Ring opening of epoxidized methyl oleate using a novel acid-functionalized iron nanoparticle catalyst†
B.
Kollbe Ahn
a,
Hongwang
Wang
b,
Shona
Robinson
c,
Tej B.
Shrestha
d,
Deryl L.
Troyer
d,
Stefan H.
Bossmann
b and
Xiuzhi Susan
Sun
*a
aBio-Materials & Technology Lab, Department of Grain Science & Industry, Kansas State University, Manhattan, KS 66506, USA
bDepartment of Chemistry, Kansas State University, Manhattan, KS 66506, USA
cDepartment of Chemistry, University of Toronto, ON M5S 3H6, Canada
dAnatomy & Physiology, Manhattan, KS 66506, USA
First published on 31st October 2011
Abstract
Hydroxyl soybean oils, also called soy polyols, are biobased chemicals designed to replace petroleum-based polyols mainly for polyurethane (PU) applications. Soy polyols are obtained by acid-catalyzed ring opening of expoxidized soybean oils or other epoxidized plant oils by nucleophilic SN2 attack of methanol. Recyclable heterogeneous catalysts are preferred for the ring-opening reactions over non-recyclable homogeneous catalysts because they minimize environmental impact. The drawbacks of current solid catalysts such as SAC 13 and Amberlite 15 are low production yield and high energy consumption. Here, we demonstrate a greener synthetic pathway of soy polyols with low energy consumption and excellent atom economy and environment (E) factor by using novel sulfamic acid-functionalized iron (iron/iron oxide core shell) nanoparticles (NPs) as a heterogeneous catalyst. The excellent selectivity of the reaction with the recyclable NPs was confirmed by 1H NMR, 1H-1H COSY NMR, and ESI-MS comparable to non-recyclable H2SO4. The synthetic route with the NPs resulted in higher product yield (almost 100%) as H2SO4 at room temperature for 30 min over the SAC 13 (83% yield at 60 °C for 60 min) and Amberlite 15 (87% yield at 60 °C for 100 min). Life cycle assessment (LCA) revealed that the NP synthetic technology for soy polyol production is superior or equal to the competing routes (H2SO4, SAC 13, and Amberlite 15 methods) with respect to 9 environmental impacts (acidification potential, ozone depletion potential, smog formation potential, global warming potential, human toxicity by ingestion, human toxicity by inhalation, persistence, bioaccumulation, and abiotic resource depletion potential).
Introduction
Epoxidized soybean oil (ESO), a well-known and commercially available functionalized plant oil as a plasticizer in the plastics industry, is amenable to hydrolysis using conventional chemical methodology of ring-opening reaction of oxirane moities.1–4Hydroxyl soybean oil called soy polyol is produced from ESO via α-methoxy-hydroxylation (Scheme 1) and is widely used for PU applications, with a potential market of 8 billion pounds.3,5 Plant oil-based polyol showed better environmental benefits than petroleum-based polyols because of the former's significantly lowered greenhouse gas emissions.6 The global market for polyols is forecasted to reach 4 billion pounds by the year 2015. Recently, Recticel (Evere, Belgium), the largest PU manufacturer in Europe, has started to produce foams using BiOH® (Cargill Inc., Minneapolis, MN).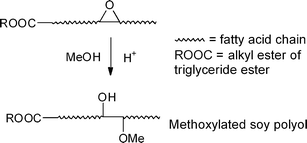 | ||
| Scheme 1 Mid-chain α -methoxy-hydroxylation of ESO or other epoxidized fatty acid esters. | ||
Although soy polyols were derived from biobased feedstocks, synthetic methods for most soy polyol production do not emphasize green chemistry, which has been spotlighted recently because of stricter government regulations regarding sustainability.7–9 The term green chemistry was comprehensively defined by Anastas and Warner as “the utilization of a set of principles that reduces or eliminates the use or generation of hazardous substance in the design, manufacture and application of chemical products”10 with 12 principles: 1) prevention, 2) atom economy, 3) less hazardous chemical syntheses, 4) safer chemicals, 5) safer solvents and auxiliaries, 6) energy efficiency, 7) use of renewable feedstocks, 8) derivatives reduction, 9) catalysis, 10) design for degradation, 11) real-time analysis for pollution prevention, and 12) inherently safer chemistry for accident prevention.10 In terms of green chemistry, a common problem in the synthesis of α-methoxy-hydroxylation from epoxidized oleo-chemicals is the need for strong Brønsted acids to activate the epoxide ring toward the attack of weakly nucleophilic alcohols. This has been accomplished through the action of strong homogeneous acids such as HCl, HBr, or p-toluenesulfonic acid.4,11 Even formic acid (a milder acid) accomplished one-pot synthesis of soy polyols, although this reaction presented low reaction selectivity (non-homogeneous distribution and oligomerization) and low conversion yield (residual epoxides).5,12 All cases require the removal of acid, solvent purification steps,3 and high temperature, which generates carbon emissions and undesirable byproducts such as ketones.13 Green chemistry researchers have become interested in solid acid catalysts (e.g., zeolites, heteropolyacids, and ion-exchange resins), which, if they replaced the numerous tons of non-recyclable homogeneous acid catalysts consumed annually in current industrial processes, would minimize environmental defects including waste generation.14,15 Heterogeneous catalysts have shown potential as replacements for traditional homogeneous acid-catalyzed processes, including biodiesel production.16–18 Rios et al.13 presented ring opening of epoxidized methyl oleate using commercial heterogeneous acid resin catalysts such as SAC 13 and Amberlite 15; however, the commercial heterogeneous solid catalysts recorded lower product yield and higher energy consumption than homogeneous sulfuric acid in the ring opening of epoxidized methyl oleate (EMO). Although Melero et al.18 showed increased activity and stability using the sulfonic acid-functionalized mesostructured silica, the technique carries the inconvenience of the resin swelling.19 Therefore, more studies on ring opening of epoxidized plant oils using a solid acid catalyst are needed with respect to selectivity and product yield.
To this end, in this work we synthesized 3-aminopropyl triethoxysilane (APTES) coated Fe/Fe3O4 nanoparticles; the NPs circumvent resin swelling19 and are efficiently removed with a magnetic field. We achieved a greener synthesis of mid-chain α-methoxy-hydroxylation of EMO for soy polyol production applications by using the new sulfamic acid-functionalized APTES coated Fe/Fe3O4 nanoparticles (NPs). NMR, ESI-MS analyses, and a life cycle assessment (LCA) on synthetic methods demonstrated that our NP technology has excellent atom economy, E factor, low energy consumption, and predominant environmental advantages compared with previously reported syntheses using sulfuric acid and other solid catalysts; the LCA comparison was carried out with respect to 9 environmental impacts and thoroughly quantifies the greenness of our NP method,20 including acidification potential, ozone depletion potential, smog formation potential, global warming potential, human toxicity by ingestion, human toxicity by inhalation, persistence, bioaccumulation, and abiotic resource depletion potential.
Results and discussion
Synthesis and characterization of sulfamic acid-functionalized core/shell Fe/Fe3O4 nanoparticles
Although several successful syntheses of magnetite NPs have been reported,21,22 they have not been applied as recyclable catalysts in soy polyol synthesis. Nanoparticles featuring an iron(0)-core have not been reported. Iron(0) is a superior magnetic material compared with magnetite.23 Strong acid catalysts such as sulfuric acid can be replaced with acid-functionalized magnetite NPs. The NPs allow not only a reduced amount of catalyst with higher surface area that can lower activation energy, but also a more convenient process in agitating and recycling (using a magnetic field) than other heterogeneous catalysts. The usage of magnetic property to achieve simple magnetic separation of catalysts had been reported in water treatments.24–27To achieve convenient reaction and catalyst recycling in soy polyol production, we developed acid-functionalized iron/iron oxide core/shell NPs. We first synthesized superparamagnetic core/shell Fe/Fe3O4 nanoparicles,28 then coated the NPs with APTES (3-aminopropyl triethoxysilane),29 and the NPs were subsequently functionalized with sulfamic acid30 (Scheme 2). TEM imaging clearly showed spherical core/shell structure of the acid-functionalized nanoparticles (Fig. 1). The average core diameter was 3 ± 1 nm and the thickness of the shell is around 2 nm, respectively.
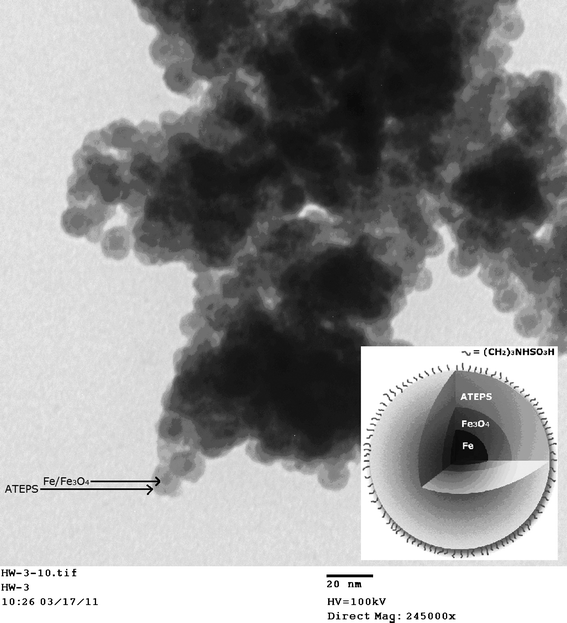 | ||
| Fig. 1 TEM image of APTES-coated Fe/Fe3O4 nanoparticles bearing terminal sulfamic acid groups. | ||
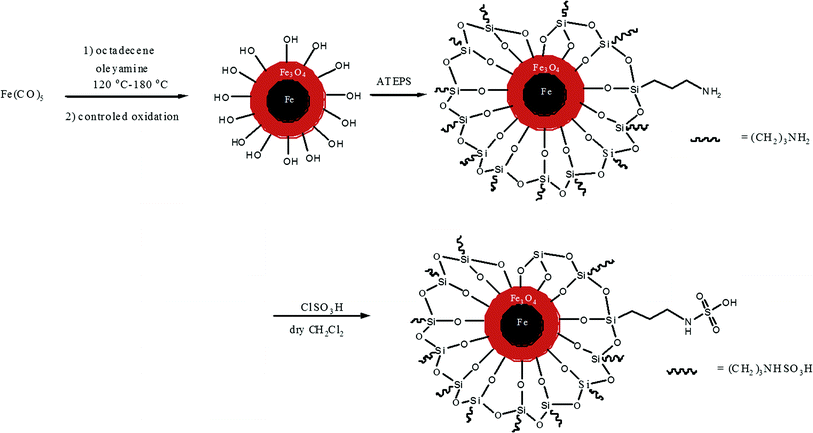 | ||
| Scheme 2 Synthesis of sulfamic acid-functionalized APTES coated core/shell Fe/Fe3O4 nanoparticles. | ||
The sulfamic acid-functionalized Fe/Fe3O4 NPs were highly monodisperse (polydispersity = 0.229) based on the dynamic light scattering (DLS) measurement (Fig. S1, ESI†). The effective diameter was around 58 nm. The different diameters between TEM and DLS measurements can be explained by clustering of the acid-functionalized NPs in water used in DLS measurement. Zeta potential measurement clearly indicated that the sulfamic acid-functionalized Fe/Fe3O4 nanoparticles carry negative charges on their surface. The zeta potential value was −30 ± 2 eV, indicating the nanoparticles prepared by this method are stable in water. Because the terminal amine groups present after APTES functionalization carry positive charges, the recording of a stable negative potential is an indication for a high conversion ratio of the terminal amino to sulfamic acid groups. The acid functionalized nanoparticles are amorphous as indicated by the absence of specific diffraction peaks in their X-ray diffraction (XRD) pattern (Fig. 2A).28 Typical Fe3O4 diffraction peaks are observed after annealing the nanoparticles at 300 °C under argon for 2 h (Fig. 2B), and further annealing of the nanoparticles at 400 °C under argon for 1 h led to the appearance of a sharp bcc-Fe diffraction peak.28 The XRD characterization gives more supportive data for the existence of Fe/Fe3O4 core/shell structure as indicated by the TEM image. Acid loading of the nanoparticle (0.36 mmol g−1) was determined by acid–base titration according to a literature reported method.28
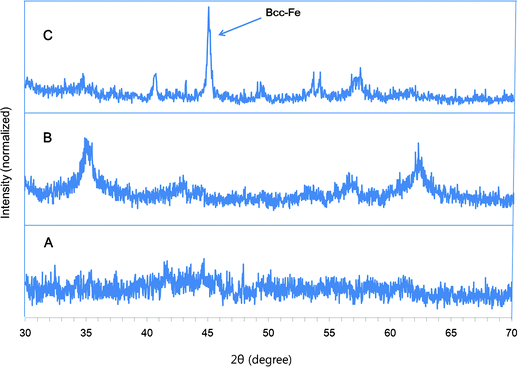 | ||
| Fig. 2 XRD patterns of A) APTES-coated Fe/Fe3O4 nanoparticles, B) annealed APTES-coated Fe/Fe3O4 nanoparticles under Ar at 300 °C for 2 h, C) annealed APTES-coated Fe/Fe3O4 nanoparticles under Ar at 400 °C for 1h. | ||
α-Methoxy-hydroxylation of EMO using APTES-coated Fe/Fe3O4 nanoparticles
EMO was used as a model for ESO to avoid the complexity of macromolecules in NMR and ESI-MS analysis.1,31 The ring opening of EMO was conducted using the sulfamic acid-functionalized Fe/Fe3O4 NPs. EMO was agitated in methanol (10 mass equivalents) with 10 w/w % of the NPs and H2SO4, respectively. We evaluated atom economy (the mass percentage of atoms from reactants that appear in the desired product of a balanced reaction equation)32 and E factor (the ratio of mass of waste over mass of product)33 using quantitative NMR (Fig. 3) analysis. 1H-1H COSY NMR (Fig. S3, ESI†) and ESI-MS (Fig. S4, ESI†) revealed the scaffold of α-methoxy-hydroxylation of EMO. H2SO4 was also used under the same reaction conditions for comparison.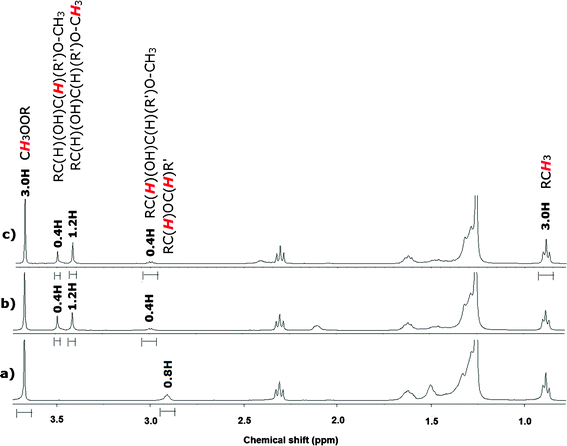 | ||
| Fig. 3 1H NMR of a) EMO, b) α-methoxy-hydroxylation of EMO by the sulfamic acid-functionalized Fe/Fe3O4 NPs, and c) α-methoxy-hydroxylation of EMO by H2SO4. | ||
As a result, the reaction efficiency of sulfamic acid-functionalized Fe/Fe3O4 NPs was comparable to that of sulfuric acid; the NPs produced identically clean product to H2SO4 with reduced reaction time (30 min) at lower temperature (40 °C) than published data using solid catalysts of SAC 13 (60 min at 60 °C) and Amberlite 15 (100 min at 60 °C)13 The NPs also showed excellent atom economy with, as for H2SO4, 100% yield compared with SAC 13 (yield: 83%) and Amberlite 15 (yield: 87%) from NMR integration using a methyl group (3H) at δ 3.67 and 0.89 as a quantitative internal standard (Fig. 3). The oxirane ring (0.8H) was completely converted to α-methoxy (0.4H) [RC(H)(OH)C(H)(R′)O-CH3] signal at δ 3.49 coupled with vicinity hydroxyl (0.4H) [RC(H)(OH)C(H)(R′)O-CH3] signal at δ 3.00 (Fig. 3). Methyl site (1.2H) of α-methoxy was also shown at δ 3.42. 1H-1H COSY NMR (Fig. S3, ESI†) and showed a clear cross peak between α-methoxy and its vicinal hydroxyl. ESI-MS (Fig. S4, ESI†) revealed the only presence of α-methoxy-hydroxylation (m/z = 367.2) without ketone byproduct13 (m/z = 335.5).
In terms of recycling efficiency, the NPs were observed to precipitate in 5 min on a supermagnet (Fig. 4), and the recycled NPs have shown a steady 100% epoxy ring-opening conversion and 100% selectivity of α-methoxy-hydroxylation in 5 consecutive uses. In this study, we confirmed that the NPs provided not only excellent catalysis, as much as H2SO4, but also environmental benefits deriving from their recyclability. These advantages of the NPs will be a green catalysis milestone for soy polyol applications with low energy consumption, excellent atom economy, and waste prevention.
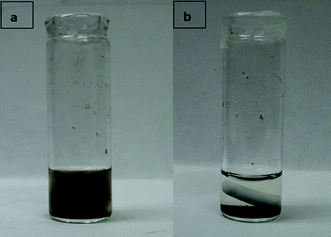 | ||
| Fig. 4 Images of a) right after reaction of the NPs in reaction solution, b) 5 min after the solution was placed on the supermagnet. | ||
LCA on the syntheses of the soy polyol
Life cycle assessment (LCA, also known as life cycle analysis) is an important tool that allows evaluation of the environmental impacts associated with a chemical process or a material's entire life cycle;6,34 however, research is limited that integrates a comprehensive LCA, and the scope of environmental impacts and unknowns presents a significant challenge.20,34 Using renewable resources is merely one of the 12 principles of green chemistry,10 so biobased materials scientists and engineers need more comprehensive LCA to designate their processes as properly green.20 The practical development of greener solutions will become more prevalent as awareness of environmental concerns increases. Multivariate metrics used in LCAs to assess environmental impacts are informative to determine overall greenness. In this study, we conducted LCA based on nine environmental impact metrics to select the greenest synthesis from the four different catalysts (the NPs, H2SO4, SAC 13, and Amberlite 15). The reaction impact assessment for epoxide ring opening was conducted using a procedure based on the ISO life-cycle assessment method,35,36 an approach that includes both the amount of waste as well as the harmfulness of the waste. Our procedure was adapted from a private communication with Sean Mercer and Philip Jessop (Queens Univ., Canada) based on the impact assessment “Choosing the Greenest Synthesis”. We compare the four reactions with respect to nine environmental risks: acidification potential (AP), ozone depletion potential (ODP), smog formation potential (SFP), global warming potential (GWP), human toxicity by ingestion (INGTP), human toxicity by inhalation (INHTP), persistence (PER), bioaccumulation (ACCU), and abiotic resource depletion potential (ADP). For most of these risks, the chemical outputs are compared with reference chemicals, which allow addition of all contributors to the risk for comparison within a synthetic route as well as between routes. In our assessment, we built a multimedia compartmental model (MCM) to assess the partitioning behavior of each chemical into four environmental compartments (air, water, soil, and sediment). This was implemented in assessing the human toxicity risk of each procedure.The ring opening of EMOvia the four processes (1-NPs, 2-H2SO4, 3-SAC13, and 4-Amberlyst-15) poses a relatively small set of environmental concerns, but the low energy requirements and high yield of our NP process set it ahead of the other processes. In addition, recovery and reusability of the iron NPs would likely be less involved than recovery of catalysts for routes 3 and 4 (Table 1). The global warming risk is lowest with route 1 and route 2, mainly due to the lower reaction temperature and slight solvent reduction manifested in the distillation contribution. The inhalation risk of all processes is similar, biased mainly by the increased solvent use in processes 3 and 4 for byproduct purification. The ingestion toxicity risk is very high for route 2 because of the environmental release of sulfuric acid. The risk of releasing environmentally persistent chemicals is similar for all reactions, an average half-life on the order of several weeks. Potential for bioaccumulation by the ketone byproduct13 of route 3 and route 4 is significant; however, when taken in combination with the relatively short half-life and relatively low release levels, this may not be a significant concern. Although no significant resource depletion occurred, sulfur does have documented abiotic resource depletion potential. Overall, the environmental impacts are relatively similar across the board, but route 1 is superior or equal to the competing routes with respect to every risk factor (see ESI† for details).
| Route | I AP | I ODP | I SFP | I GWP | I INHTP | I INGTP | PER, t1/2 | ACCU | I ADP |
|---|---|---|---|---|---|---|---|---|---|
| 1) NPs | 0 | 0 | 1.49 × 10−4 | 5.28 × 10−2 | 4.70 × 10−5 | 0.824 | 24 d | 1 | 0 |
| 2) H2SO4 | 0 | 0 | 1.49 × 10−4 | 4.92 × 10−2 | 4.70 × 10−5 | 89.3 | 24 d | 1 | 1.09 × 10−5 |
| 3) SAC13 | 0 | 0 | 1.79 × 10−4 | 6.94 × 10−2 | 5.70 × 10−5 | 0.993 | weeks | 7.3 × 105 | 0 |
| 4) Amberlyst | 0 | 0 | 1.71 × 10−4 | 6.58 × 10−2 | 5.43 × 10−5 | 0.947 | weeks | 7.3 × 105 | 0 |
Experimental
Synthesis of acid-functionalized iron nanoparticles
All starting materials were purchased from Sigma-Aldrich, and all solvents were purchased from Fisher. All chemicals were ACS-grade.Preparation of monodisperse Fe nanoparticles28
Iron nanoparticles were prepared by extensive modification of a procedure described by Peng et al.28 20 mL octadecene and 0.3 mL oleylamine were added to a 100 mL Schlenk flask and heated to 120 °C under Argon (Ar) for 30 min. After raising the temperature to 180 °C, 0.7 mL Fe (CO)5 was added under Ar. The solution turned black within 3 min. and was kept at 180 °C for 20 min before cooling to room temperature. Under Ar, the supernatant was transferred to a centrifuge tube. Iron nanoparticles accumulated on the stir bar were washed with degassed hexane (3 × 10 mL) and combined with the supernatant. Next, 50 mL of degassed absolute ethanol was added and mixed thoroughly. Nanoparticles were collected by centrifugation (8000 rmp for 30 min). After decanting the clear solvent, the nanoparticles were re-dispersed into 15 mL degassed hexane and precipitated out by adding 20 mL of absolute ethanol. The product was dried under vacuum and stored under Ar for further use.Preparation of superparamagnetic core/shell Fe/Fe3O4 nanoparicles28
15 mL octadecene and 6 mg (CH3)2NO were added into a 50 mL Schlenk flask, and after degassing by three freeze-pump-thaw cycles, the reaction container was refilled with Ar. The reaction mixture was kept at 130 °C for 30 min, then 80 mg iron nanoparticles in 2 mL of hexane were added via a syringe. After stirring at 130 °C for 2 h, the mixture was heated to 250 °C and stirred at this temperature for another 30 min. Upon cooling to room temperature, the reaction mixture was transferred to a centrifuge tube and 25 mL isopropanol was added. The nanoparticles were collected by centrifugation (8000 rpm, 20 min). The obtained nanoparticles were re-dispersed into 5 mL hexane and precipitated out by adding 10 mL absolute ethanol. The product was dried under vacuum and stored under Ar for further use.Preparation of APTES (3-aminopropyl triethoxysilane)-coated Fe/Fe3O4 nanoparticles29
40 mg Fe/Fe3O4 nanoparticles were dispersed in 80 mL hexane. 0.40 mL (3-aminopropyltriethoxysilane [APTES]) and 8 μl acetic acid (HOAc) were added and the reaction mixture was shaken at room temperature for 72 h. The black-brown precipitate was collected by centrifugation (8000 rpm, 5 min). The obtained product was further washed with 5 mL of hexane 3 times. After drying under vacuum, 38 mg APTES-functionalized nanoparticles were obtained.Preparation of sulfamic acid-functionalized Fe/Fe3O4 nanoparticles30
30 mg APTES-coated Fe/Fe3O4 nanoparticles were dispersed in 15 mL dry methylene chloride in an ultrasonic bath (10 min of sonication). With a gentle stream of Ar, a solution of 0.1 mL chlorosulfuric acid in 1 mL of dry methylene chloride was added dropwise in 10 min. Nanoparticles were collected by centrifugation (8000 rpm, 5 min), and further washed consecutively with dry methylene chloride (3 × 5 mL) and tetrahydrofuran (THF) (3× 5 mL). After drying under vacuum, 31 mg of nanoparticles were obtained.1) Mixture of EMO (starting material) 0.2 g (0.64 mmol), the NPs 0.02 g, methanol 0.2 g (6.40 mmol) was agitated for 30 min at 40 °C.
2) Mixture of EMO 0.2 g (0.64 mmol), H2SO4 0.02 g, methanol 0.2 g (6.40 mmol) was agitated for 30 min at room temperature.
3) EMO 0.2 g (0.64 mmol), SAC 13 0.02 g, methanol 0.2 g (6.40 mmol) for 60 min at 60 °C.
4) EMO 0.2 g (0.64 mmol), Amberlyst 15 0.02 g, methanol 0.2 g (6.40 mmol) for 100 min at 60 °C.
Quantities/routes were compared in LCA, and they were scaled up in the LCA.
Conclusions
In this study, we synthesized novel sulfamic acid-functionalized APTES coated Fe/Fe3O4 nanoparticles and applied the NPs for the ring opening of EMO as a recyclable catalyst for potential soy polyol applications. The NPs yielded 100% selectivity on α-methoxy-hydroxy methyl oleate, an identical yield to non-recyclable strong acid catalyst (H2SO4). A greener synthetic route for soy polyol production was proposed via NPs with excellent atom economy, E factor, and dominance in nine environmental impacts over H2SO4 and commercial recyclable resin catalysts such as SAC 13 and Amberlyst 15. This synthetic strategy using NPs has great potential for replacement of non-recyclable homogeneous acid catalysts with several economic and environmental benefits to the practical industrial applications: 1) the Fe core of the NPs responded strongly to a magnet that can provide more convenient reaction and recycling processes in a magnetic field, 2) APTES coating enabled great stability on the soy polyol process, and 3) high surface area provided very strong catalytic strength in the reaction comparable to H2SO4. The sustainability of this chemical reaction pathway to epoxidized soy oil (ESO) for soy polyol production and broader applications of NPs are being explored by our research team.Acknowledgements
The authors gratefully acknowledge the USB (United Soybean Board) and KSC (Kansas Soybean Commission) for financial support of this work. Contribution no. 12-048-J from the Kansas Agricultural Experiment Station, Manhattan, Kansas 66506. We also acknowledge financial support from the National Science Foundation under Award No. EPS-0903806 and matching support from the State of Kansas through the Kansas Technology Enterprise Corporation.References
- B. K. Ahn, S. Kraft, D. Wang and X. S. Sun, Biomacromolecules, 2011, 12, 1839–1843 CrossRef CAS.
- A. Guo, I. Javni and Z. Petrovic, J. Appl. Polym. Sci., 2000, 77, 467–473 CrossRef CAS.
- G. Lligadas, J. C. Ronda, M. Galia and V. Cadiz, Biomacromolecules, 2010, 11, 2825–2835 CrossRef CAS.
- A. Guo, Y. J. Cho and Z. S. Petrovic, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 3900–3910 CrossRef CAS.
- M. A. R. Meier, J. O. Metzger and U. S. Schubert, Chem. Soc. Rev., 2007, 36, 1788–1802 RSC.
- R. K. Helling and D. A. Russell, Green Chem., 2009, 11, 380–389 RSC.
- J. O. Metzger, Eur. J. Lipid Sci. Technol., 2009, 111, 865–876 CrossRef CAS.
- A. S. Carlsson, Biochimie, 2009, 91, 665–670 CrossRef CAS.
- J. J. Bozell, CLEAN, 2008, 36, 641–647 Search PubMed.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998 Search PubMed.
- Z. Lozada, G. J. Suppes, Y. C. Tu and F. H. Hsieh, J. Appl. Polym. Sci., 2009, 113, 2552–2560 CrossRef CAS.
- L. L. Monteavaro, E. O. da Silva, A. P. O. Costa, D. Samios, A. E. Gerbase and C. L. Petzhold, J. Am. Oil Chem. Soc., 2005, 82, 365–371 CrossRef CAS.
- L. A. Rios, P. P. Weckes, H. Schuster and W. F. Hoelderich, Appl. Catal., A, 2005, 284, 155–161 CrossRef CAS.
- M. Hara, T. Yoshida, A. Takagaki, T. Takata, J. N. Kondo, S. Hayashi and K. Domen, Angew. Chem., Int. Ed., 2004, 43, 2955–2958 CrossRef CAS.
- P. L. Dhepe, M. Ohashi, S. Inagaki, M. Ichikawa and A. Fukuoka, Catal. Lett., 2005, 102, 163–169 CrossRef CAS.
- X. H. Mo, E. Lotero, C. Q. Lu, Y. J. Liu and J. G. Goodwin, Catal. Lett., 2008, 123, 1–6 CrossRef CAS.
- J. A. Melero, L. F. Bautista, G. Morales, J. Iglesias and D. Briones, Energy Fuels, 2009, 23, 539–547 CrossRef CAS.
- J. A. Melero, L. F. Bautista, G. Morales, J. Iglesias and R. Sanchez-Vazquez, Chem. Eng. J., 2010, 161, 323–331 CrossRef CAS.
- D. E. Lopez, J. G. Goodwin and D. A. Bruce, J. Catal., 2007, 245, 381–391 CrossRef CAS.
- P. G. Jessop, Green Chem., 2011, 13, 1391–1398 RSC.
- S. Mohapatra, S. K. Mallick, T. K. Maiti, S. K. Ghosh and P. Pramanik, Nanotechnology, 2007, 18, 385102 CrossRef.
- T. Theppaleak, G. Tumcharern, U. Wichai and M. Rutnakornpituk, Polym. Bull., 2009, 63, 79–90 CrossRef CAS.
- S. H. Bossmann, in Fabrication and Bio-Application of Functionalized Nanomaterials, ed. X. Wang and E. Katz, Research Signpost, Trivandrum, Kerala, India, 2009, pp. 171–206 Search PubMed.
- L. C. A. Oliveira, R. Rios, J. D. Fabris, V. Garg, K. Sapag and R. M. Lago, Carbon, 2002, 40, 2177–2183 CrossRef CAS.
- R. C. C. Costa, M. de Fatima, F. Lelis, L. C. A. Oliveira, J. D. Fabris, J. D. Ardisson, R. Rios, C. N. Silva and R. M. Lago, Catal. Commun., 2003, 4, 525–529 CrossRef CAS.
- L. C. A. Oliveira, D. I. Petkowicz, A. Smaniotto and S. B. C. Pergher, Water Res., 2004, 38, 3699–3704 CrossRef CAS.
- L. C. A. Oliveira, R. Rios, J. D. Fabris, K. Sapag, V. K. Garg and R. M. Lago, Appl. Clay Sci., 2003, 22, 169–177 CrossRef CAS.
- S. Peng, C. Wang, J. Xie and S. H. Sun, J. Am. Chem. Soc., 2006, 128, 10676–10677 CrossRef CAS.
- R. De Palma, S. Peeters, M. J. Van Bael, H. Van den Rul, K. Bonroy, W. Laureyn, J. Mullens, G. Borghs and G. Maes, Chem. Mater., 2007, 19, 1821–1831 CrossRef CAS.
- M. Z. Kassaee, H. Masrouri and F. Movahedi, Appl. Catal., A, 2011, 395, 28–33 CrossRef CAS.
- B. J. K. Ahn, S. Kraft and X. S. Sun, J. Mater. Chem., 2011, 21, 9498–9505 RSC.
- B. M. Trost, Science, 1991, 254, 1471–1477 CrossRef CAS.
- R. A. Sheldon, Comptes Rendus Acad. Sci. Ser. II C, 2000, 3, 541–551 Search PubMed.
- J. S. Luterbacher, M. Froling, F. Vogel, F. Marechal and J. W. Tester, Environ. Sci. Technol., 2009, 43, 1578–1583 CrossRef CAS.
- J. Guinee, Handbook on life cycle assessment - Operational guide to the ISO standards, Kluwer Academic Publishers, Dordrecht, 2002 Search PubMed.
- D. T. Allen and D. R. Shonnard, Green engineering: Environmentally conscious design of chemical processes and products, Prentice-Hall, Upper Saddle River, 2001 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1gc16043e |
| This journal is © The Royal Society of Chemistry 2012 |
