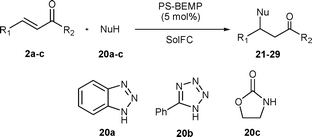
E-factor minimized protocols for the polystyryl-BEMP catalyzed conjugate additions of various nucleophiles to α,β-unsaturated carbonyl compounds†
Simona
Bonollo
,
Daniela
Lanari
,
Julie M.
Longo
and
Luigi
Vaccaro
*
Laboratory of Green Synthetic Organic Chemistry, CEMIN - Dipartimento di Chimica, Università di Perugia, Via Elce di Sotto, 8, Perugia, Italia. E-mail: luigi@unipg.it; Fax: +39 075 5855560; Tel: +39 075 5855541
First published on 3rd November 2011
Abstract
Efficient protocols for the addition of carbon-, sulphur- and nitrogen-nucleophiles to α,β-unsaturated carbonyl compounds catalyzed by PS-BEMP have been reported. The adoption of solvent-free conditions (SolFC) was crucial for improving the efficiency of all the processes, while by using an organic reaction medium poor results were obtained. Addition reactions were performed by using equimolar amounts of reagents, and the products were isolated by simple filtration with the minimal amount of organic solvent. This approach allowed the E-factor, a measure of the waste of a reaction, to be minimized. Further waste minimization (95.7% compared to batch protocol) has been accomplished by defining a larger scale continuous-flow protocol operating under SolFC.
Introduction
The Michael addition is one of the more important carbon–carbon bond forming reactions in organic chemistry.1 The aza- and sulfa-Michael additions2 are equally important carbon-nitrogen and carbon-sulfur bond forming reactions, respectively, which are key steps in the preparation of several intermediates. In general, these reactions must take place in either strongly basic or strongly acidic conditions.Recently, we have focused our work on the optimization of synthetic procedures, including Michael additions, by employing eco-friendly reaction protocols based on the use of water,3 solvent-free conditions (SolFC),4 and polymer-supported organocatalysts.5 With our approach we have achieved efficient waste minimization (E-factor minimization)6 by setting single- and multistep cyclic continuous-flow reactors.5a–d,f
The development of polymer-supported material as reagents or organocatalysts has greatly increased in recent years.7 These supported catalysts are a more sustainable and chemically efficient alternative to traditional basic catalysts used in Michael additions because they are easily recoverable and recyclable.
The use of SolFC together with polymer-supported organocatalysts has been shown to increase the effectiveness of the catalysts, which are generally but not always,7c–e less efficient than their non-supported counterparts. In the past, this group has shown that polystyrene-supported 1,5,7-triazabicyclo[4.4.0]dec-5-ene (PS-TBD, 1b) is more effective under SolFC than in typical organic solvents.5h,i Further efforts have been made to reduce the environmental impact of these processes by using an equimolar ratio of reactants to reduce waste. This, in turn, helps to reduce the E-factor of these addition reactions.
In particular, our attention has been focused on 2-tert-butylimino-2-diethylamino-1,3-dimethylperhydro-1,3,2-diazaphosphorine supported on polystyrene (PS-BEMP, 1a) (Fig. 1), a very strong base (MeCNpKa is 27.63) and a member of the class of Schwesinger's phosphazene bases that have proved to be widely useful, uncharged auxiliary bases.8 By using a catalytic amount of PS-BEMP, we have reported the nucleophilic addition of nitroalkanes to α,β-unsaturated carbonyl compounds5f and the addition of carbon nucleophiles5e and phenols5c to epoxides under solvent-free conditions, in batch condition and also by using a cyclic continuous flow reactor.
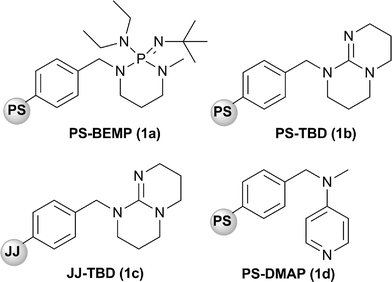 | ||
| Fig. 1 Supported bases used in this study. | ||
In this paper, we present the application of our approach to the use of PS-BEMP as catalyst for the Michael and hetero-Michael addition of a variety of nucleophiles to α,β-unsaturated carbonyl compounds.
Results and discussion
Initially, the efficiency of PS-BEMP (1a) was compared to that of PS-TBD (1b), JJ-TBD (1c), and PS-DMAP (1d) (Fig. 1) in the reaction of equimolar amounts of benzylidene acetone (2a) and dimethylmalonate (3a) under SolFC with 5 mol% of catalyst (Table 1).|
|
|||
|---|---|---|---|
| Entry | Catalyst | Solvent (M) | Conversion (%)b |
| a Reaction conditions: 2a (1.0 mmol), 3a (1.0 mmol), catalyst (5 mol%), 30 °C. b Determined by GC analyses. c Reaction conducted at 60 °C; isolated yield for 4 reported in parentheses. | |||
| 1 | PS-BEMP | — | 86 |
| 2 | PS-BEMP | — | 100 (97)c |
| 3 | PS-TBD | — | 2 |
| 4 | JJ-TBD | — | 54 |
| 5 | PS-DMAP | — | 0 |
| 6 | PS-BEMP | CH3CN (0.5) | 10 |
| 7 | PS-BEMP | CH2Cl2 (0.5) | 9 |
| 8 | PS-BEMP | THF (0.5) | 2 |
| 9 | PS-BEMP | MeOH (0.5) | 63 |
After 24 h at 30 °C, the reaction with PS-BEMP was at 86% conversion (Table 1, entry 1), while other supported bases, PS-TBD and PS-DMAP respectively, gave only traces of product 4 (Table 1, entries 3,5). JJ-TBD, a more efficient catalyst than PS-TBD,5a allowed us to obtain product 4 with a 54% conversion (Table 1, entry 4).
PS-BEMP was then tested in a variety of reaction media and the best isolated yield was obtained under SolFC (Table 1, entries 1–2 vs. entries 6–9). Also, in the presence of dichloromethane, which is reported to be the most appropriate swelling solvent for polystyrene resins, an unsatisfactory result was obtained (Table 1, entry 7).
The applicability of this protocol was then tested in the Michael addition of carbon (3a–c), sulfur (13a–b), and nitrogen nucleophiles (20a–c) with three representative α,β-unsaturated carbonyl compounds 2a–c.
Table 2 shows the results for the reactions of 2a–c with 3a–c.
|
|
|||||||
|---|---|---|---|---|---|---|---|
| Entry | A | NuH | T/°C | t (h) | Product | Yield (%)b | E-factorc |
| a Reaction conditions: acceptor (1.0 mmol), donor (1.0 mmol), catalyst (5 mol%). b Isolated yield of the pure products. c For calculation see Electronic Supplementary Information.† d Diastereoisomeric mixture. e 20 mol% of catalyst were used. f 3 equiv. of nucleophiles were used. g Column chromatography purification was necessary. | |||||||
| 1 | 2a | 3a | 60 | 24 |

|
97 | 5.9 |
| 2 | 2a | 3b | 60 | 120 |

|
95d | 9.0 |
| 3e | 2a | 3c | 80 | 48 |
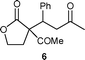
|
90d | 6.5 |
| 4 | 2b | 3a | 30 | 24 |

|
95 | 11.0 |
| 5 | 2b | 3b | 30 | 24 |

|
93d | 7.5 |
| 6 | 2b | 3c | 30 | 24 |

|
94d | 11.3 |
| 7f,g | 2c | 3a | 30 | 72 |
|
85 | 10.1 |
| 8f,g | 2c | 3b | 30 | 3.5 |

|
90 | 9.5 |
| 9 | 2c | 3c | 30 | 3 |

|
92 | 12.1 |
As with the reaction of 2a with 3a, PS-BEMP proved to be an effective catalyst for the Michael additions of the other carbon nucleophiles furnishing products 4–12 in high yields (85–97%). The reactions proceeded with 5 mol% of the catalyst and equimolar amounts of reactants (for exceptions see entries 3,7 and 8). These conditions, together with the use of SolFC, allowed us to define a simple and chemically efficient protocol for minimizing the amount of waste and consequently the E-factor of the processes (for calculation of E-factor see Electronic Supplementary Information†).
Waste was further minimized through the work-up procedure consisting of a simple filtration of the reaction mixture using the minimum possible amount of solvent. In the case of the carbon nucleophiles, the E-factor ranged from 5.9 to 12.1. When methylacrylate (2c) was used in the reaction with dimethylmalonate (3a) and ethylnitroacetate (3b) an additional bis-adduct product was formed when the reactants were used in a one to one ratio (37% and 20% respectively).
To suppress this side product, three equivalents of the nucleophile were used, though for the production of 10, 12% of the product was still the bis-adduct.
The two sulfur nucleophiles, benzenethiol (13a) and butanethiol (13b), were very reactive (Table 3). In all cases, the reactions reached 100% conversion at 30 °C in a very short time and the products 14–19 were isolated in yields ranging from 92–97%.
|
|
||||||
|---|---|---|---|---|---|---|
| Entry | A | R3SH | t (h) | Product | Yield (%)b | E-Factorc |
| a Reaction conditions: acceptor (1.0 mmol), donor (1.0 mmol), catalyst (0.5 mol%), 30 °C. b Isolated yield of the pure products. c For calculation see Electronic Supplementary Information.† d 2 mol% of catalyst were used. | ||||||
| 1 | 2a | 13a | 5 |

|
97 | 5.7 |
| 2d | 2a | 13b | 1 |

|
95 | 6.4 |
| 3 | 2b | 13a | 5 |

|
92 | 7.6 |
| 4 | 2b | 13b | 3 |
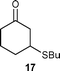
|
96 | 8.0 |
| 5 | 2c | 13a | 2 |

|
96 | 7.6 |
| 6 | 2c | 13b | 1 |

|
93 | 8.8 |
These nucleophiles also required the least amount of catalyst (0.5 mol%), except for the formation of 15 (Table 3, entry 2), in which 2 mol% of PS-BEMP was used. The E-factor for the formation of 14–19 ranged from 5.7–8.8.
The results of the Michael additions of nitrogen nucleophiles, 20a–c are shown in Table 4. Due to the low nucleophilicity of these heterocycles, temperatures from 60 to 80 °C were generally required, except in the formation of 27a and 27b in which the temperature was kept at 30 °C (Table 4, entry 7). These reactions also proceeded much more slowly than the sulfur nucleophiles, with reaction times ranging from 24 to 120 h. The E-factor ranged from 9.5 to 12.7.
|
|
|||||||
|---|---|---|---|---|---|---|---|
| Entry | A | NuH | T/°C | t (h) | Product | Yield (%)b | E-Factorc |
| a Reaction conditions: acceptor (1.0 mmol), donor (1.0 mmol), catalyst (5 mol%). b Isolated yield of the pure products. c For calculation see Electronic Supplementary Information.† d Regioisomeric mixture. e 3 equiv. of acceptor were used. f 2 equiv. of acceptor were used. | |||||||
| 1 | 2a | 20a | 60 | 120 |
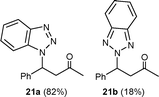
|
90d | 9.5 |
| 2 | 2a | 20b | 80 | 24 |
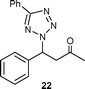
|
75 | 11.2 |
| 3 | 2a | 20c | 60 | 24 |

|
72 | 12.2 |
| 4 | 2b | 20a | 80 | 24 |

|
92d | 12.1 |
| 5 | 2b | 20b | 80 | 24 |

|
91 | 10.9 |
| 6 | 2b | 20c | 60 | 24 |

|
83 | 12.7 |
| 7 | 2c | 20a | 30 | 72 |

|
94d | 12.4 |
| 8e | 2c | 20b | 60 | 96 |

|
96 | 11.5 |
| 9f | 2c | 20c | 60 | 72 |

|
99 | 9.8 |
Finally, the recovery of the catalyst was also considered. PS-BEMP was able to be reused for five consecutive runs without any decrease of its catalytic efficiency.
In addition, we have also set a continuous-flow procedure for the reaction of 2c with 3c (Scheme 1) to show that by this approach it is also possible to even further reduce the waste of processes performed under SolFC. According to our previous reports in this field, the reactor is also a very effective tool to maintain the catalyst integrity (chemical and physical) and perform the process at large scale without further optimization.5a–d,f The schematic representation of the reactor is depicted in Scheme 1 (thermostated box is not showed for clarity).
 | ||
| Scheme 1 Continuous-flow protocol for the representative reaction of 2c with 3c. | ||
The equimolar mixture (50 mmol) of 2c and 3c was charged into a glass column functioning as a reservoir. The catalyst PS-BEMP (5 mol%, 2.5 mmol of BEMP) was charged into a glass column and the reaction mixture was continuously pumped through it at 30 °C for 3.0 h (the time necessary for the complete conversion to 12. At this point, the pump was left to run in order to recover the reaction mixture into the reservoir. Then MeOH was added (3 × 2 mL) to wash the catalyst and to isolate product 12 in 95% yield after removal of the solvent under reduced pressure.
The same protocol was repeated for five consecutive runs and the efficiency of the catalyst was unchanged. After 3 h the conversion of 2c and 3c to 12 was always complete and the final product was always recovered in very high yields (93–95%).
The E-factor of the process in flow is 0.52 (see Electronic Supplementary Information† for details) with a reduction of 95.7% compared to the 12.1 value obtained in the batch process (Table 2, entry 9).
Conclusions
In conclusion, we have reported an efficient protocol for the Michael addition of carbon-, sulphur- and nitrogen-nucleophiles to α,β-unsaturated carbonyl compounds employing PS-BEMP as catalyst. Reactions were conducted under SolFC with equimolar amounts of reagents and the products were isolated by simple filtration using the minimal amount of organic solvent necessary for the workup. This approach allowed us to reduce dramatically the reaction's waste, thus minimizing the E-factor. We have also defined a continuous-flow protocol for the representative reactions of 2c with 3c operating under SolFC on a 50 mmol scale. Under these conditions the same catalyst can be used for at least 5 consecutive runs with no decrease of its efficiency. E-factor has been reduced by 95.7%. This approach has allowed the physical integrity of the catalyst to be conserved and proves that continuous-flow reactors operating under SolFC is a promising tool to reduce waste.Experimental section
Compounds 4,95,107,98,1011,1112,1214,1316,1417,1518,1619,1721a–b,1822,1924a–b,2026,21 are known compounds, compounds 10, 23, 27a–b, 28a, 29 have been already prepared but spectroscopic data have not been reported, while compounds 6, 9, 15, 25, 28b are new compounds. Characterization data (1H NMR, 13C NMR, GC-EIMS, mp, and elemental analyses) for compounds 6, 9, 10, 15, 23, 25, 27a, 27b, 28a, 28b, 29 are reported in Electronic Supplementary Information (ESI).†Representative experimental procedure
In a screw capped vial equipped with a magnetic stirrer PS-BEMP (0.048 g, 0.1 mmol, 2.1 mmol g−1), trans-4-phenyl-3-buten-2-one (2a) (0.292 g, 2.0 mmol) and dimethylmalonate (3a) (0.229 ml, 2.0 mmol) were consecutively added and the resulting mixture was left under vigorous stirring at 60 °C. After 24 h, methanol was added, the catalyst was recovered by filtration and the organic solvent was evaporated under vacuum to give pure dimethyl-2-(1′-phenyl-3′-oxo-butyl)malonate (4) as a colourless oil (0.541 g, 97% yield).Acknowledgements
This work was supported by the REU program of the National Science Foundation under award number CHE-0755206. Thanks to the American Chemical Society for coordinating the IREU exchange program. We gratefully acknowledge the Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR) within the projects PRIN 2008 and “Firb-Futuro in Ricerca” and the Università degli Studi di Perugia for financial support.Notes and references
- For a review on asymmetric Michael additions: N. Krause and A. Hoffmann-Röder, Synthesis, 2001, 171–196 Search PubMed.
- For recent reviews on asymmetric aza Michael addition: (a) D. Enders, C. Wang and J. X. Liebich, Chem.–Eur. J., 2009, 15, 11058–11076 CrossRef CAS; (b) P. R. Krishna, A. Sreeshailam and R. Srinivas, Tetrahedron, 2009, 65, 9657–9672 CrossRef CAS. Sulfa-Michael: (c) D. Enders, K. Lüttgen and A. A. Narine, Synthesis, 2007, 959–980 CrossRef CAS; (d) G. Bartoli, M. Bartolacci, A. Giuliani, E. Marcantoni, M. Massaccesi and E. Torregiani, J. Org. Chem., 2005, 70, 169–174 CrossRef CAS.
- As representative examples see: (a) S. Bonollo, D. Lanari, F. Pizzo and L. Vaccaro, Org. Lett., 2011, 13, 2150–2152 CrossRef CAS; (b) S. Bonollo, D. Lanari and L. Vaccaro, Eur. J. Org. Chem., 2011, 2587–2598 CrossRef CAS; (c) S. Bonollo, D. Lanari, A. Marrocchi and L. Vaccaro, Curr. Org. Synth., 2011, 8, 319–329 Search PubMed.
- As representative examples see: (a) D. Lanari, R. Ballini, A. Palmieri, F. Pizzo and L. Vaccaro, Eur. J. Org. Chem., 2011, 2874–2884 CrossRef CAS; (b) L. Castrica, F. Fringuelli, F. Pizzo and L. Vaccaro, Lett. Org. Chem., 2008, 5, 602–606 CrossRef; (c) F. Fringuelli, R. Girotti, F. Pizzo and L. Vaccaro, Org. Lett., 2006, 8, 2487–2489 CrossRef CAS.
- (a) D. Lanari, R. Ballini, S. Bonollo, A. Palmieri, F. Pizzo and L. Vaccaro, Green Chem., 2011 10.1039/C1GC15790F; (b) S. Bonollo, D. Lanari, T. Angelini, A. Marrocchi, F. Pizzo and L. Vaccaro, J. Catal., 2011 DOI:10.1016/j.jcat.2011.09.032; (c) A. Zvagulis, S. Bonollo, D. Lanari, F. Pizzo and L. Vaccaro, Adv. Synth. Catal., 2010, 352, 2489–2496 CrossRef CAS; (d) F. Fringuelli, D. Lanari, F. Pizzo and L. Vaccaro, Green Chem., 2010, 12, 1301–1305 RSC; (e) T. Angelini, F. Fringuelli, D. Lanari and L. Vaccaro, Tetrahedron Lett., 2010, 51, 1566–1569 CrossRef CAS; (f) R. Ballini, L. Barboni, L. Castrica, F. Fringuelli, D. Lanari, F. Pizzo and L. Vaccaro, Adv. Synth. Catal., 2008, 350, 1218–1224 CrossRef CAS; (g) F. Fringuelli, D. Lanari, F. Pizzo and L. Vaccaro, Eur. J. Org. Chem., 2008, 3928–3932 CrossRef CAS; (h) F. Fringuelli, F. Pizzo, C. Vittoriani and L. Vaccaro, Eur. J. Org. Chem., 2006, 1231–1236 CrossRef CAS; (i) F. Fringuelli, F. Pizzo, C. Vittoriani and L. Vaccaro, Chem. Commun., 2004, 2756–2757 RSC.
- (a) R. A. Sheldon, Chem Ind. (London, U. K.), 1997, 12–15 Search PubMed; (b) R. A. Sheldon, Green Chem., 2007, 9, 1273–1283 RSC; (c) J. Augé, Green Chem., 2008, 10, 225–231 RSC; (d) R. A. Sheldon, Chem. Commun., 2008, 3352–3365 RSC.
- (a) T. E. Kristensen and T. Hansen, Eur. J. Org. Chem., 2010, 3179–3204 CAS; (b) J. Lu and P. H. Toy, Chem. Rev., 2009, 109, 815–838 CrossRef CAS. For some specific examples where the reduction of efficiency has not been observed see: (c) B. Altava, M. I. Burguete, E. García-Verdugo, S. V. Luis, M. J. Vicent and J. A. Mayoral, React. & Funct. Polym., 2001, 48, 25–35 Search PubMed; (d) N. Madhavan, C. W. Jones and M. Weck, Acc. Chem. Res., 2008, 41, 1153–1165 CrossRef CAS; (e) C. Aranda, A. Cornejo, J. M. Fraile, E. García-Verdugo, M. J. Gil, S. V. Luis, J. A. Mayoral, V. Martinez-Merino and Z. Ochoa, Green Chem., 2011, 13, 983–990 RSC.
- (a) R. Schwesinger, et al., Liebigs Ann., 1996, 1055–1081 Search PubMed; (b) R. Schwesinger, J. Willaredt, H. Schlemper, M. Keller, D. Schmitt and H. Fritz, Chem. Ber., 1994, 127, 2435–2454 CrossRef CAS.
- P. Li, S. Wen, F. Yu, Q. Liu, W. Li, Y. Wang, X. Liang and J. Ye, Org. Lett., 2009, 11, 753–756 CrossRef CAS.
- C. Liu and Y. Lu, Org. Lett., 2010, 12, 2278–2281 CrossRef CAS.
- E. Trogu, F. De Sarlo and F. Machetti, Chem. Eur. J., 2009, 15, 7940–7948 CrossRef CAS.
- D. Bensa, T. Costantieux and J. Rodriguez, Synthesis, 2004, 923–927 CAS.
- C.-M. Chu, S. Gao, M. N. V. Sastry, C.-W. Kuo, C. Lu, J.-T. Liu and C.-F. Yao, Tetrahedron, 2007, 63, 1863–1871 CrossRef CAS.
- M. Bandini, P. G. Cozzi, M. Giacomini, P. Melchiorre, S. Selva and Umani-Ronchi, J. Org. Chem., 2002, 67, 3700–3704 CrossRef CAS.
- N. Srivastava and B. K. Banik, J. Org. Chem., 2003, 68, 2109–2114 CrossRef CAS.
- W. Guo, G. Lv, J. Chen, W. Gao, J. Ding and H. Wu, Tetrahedron, 2010, 66, 2297–2300 Search PubMed.
- B. M. Fetterly, N. K. Jana and J. G. Verkade, Tetrahedron, 2006, 62, 440–456 CrossRef CAS.
- M. Gandelman and E. N. Jacobsen, Angew. Chem. Int. Ed., 2005, 44, 2393–2397 CrossRef CAS.
- G. Luo, S. Zhang, W. Duan and W. Wang, Synthesis, 2009, 1564–1572 CAS.
- J. Lv, H. Wu and Y. Wang, Eur. J. Org. Chem., 2010, 2073–2083 CrossRef CAS.
- Y.-D. Lin, J.-Q. Kao and C.-T. Chen, Org. Lett., 2007, 9, 5195–5198 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization data and copies of the 1H and 13C NMR spectra for compounds 6, 9, 10, 15, 23, 25, 27a, 27b, 28a, 28b, 29. See DOI: 10.1039/c1gc16088e |
| This journal is © The Royal Society of Chemistry 2012 |