry. It was also observed that different plasmas exerted various influences on the dispersive and polar components of the surface free energy on NCD. Compared to hydrogen plasma, oxygen plasma increased both the dispersive and polar components of surface free energy on NCDO, which has also been reported by others.12 In contrast, –NH2 terminations on NCDN significantly decreased the dispersive component but dramatically increased the polar component of surface free energy compared to –H terminations.
Lastly, critical surface energy (γC) of each sample was determined by the Zisman plot shown in Fig. 3b. A linear regression of fitting the data obtained from the various test liquids gave a reading of γC when cosθ = 1, as also shown in Fig. 3b. As expected, γC of NCD films revealed the same trend as the surface free energy or hydrophilicity measurements: NCDN > NCDO > NCDH. Interestingly, BG had a higher γC compared to Si, but the surface free energy measurements showed an opposite result in which surface free energy of Si was higher. This discrepancy again suggests that surface roughness and bulk chemistry have complicated effects on material hydrophilicity, surface free energy and γC.
Osteoblast attachment on plasma treated NCD
Results from the 12-hr osteoblast attachment assay on the various NCD films are shown in Fig. 4. Significantly higher (69% more) osteoblasts attached to NCDN than that to NCDH. More osteoblasts also attached to NCDO than NCDH (41% more when comparing the mean values), although the difference was not statistically significant. More osteoblasts attached to NCDN than both of the controls (Si and BG), while NCDH had less osteoblasts attached than controls. This result indicated that ammonia plasma treatment could significantly enhance osteoblast attachment on NCD after 12 h.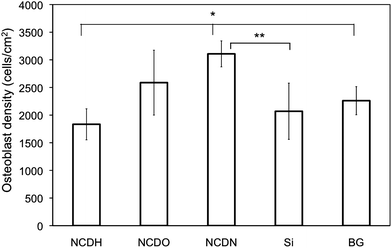 | ||
| Fig. 4 Osteoblast attachment on NCDH, NCDO, NCDN and controls of Si and borosilicate glass (BG) after12 h. Osteoblast seeding density was 104cells cm−2. Data are expressed as the mean ± standard deviation of the mean (N = 3). *p < 0.05, **p < 0.1. | ||
Osteoblast density and morphology on the NCD films observed by fluorescence microscopy (Fig. 5) confirmed the above results. Specifically, osteoblast spreading morphologies on the various substrates were different and osteoblasts on NCDN and NCDO spread more than those on NCDH. Spreading area per cell was quantified on each substrate and the results are shown in Fig. 6. Each osteoblast on average spread 112% and 100% more on NCDN and NCDO than on NCDH, respectively, and osteoblasts on NCDN and NCDO also spread more than those on the controls. Osteoblasts also exhibited more and thicker contractile filaments on NCDN and NCDO than on any other sample (Fig. 5), indicating possible stronger cell-substrate interactions or active cell movements on these two substrates. In addition, osteoblasts on these two substrates had more cell-cell interactions than those on the other substrates.
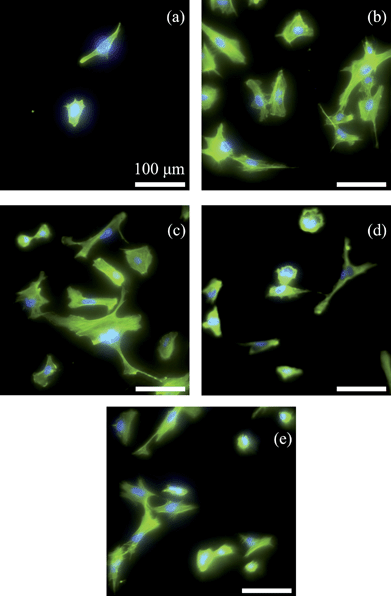 | ||
| Fig. 5 Fluorescence microscopy images showing osteoblast morphology on NCDH (a), NCDO (b), NCDN (c), Si (d) and borosilicate glass (e) after culturing for 12 h (bar = 100 μm). Osteoblasts were stained by rhodamine phalloidin and the nuclei were stained by DAPI. | ||
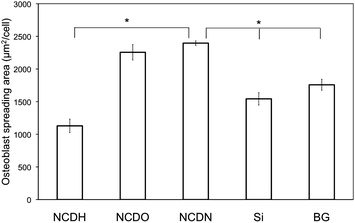 | ||
| Fig. 6 Average spreading area of osteoblasts on NCDH, NCDO, NCDN and Si and borosilicate glass (BG) controls after culturing for 12 h. The area per cell was calculated by dividing the rhodamine phalloidin-stained cytoskeleton area by the number of DAPI-stained nuclei. Over 75 cells were measured for each substrate. Data are expressed as the mean ± standard deviation of the mean. *p < 0.01. | ||
Osteoblast differentiation on plasma treated NCD
A variety of long-term osteoblast differentiation markers (alkaline phosphatase (ALP) activity, collagen synthesis and calcium deposition) were examined here. Osteoblasts on NCDN revealed the highest unit ALP activity than any other sample after 14 days, while NCDO and NCDH had almost the same level of unit ALP activity (Fig. 7).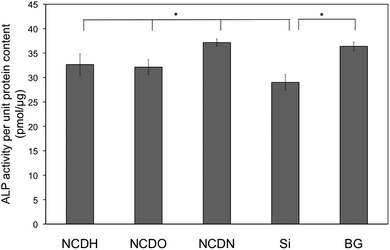 | ||
| Fig. 7 Alkaline phosphatase (ALP) activity of osteoblasts on NCDH, NCDO, NCDN and Si and borosilicate glass (BG) controls after 14 days. The ALP activity was normalized by total intracellular protein synthesis on the substrates. Osteoblast seeding density was 105cells cm−2. Data are expressed as the mean ± standard deviation of the mean (N = 3). *p < 0.05. | ||
Results of osteoblast collagen synthesis exhibited a similar trend, in which NCDN promoted osteoblast collagen synthesis the most among all the NCD films (Fig. 8). NCDO also revealed a significant promotion in collagen synthesis compared to NCDH. In this test, osteoblasts on Si revealed very high collagen synthesis, even a little higher than that on NCDN. The reason behind this promoted collagen synthesis on Si needs further investigation.
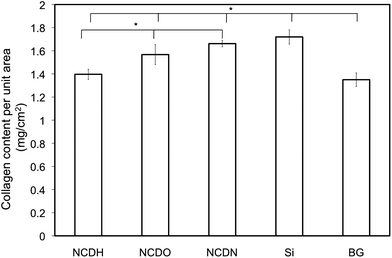 | ||
| Fig. 8 Osteoblast collagen synthesis on NCDH, NCDO, NCDN and Si and borosilicate glass (BG) controls after 14 days. The collagen content was normalized by substrate area. Osteoblast seeding density was 105cells cm−2. Data are expressed as the mean ± standard deviation of the mean (N = 3). *p < 0.05. | ||
The most important biomarker of osteoblast differentiation is extracellular calcium deposition. The results demonstrated that NCDN enhanced osteoblast calcium deposition more than any other sample after 14 days (Fig. 9). In this test, Si and BG controls were less effective at inducing osteoblast calcium deposition than NCD films.
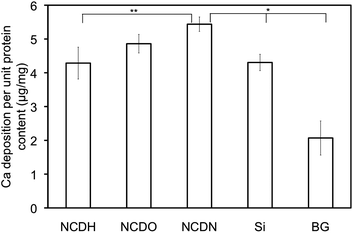 | ||
| Fig. 9 Osteoblast extracellular calcium deposition on NCDH, NCDO, NCDN and Si and borosilicate glass (BG) controls after 14 days. The calcium deposition was normalized by total intracellular protein synthesis on the substrates. Osteoblast seeding density was 105cells cm−2. Data were expressed as the mean ± standard deviation of the mean (N = 3). *p < 0.01 and **p < 0.1. | ||
All the osteoblast differentiation results revealed the same trend among the three NCD films: NCDN promoted the best osteoblast functions and NCDH was the worst. Considering previous trends in osteoblast attachment (Fig. 4) and cell spreading area (Fig. 6), consistent results of osteoblast responses to plasma treated NCD were observed here. More importantly, a strong correlation between surface free energy or γC of plasma treated NCD and osteoblast responses was established. In other words, the osteoblast short and long-term functions (attachment, morphology and differentiation) on NCD had a strong dependence on NCD surface free energy or γC that can be easily altered by different surface terminations.
Discussion
Direct plasma treatment has been demonstrated here to be a facile and versatile technique for the chemical modification of NCD or other diamond films by eliminating complicated, lengthy and multi-step chemical reactions or processes.2,19–21 As demonstrated in the present study, plasma treatment only added one extra 15-min step after MPCVD fabrication of diamond films. In terms of efficiency, plasma treatment is probably the most efficient method for the oxidization or amination of diamond surfaces.20,22 Moreover, the results here indicated that plasma treatment using different gases could readily alter NCD surface wettability and surface energetics (surface free energy and γC). With further investigation on plasma treating time and surface termination coverage, complete control of NCD surface hydrophilicity (e.g., θ altering from ∼10° to ∼100°) and corresponding surface free energy or γC can be achieved by using H2, O2 or NH3 plasmas. This control rendered the specific study on the effects of NCD surface free energy on osteoblast responses possible. In addition, chemically active terminal groups on NCD such as –OH and –NH2 provided exceptional capability to further immobilize or conjugate biological or chemical molecules.2,20,23,24Stability of the surface terminations on the NCD films produced by H2, O2 or NH3 plasmas remains a question. A previous study and our experience suggests that –H terminations on NCD are stable for several months but are partially lost when cleaned by a series of solutions of acetone, ethanol and deionised water.5 A preliminary study showed that –OH and –NH2 terminations on NCD were only stable for a few weeks and the water contact angles of NCDO and NCDN increased to similar values as NCDH after storage in ambient conditions for 4 weeks.25 It was speculated that the lower stability of –OH and –NH2 terminations on NCD were due to their high chemical activities. Studies on this speculation are out of the scope of the present study, but are worth further investigation.
The most significant finding from this study was the establishment of a strong correlation between the surface energetics of NCD and osteoblast responses/functions. For years, material hydrophilicity has been speculated to influence cell-adhesive protein (fibronectin, vitronectin, etc.) adsorption and bioactivity as well as subsequent cell responses; however, no ubiquitous results have been reported.26 In the present study, variable NCD hydrophilicity and underlying surface free energy were obtained by introducing various surface terminations, and moreover, the influence of surface roughness or topography on hydrophilicity and surface free energy was eliminated through carefully designed experiments. A consistent relationship between contact angles and surface free energy of NCD revealed monotonically increased values of surface free energy and γC when NCD hydrophilicity increased. This relationship can be thermodynamically understood since the surface with higher surface free energy tends to lower its total free energy by wetting of liquid. More importantly, both short- and long-term osteoblast responses demonstrated a clear correlation to surface free energy or γC of NCD: significantly increased osteoblast attachment, spreading and differentiation were found on NCD with higher surface free energy or γC.
To explain this strong correlation, the possible differential adsorption of proteins from serum (i.e., fetal bovine serum in the culture medium) on plasma treated NCD needs to be considered. Fibronectin adsorption was quantified by an enzyme-linked immunosorbent assay (ELISA). However, no difference in fibronectin adsorption on NCDH, NCDO and NCDN was observed.25 Further investigation into the reasons behind this similar fibronectin adsorption and studies which compare the adsorption (and conformation) of other proteins need to be completed.
Instead, here we can offer a surface tensiometric approach comparing cell-cell cohesivity and cell-substrate adhesivity to understand the NCD surface free energy-dependent responses of osteoblasts. Also for cells like osteoblasts, because proliferation and differentiation (characterized by ALP activity, collagen and calcium synthesis) is closely related to their initial cell shape and spreading,4,27 the focus here was to highlight the effects of NCD surface free energy on osteoblast spreading. According to Ryan et al., competition between cell-cell cohesivity and cell-substrate adhesivity determines tissue spreading on implantable substrates and two criteria can be used to evaluate cell adhesivity to substrates: the rate (density) of attachment and the degree of cell spreading over time.28
Clearly, results from this study revealed that NCDN had the highest density of cell attachment and degree of cell spreading, suggesting the highest cell adhesivity to the NCD with the highest surface free energy (or γC). Similarly, one can obtain osteoblast adhesivity on the various NCD films following the relationship NCDN > NCDO > NCDH, which agrees with the relationship of surface free energy and γC. Therefore, NCD with higher surface free energy or γC possesses better adhesiveness to overcome the cohesivity of osteoblast aggregates, allowing osteoblasts to remain spread and consequently, to promote cell proliferation and differentiation. This relationship can be further understood by comparing cell-cell cohesivity (i.e., surface tension of cell aggregates) and the surface free energy of the substrates (i.e., surface tension between substrates and air). The aggregate cohesivity of anchorage-dependent cells is usually between ∼2 mN m−1 and ∼9 mN m−1,28,29 which is much less than the surface free energy or γC of NCD films (surface free energy between 40.2 mN m−1 and 62.9 mN m−1 and γC between 46.8 mN m−1 and 53.8 mN m−1 for NCD films in this study). Lower surface tension promotes cell aggregates to “wet” the substrate surfaces with higher surface free energy rather than clustering together, resulting in strong cell-substrate adhesivity revealed in the present study. Moreover, thermodynamically, substrates with higher surface free energy have more potential to cause “wetting” of cell aggregates on their surfaces. Thus, this increased potential explains the improved osteoblast spreading and subsequent cell functions on NCD with higher surface free energy or γC. This thermodynamic mechanism has been experimentally verified by comparing the spreading of osteoblast aggregates (aggregate sizes 100–150 μm, containing 50–100 cells) on NCDH, NCDO and NCDN.25 The results revealed that osteoblast aggregates on NCDN and NCDO spread much faster than that on NCDH, indicating strong cell-substrate adhesivity on NCD with higher surface free energy.
Based on this mechanism, the observations of less osteoblast responses to controls (BG and Si) than NCDN and NCDO may also be explained, because Si and BG had less surface free energy than NCDN and NCDO. However, this correlation did not stand when comparing to NCDH or between Si and BG, probably due to large differences in their surface roughness and bulk chemistry.
Conclusions
This study demonstrated the feasibility and efficiency of using direct plasma treatments to modify surface chemistry and control surface energetics of NCD films. Osteoblast responses to NCD films modified by H2, O2 and NH3 plasmas were systematically studied from 12 h to 14 days, and for the first time, the best osteoblast responses were found on NH2-terminated NCD for all the cell functions investigated here. Most importantly, a strong correlation between surface free energy (or γC) of NCD and osteoblast responses (attachment, morphology and spreading, and differentiation) was established: without altering surface roughness and topography, NCD with higher surface free energy or γC promoted osteoblast functions more than NCD which had less surface free energy or γC. These results provide important information to create appropriate NCD bio-interfaces for numerous applications, most significantly, orthopaedic implant coatings.Experimental section
NCD films
NCD films were deposited on polished silicon wafers (phosphorus doped, (100) face polished, Virginia Semiconductor, PA) through microwave plasma enhanced CVD (MPCVD). The Si wafers were cut and cleaned with acetone and methanol each for 10 mins, and then were seeded with nanosized diamond powder (particle size distribution 1–50 nm) dispersed in methanol for 30 mins. NCD films were deposited on the seeded Si substrates at 800 °C using a plasma of 94% Ar, 5% H2 and 1% CH4 (volume percentage) at 120 Torr for 2 h. A microwave power of 800 W was used for film deposition. After film growth, the NCD sample was kept in the chamber and pumped to a vacuum of 20 mTorr for further surface modification.Surface modification by plasma treatment
Three different plasma treatments were performed on the NCD. Hydrogen terminated NCD (NCDH) was obtained by treating NCD with 100% H2 plasma after film growth. The plasma was generated and maintained at 13 Torr and 800 °C for 15 mins. Similarly, oxygen terminated NCD (NCDO) was obtained using 90% H2–10%O2 (volume percentage) plasma at 13 Torr to treat NCD for 15 mins. For preparing amine terminated NCD (NCDN), a plasma of 90% Ar, 5% H2 and 5% NH3 (volume percentage) at 120 Torr was used for 15 mins. A lower substrate temperature of 400 °C was used to reduce the decomposition of the amine group (–NH2) on NCDN.30 For all the plasma treatments, a microwave power of 800 W was used.Surface characterisation
| Liquid | Specifications | γL (mN m−1) | γLd (mN m−1) | γLp (mN m−1) |
|---|---|---|---|---|
| Deionized water | Milipore systems | 72.8 | 21.8 | 51 |
| Glycerol | Sigma-Aldrich, >99% | 64 | 34 | 30 |
| Formamide | Fisher, ≥99.5% | 58 | 39 | 19 |
| Tetradecane | Fisher, ≥99.5% | 26.56 | 26.56 | ∼0 |
The surface free energy of the samples were calculated by the Owens-Wendt model following the method reported by Lamour et al.31 Briefly, water and formamide contact angles on the substrates of interest to this study were used to calculate the surface free energy by the following equation:
| γSL = (1 + cosθ) γL = 2 (γSd γLd)1/2 + 2 (γSp γLp)1/2 | (1) |
The critical surface energy (γC, or critical surface tension) was calculated using a first order approximation of the Good-Girifalco equation.31 A linear regression analysis, also known as the Zisman plots (cosθ = f(γL)), was conducted for each sample by fitting the γL and cosθ data obtained from the different test liquids. The γC value of each sample was read where the line of the best fit approximation intersected cosθ = 1 (i.e., θ = 0).
Cells
Non-transformed human femur osteoblasts (CRL-11372, ATCC) were used at population numbers of 9 and 10. The osteoblasts were cultured in Dulbecco's Modified Eagle's Medium (DMEM, Hyclone) supplemented with 10% fetal bovine serum (FBS, Hyclone) and 1% penicillin/streptomycin (P/S, Hyclone) under standard cell culture conditions (5% CO2, humidified air at 37 °C). The osteoblasts were cultured until 80–100% confluence before cell tests.Evaluation of osteoblast attachment
Before cell tests, NCD films and controls (Si and BG) were ultrasonically cleaned in 100 vol.% ethanol, 70 vol.% ethanol and deionised water, and then were sterilized by UV light exposure for 1 h. Osteoblasts were seeded on the sample surfaces at a density of 104cells cm−2. After seeding, osteoblasts were cultured in DMEM supplemented with 10% FBS and 1% P/S under standard cell culture conditions. After 12 h, the samples were washed three times with phosphate buffered saline solution (PBS) and then the adherent cells were fixed with 10% formaldehyde (Fisher Scientific) and their nuclei stained by 4′,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich). The stained cells were counted under a fluorescence microscope (Leica DM5000B) to calculate osteoblast density (cells per unit area of the substrate). Five areas were counted and averaged per substrate. Experiments were performed in triplicate and repeated three times (N = 3).Osteoblast morphology
Osteoblast morphology on NCD films and controls was observed by fluorescence microscopy. Osteoblasts were seeded and cultured for 12 h on the substrates as previously described. Adherent osteoblasts on the substrates were washed with PBS and fixed with 4% formaldehyde for 10 mins. After fixation, the cells were stained with rhodamine phalloidin conjugates (Alexa Fluor® 555 phalloidin, Invitrogen) following the instructions provided by the manufacturer. The fluorescent phalloidin conjugates can label F-actin in cells, enabling the observation of cytoskeleton morphology as well as F-actin alignment in cells. For indicating cell density on the substrate, the cell nuclei were further stained with DAPI. Images of stained cells on the substrates were collected at 20× and 40× magnifications for quantitative and qualitative purposes, respectively.20× images were analyzed by Image J (free resource from http://rsbweb.nih.gov/ij/) for the quantification of each osteoblast spreading area (area per cell). For each image, the total osteoblast spreading area was quantified by threshold processing on the rhodamine phalloidin stained image and cell density was counted from the DAPI stained images, and then spreading area per cell was calculated. At least three images per sample were analyzed and over 75 cells per sample were averaged.
Osteoblast differentiation assays
Osteoblast alkaline phosphatase (ALP) activity, collagen synthesis and extracellular calcium deposition on the different NCD films and controls were evaluated here. For this, osteoblasts were seeded on the NCD films, Si and BG at a density of 105cells cm−2 and cultured in DMEM supplemented with 10% FBS, 1% P/S, 50 μg mL−1L-ascorbate (Sigma-Aldrich) and 10 mM β-glycerophosphate (Sigma-Aldrich) under standard cell culture conditions for 14 days. Cell culture medium was replaced every other day. At day 14, the samples were moved to new cell culture plates and rinsed with tris buffered saline (pH 7.2, Sigma-Aldrich) three times. Osteoblasts on the substrates were lysed in 1 mL deionised water using three freeze-thaw cycles. The freeze-thaw protocol used for osteoblast lysis removes only intracellular proteins not extracellular matrix (ECM) proteins.32 The supernatant lysates were then transferred into microtubes for determining intracellular protein synthesis, ALP activity and collagen synthesis, while the remaining substrates were used for determining extracellular calcium deposition as described below. Experiments were performed in triplicate and repeated three times (N = 3).Statistical analysis
Statistical results were analyzed using one-way analysis of variance (ANOVA) and the data expressed as the mean ± standard deviation of the mean.Acknowledgements
The authors would like to acknowledge the Hermann Foundation (primary funding) and also the National Science Foundation (award DMR-0805172).References
- A. M. Schrand, S. A. C. Hens and O. A. Shenderova, Crit. Rev. Solid State Mat. Sci., 2009, 34, 18 Search PubMed.
- W. S. Yang, O. Auciello, J. E. Butler, W. Cai, J. A. Carlisle, J. Gerbi, D. M. Gruen, T. Knickerbocker, T. L. Lasseter, J. N. Russell, L. M. Smith and R. J. Hamers, Nat. Mater., 2002, 1, 253 CrossRef CAS.
- D. Steinmuller-Nethl, F. R. Kloss, M. Najam-U-Haq, M. Rainer, K. Larsson, C. Linsmeier, G. Koehler, C. Fehrer, G. Lepperdinger, X. Liu, N. Memmel, E. Bertel, C. W. Huck, R. Gassner and G. Bonn, Biomaterials, 2006, 27, 4547 CrossRef CAS.
- L. Yang, B. W. Sheldon and T. J. Webster, Biomaterials, 2009, 30, 3458 CrossRef CAS.
- L. Yang, B. W. Sheldon and T. J. Webster, J. Biomed. Mater. Res., Part A, 2009, 91A, 548 CrossRef CAS.
- Y. C. Chen, D. C. Lee, T. Y. Tsai, C. Y. Hsiao, J. W. Liu, C. Y. Kao, H. K. Lin, H. C. Chen, T. J. Palathinkal, W. F. Pong, N. H. Tai, I. N. Lin and I. M. Chiu, Biomaterials, 2010, 31, 5575 CrossRef CAS.
- W. C. Clem, S. Chowdhury, S. A. Catledge, J. J. Weimer, F. M. Shaikh, K. M. Hennessy, V. V. Konovalov, M. R. Hill, A. Waterfeld, S. L. Bellis and Y. K. Vohra, Biomaterials, 2008, 29, 3461 CrossRef CAS.
- C. Popov, H. Vasilchina, W. Kulisch, F. Danneil, M. Stuber, S. Ulrich, A. Welle and J. P. Reithmaier, Diamond Relat. Mater., 2009, 18, 895 CrossRef CAS.
- M. Kalbacova, B. Rezek, V. Baresova, C. Wolf-Brandstetter and A. Kromka, Acta Biomater., 2009, 5, 3076 CrossRef CAS.
- F. R. Kloss, R. Gassner, J. Preiner, A. Ebner, K. Larsson, O. Hachl, T. Tuli, M. Rasse, D. Moser, K. Laimer, E. A. Nickel, G. Laschober, R. Brunauer, G. Klima, P. Hinterdorfer, D. Steinmuller-Nethl and G. Lepperdinger, Biomaterials, 2008, 29, 2433 CrossRef CAS.
- P. Ariano, O. Budnyk, S. Dalmazzo, D. Lovisolo, C. Manfredotti, P. Rivolo and E. Vittone, Eur. Phys. J. E, 2009, 30, 149 CrossRef CAS.
- F. Klauser, M. Hermann, D. Steinmuller-Nethl, O. Eiter, A. Pasquarelli, E. Bertel, T. Seppi, P. Lukas and T. Lechleitner, Chem. Vap. Deposition, 2010, 16, 42 CrossRef CAS.
- T. J. Webster and J. U. Ejiofor, Biomaterials, 2004, 25, 4731 CrossRef CAS.
- R. Pareta, L. Yang, A. Kothari, S. Sirinrath, X. Xiao, B. W. Sheldon and T. J. Webster, J. Biomed. Mater. Res. A, 2010, 95, 129 CrossRef.
- L. Yang, V. Chinthapenta, Q. Li, D. Stout, A. Liang, B. W. Sheldon and T. J. Webster, J. Biomed. Mater. Res., Part A, 2011, 97A, 375 CrossRef CAS.
- F. Maier, M. Riedel, B. Mantel, J. Ristein and L. Ley, Phys. Rev. Lett., 2000, 85, 3472 CrossRef CAS.
- K. Larsson, S. Lunell and J. O. Carlsson, Phys. Rev. B: Condens. Matter, 1993, 48, 2666 CrossRef CAS.
- C. Popov, W. Kulisch, S. Bliznakov, G. Ceccone, D. Gilliland, L. Sirghi and F. Rossi, Diamond Relat. Mater., 2008, 17, 1229 CrossRef CAS.
- S. Szunerits, C. Jarna, Y. Coffinier, B. Marcus, D. Delabouglise and R. Boukherroub, Electrochem. Commun., 2006, 8, 1185 CrossRef CAS.
- S. Szunerits and R. Boukherroub, J. Solid State Electrochem., 2008, 12, 1205 CrossRef CAS.
- T. Strother, T. Knickerbocker, J. N. Russell, J. E. Butler, L. M. Smith and R. J. Hamers, Langmuir, 2002, 18, 968 CrossRef CAS.
- C. H. Goeting, F. Marken, A. Gutierrez-Sosa, R. G. Compton and J. S. Foord, Diamond Relat. Mater., 2000, 9, 390 CrossRef CAS.
- Y. Coffinier, S. Szunerits, C. Jama, R. Desmet, O. Melnyk, B. Marcus, L. Gengembre, E. Payen, D. Delabouglise and R. Boukherroub, Langmuir, 2007, 23, 4494 CrossRef CAS.
- Y. Coffinier, S. Szunerits, B. Marcus, R. Desmet, O. Melnyk, L. Gengembre, E. Payen, D. Delabouglise and R. Boukherroub, Diamond Relat. Mater., 2007, 16, 892 CrossRef CAS.
- L. Yang, PhD Thesis, Brown University, Providence, 2011.
- C. J. Wilson, R. E. Clegg, D. I. Leavesley and M. J. Pearcy, Tissue Eng., 2005, 11, 1 CrossRef CAS.
- J. Folkman and A. Moscona, Nature, 1978, 273, 345 CrossRef CAS.
- P. L. Ryan, R. A. Foty, J. Kohn and M. S. Steinberg, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 4323 CrossRef CAS.
- E. E. Robinson, K. M. Zazzali, S. A. Corbett and R. A. Foty, J. Cell Sci., 2003, 116, 377 CrossRef CAS.
- X. L. Zhou, C. R. Flores and J. M. White, Surf. Sci., 1992, 268, L267 CrossRef CAS.
- G. Lamour, A. Eftekhari-Bafrooei, E. Borguet, S. Soues and A. Hamraoui, Biomaterials, 2010, 31, 3762 CrossRef CAS.
- T. J. Webster, E. L. Hellenmeyer and R. L. Price, Biomaterials, 2005, 26, 953 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
