Effects of the sampling interface in MC-ICP-MS: Relative elemental sensitivities and non-linear mass dependent fractionation of Nd isotopes
Karla
Newman
*
Water Quality Centre, Trent University, 1600 West Bank Drive, Peterborough, ON K9J 8E5, Canada. E-mail: karlanewman@trentu.ca; Fax: +1 705 748 1625; Tel: +1 705 748 1011 x7134
First published on 18th November 2011
Abstract
Relative elemental sensitivities are reported for a number of sample and skimmer cone combinations, with standard and enhanced pumping of the interface region, for the Thermo Scientific Neptune MC-ICP-MS. An approximate two-fold sensitivity enhancement was observed for Sr, Nd, Hf, Pb and U using the Jet sample cone and an enhanced pumping configuration, compared to the standard arrangement. In the case of Li, the standard sample cone and enhanced pumping configuration gave the maximum sensitivity. In addition to improved ion transmission, the X skimmer cone geometry is also associated with additional contributions to the instrumental mass fractionation (at least in the case of Nd) that do not have a simple exponential dependence on the isotope mass. Neodymium isotope ratios measured using the X cone displayed large deviations (up to ∼750 ppm) from the reference (i.e. ‘true’) values. Similar deviations have been reported previously when using high sensitivity skimmer cone geometries on the Nu MC-ICP-MS instruments (K. Newman et al., J. Anal. At. Spectrom., 2009, 24, 742). The reported non-linear contribution to the instrumental mass fractionation with respect to Nd is not specific to a particular MC-ICP-MS instrument; it is associated with an increase in the NdO+/Nd+ ratio, due to either a change in the plasma operating conditions (e.g. an increase in the sample gas flow, absence of a secondary discharge at the interface, change in plasma gas composition) or modification of the cone geometry. The role of secondary discharge formation and the physical and chemical processes occurring in the supersonic expansion with respect to NdO+ formation are discussed.
Introduction
A number of studies have investigated the effect of sample and skimmer cone geometry1–9 and interface pumping configuration10,11 on the ion extraction efficiency in ICP-MS. Optimization of the cone geometry and a reduction in the first vacuum stage pressure (i.e. between the sample and skimmer cones) can afford an increase in the analytical sensitivity. However, this may be accompanied by unwanted effects; for example, an increase in space charge repulsion in the interface region and concomitant changes in the magnitude of matrix effects.5 The skimmer geometry and material may also influence the intensity of polyatomic interferences.1,6–8 Therefore, the design and construction of the sample and skimmer cones will make a significant contribution to the analytical sensitivity and instrumental mass fractionation behaviour.Isotope ratios can routinely be measured with parts per million (ppm) levels of precision with current multi-collector (MC)-ICP-MS instruments, accentuating the need for accurate correction of the instrumental mass fractionation. However, modified skimmer cone geometries which give an increase in the analytical sensitivity are known to be associated with increased MO+/M+ ratios, where M is a rare earth element (REE), and non-linear mass dependent fractionation. In a previous study9 using Nu Plasma HR and 1700 MC-ICP-MS instruments (referred to collectively as Nu MC-ICP-MS instruments here), neodymium isotope ratios measured using a high sensitivity skimmer cone exhibited large (per mil) deviations from their true values. This deviation was not a linear function of mass and could not be corrected for using the standard mass fractionation laws. Mass balance calculations for the Nd+ and NdO+ species were consistent with a correlation between oxide formation and non-linear mass fractionation.
This previous study9 highlighted the need for careful evaluation of the instrumental mass fractionation behaviour for individual isotope systems when using high sensitivity skimmer cones in MC-ICP-MS. In this work, the relative sensitivities for a number of sample and skimmer cone combinations are reported, together with further investigations of the instrumental mass fractionation behaviour, with respect to Nd, using the Thermo Scientific Neptune MC-ICP-MS. Neodymium has seven stable isotopes and high precision isotope ratio values are readily available, making it an ideal element for the study of instrumental mass fractionation. An improved understanding of the mass fractionation behaviour of Nd on different MC-ICP-MS instrument platforms is not only important for the application of Nd in geochronology and provenance studies, but will also contribute to a wider understanding of the origins of instrumental mass fractionation in ICP-MS.
Experimental
Mass spectrometry
Datasets were acquired using a Neptune MC-ICP-MS (Thermo Scientific, Bremen, Germany), over a period of approximately 1 year, at the Water Quality Centre, Trent University. Standard solutions were introduced as a dry aerosol using a Cetac Aridus desolvating system and Aspire PFA micro-concentric nebulizer (nominal uptake rate of 50 μL min−1). Unless otherwise stated, the Aridus was used with the Ar sweep gas only (i.e. no additional N2 gas flow). The instrument operating conditions are given in Table 1.| a Optimized for measurement of relative sensitivities, fixed at 1.1 L min−1 for Nd isotope ratio measurements,. b Pirani gauge, 2nd stage vacuum behind skimmer,. c Penning gauge (transfer lens housing),. d Ion getter pumps. | |
|---|---|
| Rf forward power/W | 1200 |
| Sample gas flow rate/L min−1 | 1.1 a |
| Auxiliary gas flow rate/L min−1 | 0.9 |
| Coolant gas flow rate/L min−1 | 16.5 |
| Fore vacuum pressure/mbar b | <6 × 10−4 |
| High vacuum pressure/mbar c | <1 × 10−7 |
| Analyser pressure/mbar d | <8 × 10−9 |
Relative sensitivities were measured using a 50–100 μg L−1 mixed element solution, in 2% (v/v) nitric acid, containing Li, Mg, Sr, Nd, Hf, Pb and U, for a number of sample and skimmer cone combinations, with both standard and enhanced pumping of the interface region. In the standard configuration, the vacuum chamber between the sample and skimmer cones (referred to here as the expansion chamber) was evacuated by a single 30 m3 hr−1 rotary pump (Pfeiffer UNO 030 B) connected via a NW25 T-piece to two ports, (180° apart) on the first vacuum chamber. In the enhanced pumping configuration, the T-piece was removed and each port was connected to a separate 30 m3 hr−1 rotary pump (Pfeiffer UNO 030B and UNO 30M), using the two existing 25 mm i.d., ∼ 1 m lengths of tubing. The relative sensitivities reported were measured with the guard electrode switched on (grounded) for maximum signal intensity. The lens voltages, and sample and (Aridus) sweep gas flows were re-optimized for each sample and skimmer cone combination and for each individual element. The measured ion intensities were corrected for isotopic abundance and are reported as the total elemental sensitivity, expressed as volts per mg L−1 of analyte.
Neodymium isotope data was obtained using 200 μg L−1 standard solutions (La Jolla and PlasmaCAL, SCP Science) in 2% (v/v) nitric acid. For the measurement of Nd isotope ratios using the NdO+ species, a 1000 μg L−1 standard solution was used to give a signal intensity >5 × 10−11 A for the less abundant 148NdO+ and 150NdO+ species. The collector arrays used are given in Table 2. Each measurement consisted of 5 blocks with 25 integrations of 8 s. A baseline measurement (ESA defocused) consisting of 60 integrations of 1 s was performed prior to each measurement. The standard exponential law was used for correction of the instrumental mass fractionation (with respect to the Nd+ and NdO+ species) with internal normalisation to 146Nd/144Nd = 0.7219.12
| L4 | L3 | L2 | L1 | Ax | H1 | H2 | H3 | H4 | |
|---|---|---|---|---|---|---|---|---|---|
| Nd | 142 | 143 | 144 | 145 | 146 | 147Sm | 148 | 149Sm | 150 |
| NdO | 158 | 159 | 160 | 161 | 162 | ||||
| 160 | 161 | 162 | 164 | 166 |
Sample and skimmer cones
Two standard skimmer cone geometries are commercially available for use with the Neptune MC-ICP-MS instrument; H cones and X cones. In this study, the standard nickel cones (T1002A-Ni and T1002X-Ni respectively) were used, in combination with a standard nickel sample cone (T1001-Ni). The X cones are characterised by an increase in the analytical sensitivity, particularly for lighter elements.13 Further enhancements in the sensitivity have been reported for combination of the X cone with the new Jet sample cone and an enhanced pumping configuration.10,11Results
Relative sensitivities
The abundance corrected sensitivities for Li, Mg, Sr, Nd, Hf, Pb and U, for a number of sample and skimmer cone combinations, with standard and enhanced pumping of the interface region, are given in Table 3. An improvement in analytical sensitivity was observed for all of the elements measured when using the X skimmer cone, compared to the standard H cone, particularly for the lighter elements, as expected.| Sampler | Skimmer | Pumping configuration | Signal intensity/V per mg L−1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Li | Mg | Sr | Nd | % NdO/Nd | Hf | Pb | U | |||
| Standard Ni | H | Standard | 21 | 33 | 36 | 41 | 2 | 46 | 103 | 69 |
| Standard Ni | X | Standard | 563 | 409 | 278 | 162 | 8 | 171 | 478 | 141 |
| Jet | X | Standard | 390 | 342 | 351 | 265 | 3 | 280 | 611 | 282 |
| Jet | X | Enhanced | 369 | 409 | 509 | 319 | 7 | 320 | 822 | 303 |
| Standard Ni | X | Enhanced | 652 | 329 | 249 | 144 | 9 | 154 | 367 | 133 |
In contrast, the relative signal intensity observed when using the Jet sample cone, in combination with the X skimmer cone and standard pumping configuration (with respect to the standard sample cone geometry) was dependent on the element (Table 3, row 3). For Li and Mg, a decrease in the signal intensity of ∼30% and ∼16% respectively was observed. The other elements measured all displayed an increase in the relative signal intensity, with the greatest enhancements observed for Nd and Hf (∼63% increase). When using the Jet sample cone and X skimmer cone with the enhanced pumping configuration (Table 3, row 4), a further increase in the signal intensity was observed for Sr, Nd, Hf, Pb, and U.
Strontium and lead displayed the greatest relative increases in the signal intensity of ∼45% and ∼28% respectively. In contrast, the relative sensitivity for Li was ∼35% lower than for the standard pumping and cone (standard Ni and X) configuration. Similarly, the Jet sample cone and enhanced interface pumping did not confer an increase in the analytical sensitivity for Mg.
The enhanced pumping configuration was also evaluated using a standard sample cone and X skimmer cone (Table 3, row 5). A decrease in the relative sensitivity was observed for Mg, Sr, Nd, Hf, Pb and U, when compared to the alternative pumping configuration and sample cone combinations used with the X skimmer cone in this study. In contrast, the relative sensitivity for Li was 652 V per mg L−1; a 16% increase in the relative sensitivity compared to the same cone combination and the standard pumping configuration.
Nd and NdO isotope data
Neodymium isotope ratios measured for different sample and skimmer cone geometries are given in Table 4. A correlation between the magnitude of the NdO+/Nd+ ratio and the absolute deviation in the xNd+/144Nd+ isotope ratio, relative to reference values, when using high sensitivity skimmer cone geometries, has been reported previously.9 This proportionality is confirmed in the present study (Table 4), and necessitates careful experimental design to elucidate effects associated solely with the ICP-MS interface. The formation of NdO+ will strongly depend on the plasma running conditions. For example, the NdO+/Nd+ ratio was observed to increase with an increase in the Ar sweep gas flow of the Aridus desolvating system used (with a concomitant increase in the optimum focus lens setting to less negative values) and/or sample gas flow rate. Under these conditions of enhanced NdO+ formation, deviations in the measured xNd+/144Nd+ isotope ratios were observed for both the standard H and enhanced sensitivity X skimmer cones, although the magnitude of the relative deviations was significantly greater for the latter.| Skimmer | 142Nd/144Nd | 143Nd/144Nd | 145Nd/144Nd | 148Nd/144Nd | 150Nd/144Nd | NdO+/Nd+ | % Bias b |
|---|---|---|---|---|---|---|---|
| a Deviation with respect to the accepted TIMS values.14 b % deviation from 146Nd/144Nd = 0.7219. c Multiple measurement sessions. | |||||||
| Reference values: (H cone, n = 9) | 1.141879 (48) | 0.512207 (21) | 0.348396 (39) | 0.241595 (24) | 0.236479 (44) | 0.7–1.0 | 2.1–2.7 c |
| La Jolla (n = 3) | 1.141873 (69) | 0.511869 (79) | 0.348409 (30) | 0.241578 (66) | 0.241578 (50) | ||
| ppm deviation a | +3 | +20 | −18 | −39 | +6 | ||
| H cone (high oxide) | 1.141674 (15) | 0.512148 (29) | 0.348382 (33) | 0.241597 (32) | 0.236443 (55) | 2.1 | 2.1 |
| ppm deviation | −180 | −116 | −41 | +10 | −152 | ||
| X cone (low oxide) | 1.141800 (26) | 0.512204 (24) | 0.348413 (18) | 0.241572 (26) | 0.236429 (37) | 0.7 | 2.2 |
| ppm deviation | −69 | −5 | +50 | −95 | −211 | ||
| X cone (high oxide) Nd | 1.141029 (36) | 0.511953 (22) | 0.348305 (7) | 0.241627 (24) | 0.236322 (19) | 5.0 | 2.1 |
| ppm deviation | −744 | −496 | −262 | +134 | −662 | ||
| NdO | 1.150949 (239) | 0.514918 (216) | 0.350055 (143) | 0.240562 (90) | 0.236094 (239) | 2.4 | |
| ppm deviation | +7943 | +5293 | +4762 | −4276 | −1628 | ||
| Nd (GE off) | 1.141787 (84) | 0.512202 (36) | 0.348387 (41) | 0.241595 (75) | 0.236451 (101) | 0.4 | 2.6 |
| ppm deviation | −81 | −10 | −25 | +3 | −119 | ||
| Nd (Jet cone) | 1.141273 (40) | 0.512035 (30) | 0.348338 (8) | 0.241608 (40) | 0.236354 (44) | 4.8 | 2.3 |
| ppm deviation | −531 | −336 | −167 | +55 | −527 |
When using the H skimmer cone (in combination with a standard sample cone), the plasma operating conditions could be optimized to minimize the formation of NdO+, with little or no decrease in the Nd+ signal intensity. Typical NdO+/Nd+ ratios under these conditions were 0.7 to 1.0%;† the fractionation corrected xNd+/144Nd+ isotope ratios are assumed to represent the ‘true’ isotope composition of the SCP Nd solution used (Table 4, row 1) and the deviations in the measured xNd+/144Nd+ isotope ratios, expressed in parts per million, given in Table 4 are relative to these reference values. The accuracy of the reported ratios under these conditions was confirmed by measurement of the La Jolla standard (Table 4, row 2), and are in agreement with the accepted TIMS values,14 within the measurement precision.
The plasma operating conditions could also be adjusted to minimize the NdO+/Nd+ ratio when using the X skimmer cone (and standard sample cone). The lowest attainable NdO+/Nd+ ratio for this cone combination was 0.7% (i.e. similar to the NdO+/Nd+ ratio when using the H cone, as described above). However, this was accompanied by a 15% decrease in the signal intensity for Nd+ (relative to the maximum attainable signal intensity) and the xNd+/144Nd+ isotope ratios measured were observed to have deviations of up to 211 ppm with respect to the reference values (Table 4, row 4). The relative deviations observed, as a function of the average mass of the isotope ratio, are of the same form as that reported by Vance and Thirlwall15 for Nd isotope ratios measured using a Micromass Isoprobe MC-ICP-MS instrument. The authors showed that there is a linear relationship between the difference in the average mass of the measured ratio and the normalizing ratio, and the deviation in the measured ratio from the accepted value. Furthermore, these authors reported similar deviations on both Nu Plasma and MAT Neptune MC-ICPMS instruments (although no details were given of the experimental set-up).15
Rows 5 to 8 of Table 4 reports Nd isotope data obtained using the X skimmer cone, with the plasma operating conditions optimized for maximum signal intensity (and hence elevated NdO+/Nd+ ratios). For the Nd+ and NdO+ ratios measured using the standard sample cone geometry, the (Aridus) sweep gas, the sample gas flow and the sample depth (torch z position) were constant. Deviations in the measured Nd isotope ratios (from the reference values) of up to 744 ppm were observed. The largest deviations of −744 ppm and −662 ppm were observed for the 142Nd/144Nd and 150Nd/144Nd ratios respectively. Large negative offsets were also observed for the 143Nd/144Nd and 145Nd/144Nd ratios (Table 4 and Fig. 1a). In contrast, a positive offset of +134 ppm, relative to the expected value, was observed for the 148Nd/144Nd ratio.
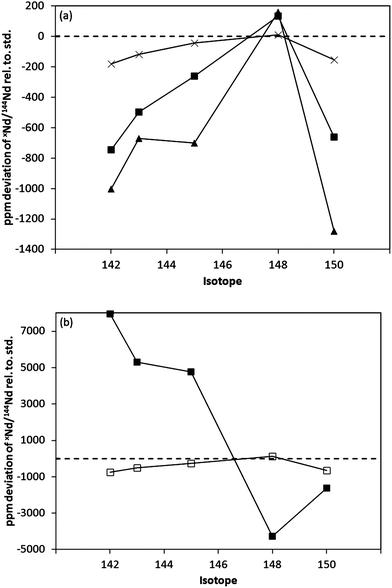 | ||
| Fig. 1 (a) Deviation in the measured xNd/144Nd isotope ratios, relative to reference values (Table 4, row 1), for (a) Nd+, for different skimmer geometries under conditions of high NdO+ formation; H cone ×, X cone ■, Nu Type C cone (ref. 9) ▲, (b) Nd+ □, and NdO+ ■, using the X cone. Isotope ratios were measured for a SCP Nd solution, using a standard sample cone and pumping configuration, and guard electrode grounded (on). | ||
With the exception of the reference values (rows 1 and 2), the isotope ratio data given in Table 4 is for a single measurement session and are typical values to illustrate the relative deviations from the reference values. The general trends observed were reproducible over a period of several months, however, the absolute values measured were dependent on the NdO+/Nd+ ratio, which varied by <3% between measurement sessions for comparable plasma operating conditions and skimmer geometry. This variation is attributed to changes in the leak rate associated with the sample introduction system (e.g. torch connections and gas lines) and erosion of the skimmer tip.
The relative deviations in the measured Nd isotope ratios, relative to the reference values, are comparable to that reported previously for the Nu MC-ICP-MS instruments,9 when using high sensitivity skimmer cone geometries (Fig. 1a). The magnitude of the deviations are smaller in this work, consistent with the lower NdO+/Nd+ ratios reported (Table 4, penultimate column). The lower NdO+/Nd+ ratio may be partly attributed to differences in the Nu Plasma Type C and Thermo Scientific X cone geometries. However, since oxide levels will also depend on the plasma operating conditions and sample introduction system used, direct comparisons of absolute oxide levels between different ICP-MS instruments is very difficult.
For comparison, Table 4 (row 3) and Fig. 1a also includes the deviation in the xNd/144Nd isotope ratios for the H cone, measured using an elevated sweep gas flow rate (and concomitant increase in the NdO+/Nd+ ratio). The variation in the relative deviations with the average isotope ratio mass are comparable to the high sensitivity skimmer cone geometries, although the absolute magnitude of the deviations is significantly smaller for the H cone geometry, and is consistent with the lower NdO+/Nd+ ratio observed.
The previous study9 also reported large deviations from the reference values for the NdO+ isotope ratios, but in the opposite direction to that observed for the same ratio measured using the Nd+ species. This inverse relationship between the measured deviations in the Nd+ and NdO+ ratios was also observed in the current study when using the X cone (Table 4, rows 5 and 6 and Fig. 1b). For example, the 143NdO+/144NdO+ ratio displayed a positive deviation of +5293 ppm, in the opposite sense to the −496 ppm deviation observed for the 143Nd+/144Nd+ ratio. This co-variation of the metal and oxide species, whereby a negative deviation from the accepted xNd/144Nd ratio is associated with a positive deviation in the same ratio for the NdO+ species (and vice versa) appears to be supported by mass balance calculations. The calculated isotope ratios agree within ± 200 ppm for the xNd/144Nd ratios, where x = 142, 143, 145 and 148. The agreement between the calculated and ‘true’ 150Nd/144Nd ratio is less (- 728 ppm). The reason for this is unknown; but the anomalous (i.e. lower than expected) 150NdO/144NdO ratio reported is reproducible over the course of several months and may be speculatively attributed to a failure in the exponential law to adequately correct for the mass fractionation behaviour of the oxide species. Indeed, this may be expected if NdO+ is formed in the supersonic expansion (i.e. after the sample cone) as opposed to in the bulk plasma.
The deviation in the measured xNd/144Nd isotope ratios for a standard sample cone and X skimmer cone, with the guard electrode grounded (on) and floating (off) are given in Table 4 (row 7) and Fig. 2. There was no change in the ion optic tuning, plasma or sample introduction parameters. When the guard electrode was switched off, a 5-fold reduction in the signal intensity was observed, with a concomitant 12.5-fold decrease in the NdO+/Nd+ ratio (Table 4). This attenuation of the NdO+ signal significantly improved the accuracy of the measured xNd/144Nd ratios (as expected) with the maximum deviation, from the reference value, of −119 ppm observed for the 150Nd/144Nd ratio.
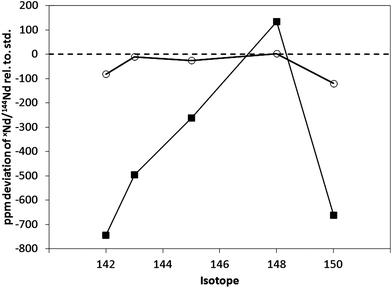 | ||
| Fig. 2 Deviation in the measured xNd/144Nd+ isotope ratio for SCP Nd+ solution, for standard sample cone and X skimmer cone, with guard electrode grounded (on), ■ and floating (off), ○. | ||
Neodymium isotope ratios measured using the X skimmer cone in combination with the Jet sample cone (Table 4, row 8), also displayed the same relative deviations in the measured xNd/144Nd ratios, but the absolute magnitude of the deviations was slightly smaller when compared to the X cone and standard sample cone combination. This has been attributed to the slightly lower (by 6%) optimum sweep gas flow rate of the Aridus system, with a concomitant decrease in the NdO+/Nd+ ratio. As described above, the plasma operating conditions could also be adjusted to minimise the NdO+/Nd+ ratio. However, for the X skimmer cone and Jet sample cone combination, reducing the NdO+/Nd+ ratio to 0.8% was accompanied by a 38% decrease in the signal intensity. For the various sample and skimmer cone combinations and plasma operating conditions evaluated in this work there was no obvious correlation between the magnitudes of the non-linear mass fractionation effects observed and the instrumental mass bias (Table 4, last column).
The addition of small quantities (<5 ml min−1) of nitrogen, admixed to the sample gas flow, was previously found to attenuate the NdO+/Nd+ ratio (and hence the deviation in the measured Nd isotope ratio from their accepted values).9 The deviation in the measured xNd/144Nd isotope ratios (standard sample cone and X skimmer cone) with the addition (via the Aridus system) of 3 to 15 mL min−1 of nitrogen is given in Fig. 3. As expected, the addition of nitrogen attenuated the NdO+ ion intensity, and improved the accuracy of the measured xNd/144Nd ratios. At N2 flow rates >15 ml min−1, the Nd+ signal intensity was observed to decrease with no further reduction in the NdO+/Nd+ ratio. The minimum NdO+/Nd+ ratio observed with the addition of nitrogen in this work was ∼0.1%, compared to <0.04% in the previous study.9 In the previous study, the nitrogen was admixed directly to the sample gas flow. In contrast, here the nitrogen was added to the sweep gas flow of the Aridus system. Nitrogen addition to the sample gas flow is, therefore, limited by passage of the nitrogen through the PTFE desolvating membrane, accounting for the smaller effects observed in this work.
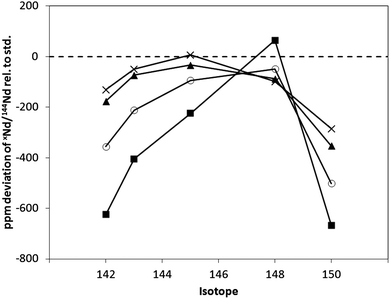 | ||
| Fig. 3 Deviation in the measured xNd/144Nd+ isotope ratio for SCP Nd+ solution with N2 addition, standard sample cone and X skimmer cone, guard electrode grounded (on), NdO+/Nd+ ratio in parentheses; Ar only (5.7%) ■, 3 ml min−1 (0.9%) ○, 10 ml min−1 (0.4%) ▲, 15 ml min−1 (0.1%) ×. | ||
Discussion
The relative elemental sensitivities reported in Table 3 clearly demonstrate that the X skimmer cone geometry confers a significant improvement in the analytical sensitivity. The optimum experimental set-up, in terms of choice of sample cone geometry and interface pumping configuration, is element dependent. For example, in the case of Li, the standard sample cone and enhanced pumping configuration gave the maximum sensitivity of 652 V per mg L−1, an increase of ∼16% in sensitivity compared to the standard pumping configuration. Absolute sensitivities for Li >3000 V per mg l−1 have been reported using a single dry interface pump with a 100 m3 hr−1 pumping capacity.10 Taking into account the higher sample uptake rate used in ref. 10, this represents a 2.3-fold relative increase in the analytical sensitivity for Li by comparison to this study.For heavier elements (Sr to U), the Jet sample cone in combination with enhanced pumping of the interface was found to give the maximum analytical sensitivity. The absolute sensitivities reported here are 2 to 4 times lower (depending on the element) than reported for the same skimmer and sample cone combination and a 100 m3 hr−1 dry interface pump.10
In addition to improved ion transmission, the X skimmer cone geometry is also associated with additional contributions to the instrumental mass fractionation (at least in the case of Nd) that do not have a simple exponential dependence on the isotope mass. Two distinct contributions to these additional mass fractionation effects can be identified. Under conditions of low NdO+ formation (e.g. low sweep and sample gas flows and/or the addition of N2), deviations in the measured xNd/144Nd ratios are a function of the average mass of the isotope pair.15 Similar deviations were reported for the standard sample and X skimmer cone combination and a 100 m3 hr−1 dry interface pump, using the Aridus II system for sample introduction (with N2 addition).10 This appears to be a general limitation of using the exponential law, over a relatively large mass range, for the correction of the large mass fractionation (relative to TIMS, for example) associated with the ICP-MS interface. The larger divergence in the instrumental mass fractionation, from the exponential law, observed for the X cone (relative to the H cone) may imply that the failure of the exponential law has its origins in space charge effects associated with the increased ion transmission in the interface region, or other changes in the properties of the sampled plasma, behind the skimmer cone.
The second contribution to deviations from the standard exponential law, observed when using the X skimmer cone geometry, is associated with an increase in the NdO+/Nd+ ratio. This work has shown that the non-linear mass dependent fractionation for Nd reported in the previous study9 is not specific to the sample–skimmer cone combination or interface configuration used on the Nu MC-ICP-MS instruments. It is a general feature of ICP-MS under conditions that favour the formation of the NdO+ species, due to either a change in the plasma operating conditions (e.g. an increase in the sample gas flow, absence of a secondary discharge at the interface, change in plasma gas composition) or modification of the sample or skimmer cone geometry.
High sensitivity skimmer cone geometries and MO+ formation
The dependence of the NdO+/Nd+ ratio (and more generally MO+/M+, where M is a REE) on the plasma operating conditions may be considered as indicative of the formation of these species in the ICP. There is some evidence for MO+ formation in the bulk plasma; YO+ emission bands have been observed in the ICP, for example.16,17 In another study,8 calculations of the source kinetic temperature (by comparison of the theoretical and experimental dissociation reactions of common polyatomic species in the ICP) suggested that strongly bound metal ions, such as CeO+, are formed in the bulk plasma. However, the results of this and preceding work9 unambiguously show that elevated MO+/M+ ratios are associated with high sensitivity skimmer cone geometries.The Neptune X cone and Nu Plasma high sensitivity (HS) cone confer an increase in the analytical sensitivity that implies that these modified skimmer geometries enhance the ion sampling efficiency with respect to the supersonic expansion. The greater signal enhancements observed for the lighter elements could suggest increased sampling of ions with a lower kinetic energy (KE) and/or from the outer edges of the expansion plume. Therefore, one possibility is that the elevated MO+/M+ ratio associated with high sensitivity skimmer cone geometries reflects the distribution, and increased sampling, of MO+ in the supersonic expansion. This would be supported by the lower KEs observed for the MO+ species compared to isobaric atomic ions.18 However, the magnitude of the relative deviations in the measured Nd isotope ratios from their reference values depends on the NdO+/Nd+ ratio, which indicates that the modified skimmer geometries are associated with an increase in the formation (or a decrease in the dissociation) of the MO+ species, as opposed to a simple increase in the sampling efficiency of MO+.
It has been proposed9 that the formation of MO+ proceeds via reaction pathways involving excited electronic states:
| M+(*) + O(m) → MO+* → MO+ + hν | (1) |
Formation of MO+ during the sampling process has been suggested previously; for example, Vaughan and Horlick1 reported an increase in the MO+/M+ ratio as the sample orifice diameter decreased and proposed that MO+ was formed in the very early stages of the supersonic expansion. This study and others19–21 suggested that the anomalously high plasma temperature determined from Boltzman plots of MO+/M+versus the MO+ bond dissociation energy were incongruent with the formation of MO+ in the bulk plasma. Whilst numerous collisions occur in the early stages of the supersonic expansion (i.e. in the vicinity of the sample cone orifice), the probability of reaction in the centreline gas flow, behind the sample cone, is generally thought to be insignificant due to the low collision frequency and short residence time (of a few μs).2 However, Nonose & Kubota22 observed molecular band emissions for N2 and N2+ behind the skimmer, that were not observed behind the sample cone, using a high resolution ICP-MS. They also demonstrated the ionisation of N2+, (presumably via charge transfer reactions with Ar atoms) and presented evidence for possible collision-induced dissociation of ArO+, occurring at the skimmer tip. Measurement of the electron density and temperature by Niu et al.23 highlighted the formation of a shock wave in front of the skimmer, that could act as a new source for a secondary expansion through the skimmer. Therefore, the skimmer cone is more than a passive obstruction in the supersonic expansion, and there is the potential for chemical processes (e.g.oxide formation, collisional quenching) to occur close to the skimmer tip that would alter the resultant mass spectrum.
Effects of a secondary discharge at the ICP interface
It is proposed that the dependence of the MO+/M+ on the plasma operating conditions reflects a change in the physical properties of the supersonic expansion, which will influence ion-atom/molecule reactions and the behaviour of excited states in the supersonic expansion. This is particularly true for a secondary discharge‡ at the sample cone. The NdO+/Nd+ ratio (and concomitant non-linear mass dependent fractionation) is strongly dependent on the status of the guard electrode (Fig. 2), inserted between the load coil and outer edge of the torch. When this electrode is grounded (on), it attenuates the formation of a secondary discharge between the plasma and the grounded sample cone.The magnitude of the secondary discharge is proportional to the plasma potential (typically a few volts) and is dependent on the plasma operating conditions. The plasma potential may be attenuated by reducing the sample gas flow rate and solvent loading of the plasma, increasing the rf power and/or reducing the sampling depth.2,24,25 These effects are associated with changing the impedance of the sample cone sheath. A number of studies have also investigated the potential associated with the micro-plasma observed immediately behind the sample and skimmer cones and in the supersonic expansion,26–29 which was observed to vary in a similar manner to the ICP potential, with respect to the plasma operating conditions.
The attenuation of a secondary discharge at the ICP interface is associated with an increase in the analytical sensitivity. Nonoseet al.30 reported a decrease in the mean and relative distribution of ion kinetics energies when using a grounded guard electrode (using ArO+ and LaO+ as probe species), which would improve ion transmission through ion optical apertures. In another study, atomic (Pb I and Ar metastables) and ionic (Ba II and Sc II) fluorescence intensities (which is proportional to the species density), measured directly behind the skimmer cone, were observed to increase significantly when using a grounded guard electrode.31 In contrast, ion and atom densities in front of the sample cone were not affected by the status of the guard electrode. The increase in the atom and ion densities behind the skimmer suggests that the magnitude of the plasma potential also influences the flow field of the supersonic expansion (presumably due to the decrease in gas kinetic temperature when the secondary discharge is attenuated and concomitant increase in the gas flow through the sample cone orifice)§, so as to increase the atom and ion densities along the centreline flow sampled by the skimmer. Spectroscopic measurements30 indicated that the attenuation of a secondary discharge was also accompanied by a decrease in the electron number density and emission intensity of atomic ionic (Mg II) and neutral (Mg I) species, in both the ICP and supersonic expansion behind the sample cone. Therefore, the presence (or absence) of a secondary discharge at the sample cone is associated with significant changes in the properties of the supersonic expansion, summarized in Table 5.
A correlation has been reported previously between a reduction in the plasma potential and an increase in the MO+/M+ ratio (where M is a REE). For example, Gray et al.24 reported a decrease in the plasma potential and a concomitant increase in the CeO+/Ce+ ratio, as the rf power was increased. Becker and Dietze32 reported an increase in the ThO+/Th+ ratio by a factor of 1.6 to 13, depending on the type of nebulizer used, when using a grounded guard electrode. This has previously been attributed to an increase in the dissociation of MO+ in the secondary discharge at the sample cone, without elaboration.24,32 The higher ion KE (which is proportional to the plasma potential) associated with the presence of a secondary discharge would result in more energetic collisions during the sampling process and in the supersonic expansion. This is reflected in the observed increase in the atomic emission intensity30 indicating the promotion of excitation pathways. In the case of molecular species such as MO+, step-wise vibrational excitation via multiple collisions can result in dissociation. However, the relative kinetic energies of M+(*) and O(m) in Reaction (1) will determine the duration of any collision, and hence, together with changes in the atom and ion densities in the vicinity of the skimmer cone,31 the magnitude of the plasma potential may also influence reaction cross sections in the supersonic expansion, so as to reduce the probability of reaction occurring. Therefore, from the results of this (and preceding studies), it is not possible to elucidate whether the attenuation of the MO+/M+ ratio in the presence of a secondary discharge is due to an increase in dissociation or a decrease in the rate of formation of MO+.
It is interesting to note that the elevated NdO+/Nd+ ratio observed on the Nu MC-ICP-MS instrument when using high sensitivity skimmer cone geometries9 would imply that the magnitude of any secondary discharge present is small, even without the use of a guard electrode. This is consistent with the results of earlier studies34 using a grounded guard electrode, in which no significant increase in the analytical sensitivity was observed (when using a desolvating nebulizer system for sample introduction). This is also in agreement with early studies of ion sampling from the ICP, which reported an increase in sensitivity when the sample and/or skimmer cone was electrically floated, and was attributed to the attenuation of the secondary discharge at the sample cone.35 Langmuir probe measurements24 confirmed that the plasma potential followed the interface potential (with a fixed offset). This enables ions to be sampled from the plasma, even if the interface is at a potential of +4 to +6 kV.
Conclusions
Enhancement of the analytical sensitivity in MC-ICP-MS can be achieved by judicious selection of the sample and skimmer cone combination, for specific applications. Signal enhancements (up to a factor of two) were observed for the new Jet sample cone, in combination with the X skimmer cone, for the heavier elements (i.e. Sr to U). Further increases in signal intensity may be realised with (even a modest) improvement in the interface pumping capacity. To achieve the maximum sensitivity reported for the Jet sample cone requires a significant increase in the pumping capacity, compared to the standard configuration.10 However, contrary to the manufacturer`s recommendations, the Jet sample cone can be used without modification of the interface pumping and confer an (albeit modest) increase in the analytical sensitivity for heavier elements.In the case of Nd, high sensitivity skimmer cone geometries are associated with additional contributions to the instrumental mass fractionation that cannot be corrected for using the standard exponential, power or linear laws. A significant component of these anomalous mass fractionation effects is associated with the formation of NdO+via isotope dependent excited state reaction pathways. Whilst elucidation of the mechanistic details is difficult due to the interrelation of plasma parameters, what is clear is that the NdO+/Nd+ ratio ultimately determines the accuracy of the measured isotope ratios, irrespective of the MC-ICP-MS instrument platform used. As such, measures to minimize the oxide ratio (i.e. by optimization of plasma parameters, sample and skimmer cones used) should be incorporated into analytical protocols. Even when the formation of NdO+ is minimized by adjustment of the plasma operating conditions (at the expense of signal intensity), the standard exponential law cannot correct for the instrumental mass fractionation, associated with the X skimmer cone geometry.
The anomalous mass fractionation effects reported here and in a previous study9 do not necessarily exclude the use of high sensitivity skimmer cone geometries for isotope ratio measurements (e.g. in Nd-depleted samples such as seawater); however additional empirical corrections are required (e.g. by setting cup efficiencies). Neodymium isotope ratios may also be accurately expressed relative to an isotopic standard provided that the standard and the sample display the same non-linear mass dependent fractionation behaviour. This would need to be verified for individual applications as any residual matrix in the sample may influence the formation of NdO+ and hence the Nd isotope ratios.
Acknowledgements
Dr Phil Freedman (Nu Instruments) is thanked for helpful comments on the manuscript. Dr R. Bastian Georg (Trent University, Water Quality Centre) is acknowledged for one Nd data set. This study was funded by Trent University Water Quality Centre. The constructive comments of three anonymous reviewers are also acknowledged.References
- M. A. Vaughan and G. Horlick, Spectrochim. Acta, Part B, 1990, 45, 1289 CrossRef.
- H. Hiu and R. S. Houk, Spectrochim. Acta, Part B, 1996, 51, 779 CrossRef and references therein.
- K. E. Jarvis, P. Mason, T. Platzner and J. G. Williams, J. Anal. At. Spectrom., 1998, 13, 689 RSC.
- I. Brenner, J. Pacheco and M. Valiente, J. Anal. At. Spectrom., 2009, 24, 1558 RSC.
- E. Colas, M. Valiente and I. Brenner, J. Anal. At. Spectrom., 2004, 19, 282 RSC.
- H. P. Longerich, B. J. Fryer, D. F. Strong and C. J. Kantipuly, Spectrochim. Acta, Part B, 1987, 42, 75 CrossRef.
- N. P. Zahran, A. I. Helal, M. A. Amr, A. Abdel-Hafiez and H. T. Mohsen, Int. J. Mass Spectrom., 2003, 226, 271 CrossRef CAS.
- J. Wisnewski Ferguson and R. S. Houk, Spectrochim. Acta, Part B, 2006, 61, 905 CrossRef.
- K. Newman, P. A. Freedman, J. Williams, N. S. Belshaw and A. N. Halliday, J. Anal. At. Spectrom., 2009, 24, 742 RSC.
- C. Bouman, M. Deerberg, J. B. Schwieters, Thermo Fischer Scientific Application Note: 30187 Search PubMed.
- A. Makishima and E. Nakamura, J. Anal. At. Spectrom., 2010, 25, 1712 RSC.
- R. K. O'Nions, P. J. Hamilton and N. M. Evensen, Earth Planet. Sci. Lett., 1977, 34, 13 CrossRef CAS.
- S. Weyer and J. B. Schwieters, Int. J. Mass Spectrom., 2003, 226, 355 CrossRef CAS.
- M. F. Thirwall, Chem. Geol., 1991, 94, 85 CrossRef.
- D. Vance and M. Thirwall, Chem. Geol., 2002, 185, 227 CrossRef CAS.
- N. Furata, Spectrochim. Acta, Part B, 1986, 41, 1115 CrossRef.
- K. P. Li, M. Dowling, T. Fogg, T. Yu, K. S. Yeah, J. D. Hwang and J. D. Winefordner, Anal. Chem., 1988, 60, 1590 CrossRef CAS.
- S. D. Tanner, J. Anal. At. Spectrom., 1993, 8, 891 RSC.
- D. J. Douglas and J. B. French, Spectrochim. Acta, Part B, 1986, 41, 197 CrossRef.
- H. P. Longerich, B. J. Fryer, D. F. Strong and C. J. Kantipuly, Spectrochim. Acta, Part B, 1987, 42, 75 CrossRef.
- A. J. R. Kent and C. A. Ungerer, J. Anal. At. Spectrom., 2005, 20, 1256 RSC.
- N. Nonose and M. Kubota, J. Anal. At. Spectrom., 2001, 16, 551 RSC.
- H. Niu, S. Luan, H. Pang and R. S. Houk, Spectrochim. Acta, Part B, 1995, 50, 1247 CrossRef.
- A. L. Gray, R. S. Houk and J. G. Williams, J. Anal. At. Spectrom., 1987, 2, 13 RSC.
- C. J. Park and S. M. Noh, J. Anal. At. Spectrom., 1998, 13, 715 RSC.
- H. B. Lim, R. S. Houk and J. S. Crain, Spectrochim. Acta, Part B, 1989, 44, 989 CrossRef.
- H. B. Lim and R. S. Houk, Spectrochim. Acta, Part B, 1990, 45, 453 CrossRef.
- D. M. Chambers, J. Poehlman, P. Yang and G. M. Hieftje, Spectrochim. Acta, Part B, 1991, 46, 741 CrossRef.
- H. Niu and R. S. Houk, Spectrochim. Acta, Part B, 1994, 49, 1283 CrossRef.
- N. S. Nonose, N. Matsuda, N. Fudagawa and M. Kubota, Spectrochim. Acta, Part B, 1994, 10, 955 CrossRef.
- B. S. Duersch, J. E. Patterson and P. B. Farnsworth, J. Anal. At. Spectrom., 1999, 14, 615 RSC.
- J. S. Becker and H. Dietze, Int. J. Mass Spectrom., 2000, 202, 69 CrossRef CAS.
- D. J. Douglas and J. B. French, J. Anal. At. Spectrom., 1988, 3, 743 RSC.
- Nu Instruments Ltd., unpublished work.
- K. Hu and R. S. Houk, J. Am. Soc. Mass Spectrom., 1993, 4, 733 CrossRef CAS.
Footnotes |
| † The CeO+/Ce+ ratio quoted by the manufacturer when using the Aridus system is <0.05%; the NdO+/Nd+ ratio will be even lower.9 This is true for the H cone/standard sample cone configuration when small quantities of N2 are added to the Ar sweep gas (i.e. the routine set-up) however, this was not used as standard in this work. |
| ‡ The plasma floats to an rf potential that is determined by the ratio of impedances associated with the sample cone sheath, and capacitive coupling of the plasma and load coil. The rf voltage is rectified by the sample cone sheath to give a net positive dc voltage, referred to as the plasma potential. |
| § The total gas flow through the sample cone orifice scales as Tgas−1/2, where Tgas is the source gas kinetic temperature.33 |
| This journal is © The Royal Society of Chemistry 2012 |
