A magnetic nanoparticle supported dual acidic ionic liquid: a “quasi-homogeneous” catalyst for the one-pot synthesis of benzoxanthenes
Qiang
Zhang
,
Hong
Su
,
Jun
Luo
* and
Yunyang
Wei
School of Chemical Engineering, Nanjing University of Science & Technology, Nanjing, 210094, PR China. E-mail: luojun@mail.njust.edu.cn; Fax: (+)86-25-84315030
First published on 17th November 2011
Abstract
A novel magnetic nanoparticle supported dual acidic ionic liquid catalyst was synthesized by anchoring 3-sulfobutyl-1-(3-propyltriethoxysilane) imidazolium hydrogen sulfate onto the surface of silica-coated Fe3O4 nanoparticles. Due to the combination of nano-support features and flexible imidazolium linkers, it acted as a “quasi-homogeneous” catalyst to effectively catalyze the one-pot synthesis of benzoxanthenes by a three-component condensation of dimedone with aldehyde and 2-naphthol under solvent-free conditions. More importantly, the catalyst could be easily recovered by an external magnet and reused six times without significant loss of catalytic activity.
Introduction
Xanthenes and benzoxanthenes are important bioactive compounds which possess analgesic,1 antiviral,2 antibacterial,3 and anti-inflammatory activities.4 Some of them have been used as antagonists for paralyzing the action of zoxazolamine,5 and in photodynamic therapy.6 Moreover, they are the source of brilliant fluorescent dyes7 and are used extensively in laser technology8 and pH sensitive fluorescent materials for visualization of biomolecules.9 Some methods for the synthesis of tetrahydrobenzo[a]xanthene-11-ones have been reported,10 which involve a three-component condensation of dimedone with aromatic aldehyde and 2-naphthol. However, each of these procedures have some drawbacks, such as harsh reaction conditions, tedious work-ups, low yields, use of halogenated solvents or difficult catalyst recycling. Benzoxanthenes have also been of interest recently due to their biological importance.Ionic liquids (ILs), as eco-friendly reaction media or catalysts, have attracted increasing attention due to their particular properties, such as negligible vapor pressure, wide liquid range, high thermal stability, and excellent solubility.11 Among them, SO3H-functionalized ILs with a hydrogen sulfate counteranion has been intensively studied as a class of dual acidic functionalized ILs during the last five years, because the existence of both SO3H-functional groups and the hydrogen sulfate counteranion can enhance their acidities.12 These strongly acidic ILs have been exploited as efficient catalysts for many reactions and generally can afford higher yields and selectivities against traditional acid catalysts.13 Inspite of their widespread use in organic reactions, a series of drawbacks such as difficult product separation, catalyst recovery and the use of large amounts of ILs in biphasic systems, which is costly and may cause toxicological concerns, still exist.14 Recently, a concept using supported ionic liquid catalysis (SILC) has been proposed,15 which combines the benefits of ILs and heterogeneous catalysts, such as high designability, high “solubility” of the catalytic site, along with ease of handling, separation and recycling. Both organic polymers, like polystyrene,16 and inorganic matrices, such as silica,17 have been frequently employed as the solid supports. Some supported acidic ILs were prepared and used as solid catalysts, e.g., in esterification, nitration,18 Baeyer–Villiger reactions,19acetal formation20 and the hydrolysis of cellulose.21 Indeed, we also successfully synthesized a silica gel supported acidic ionic liquid (AIL@SiO2) and used it as an efficient and recyclable catalyst for the preparation of amidoalkyl naphthols.22 Despite the good recyclability in most cases, reduced activities were often observed due to the poor dispersion of the supported IL in the reaction system. Moreover, the tedious recovery procedure viafiltration or centrifugation and the inevitable loss of solid catalysts in the separation process limited their application. On the other hand, magnetic nanoparticles (MNPs) have recently appeared as a new kind of catalyst support because of their good stability, easy synthesis and functionalization, high surface area, facile separation by magnetic forces, low toxicity and cost.23 Owing to these attractive properties, many MNP supported catalysts have been designed and widely applied as novel magnetically separated catalysts in traditional metal catalysis,24 organocatalysis,25 and even enzyme catalysis.26
Driven by the unique properties of magnetic nanoparticles and the valuable potential applications of acidic ILs in catalysis,27 herein, we report a novel silica-coated Fe3O4 nanoparticle supported dual acidic ionic liquid and its application as a highly efficient and magnetically recoverable catalyst for the one-pot synthesis of benzoxanthenes (Scheme 1). As a result of the combination of MNP support features and flexible imidazolium linkers, the catalyst acts as an efficient “quasi-homogeneous” catalyst, which can be highly dispersed in the reaction system, just like a homogenous one and easily separated and reused like a heterogeneous catalyst by magnetic force. This novel catalyst may be a significant bridge between homogeneous and heterogeneous catalysis. Furthermore, to the best of our knowledge, this is the first report on the preparation and application of a MNP supported acidic IL catalyst.
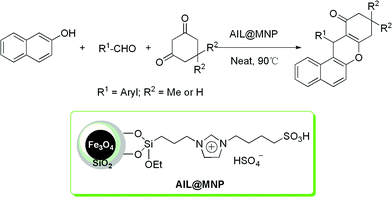 | ||
| Scheme 1 One-pot synthesis of benzoxanthenes. | ||
Results and Discussion
Preparation and characterization of the catalyst
The magnetic nanoparticle supported acidic ionic liquid catalyst (AIL@MNP) was prepared following the procedure shown in Scheme 2. The precursor ionic liquid 2 was prepared by quaternization of N-(3-propyltriethoxysilane) imidazole 1 with 1,4-butane sultone, followed by treatment with sulfuric acid. Magnetite nanoparticles, Fe3O4, easily prepared via a coprecipitation method described by Massart,28 were chosen as the support. Considering the aggregation tendency of the naked nano-Fe3O4 and acid corrosion problem, coating the magnetic nanoparticles with silica should be a good solution. Furthermore, the outer shell of silica also provides suitable sites (Si–OH groups) for surface functionalization with the precursor IL 2. The coating process was performed by suspending the magnetic nanoparticle in an ethanol–water solution and mixing with tetraethoxysilane (TEOS) to form a silica shell under basic conditions through a sol–gel method. The XRD pattern of the coated nanoparticles shows characteristic peaks and relative intensity, which match well with the standard Fe3O4 sample (JCPDS file No. 19-0629, Fig. 1). The broad peak from 2θ = 20° to 30° is consistent with an amorphous silica phase in the shell of the silica-coated Fe3O4 nanoparticles (SiO2@Fe3O4).26 In addition, TEM analysis displays a dark nano-Fe3O4 core surrounded by a grey silica shell about 3–5 nm thick and the average size of the obtained particles is 20–30 nm (Fig. 2a).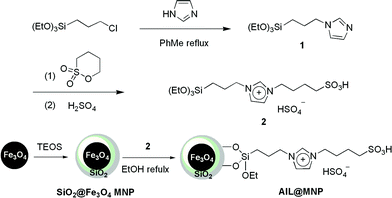 | ||
| Scheme 2 Synthesis of the magnetic nanoparticle supported IL catalyst. | ||
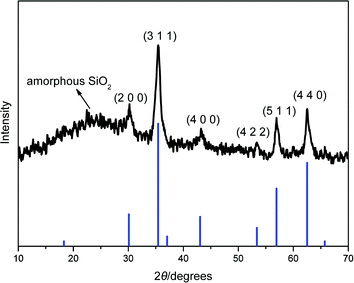 | ||
| Fig. 1 X-Ray diffraction pattern of silica-coated magnetic nanoparticles. The bottom row of tick marks indicates the reflection positions for a standard magnetite pattern (JCPDS no. 19-0629). | ||
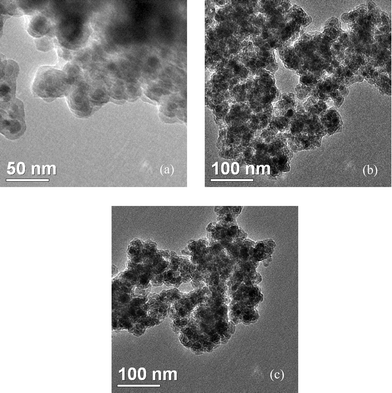 | ||
| Fig. 2 Transmission electron microscope (TEM) images of SiO2@Fe3O4 (a), AIL@MNP (b) and six-times reused catalyst (c). | ||
Ultimately, a suspension of SiO2@Fe3O4 with an ethanol solution of the precursor 2 was refluxed for 2 days to undergo a condensation reaction, which afforded AIL@MNP. The morphology of the AIL@MNP was also investigated by TEM (Fig. 2b). The particle size of the AIL@MNP is similar to that of the SiO2@Fe3O4 and the core–shell structure can also be observed. The loading amount of the acidic IL on SiO2@Fe3O4 was determined to be 0.54 mmol g−1 by elemental analysis.
In order to warrant the successful functionalization of silica-coated Fe3O4 nanoparticles with acidic IL, FT-IR was employed to give a detailed investigation of the blank MNP (SiO2@Fe3O4), AIL@MNP and free AIL (precursor 2). As can be seen from Fig. 3, all the samples show broad peaks at around 3400 and 1645 cm−1, which are attributed to the Si–OH group and adsorbed water, respectively. The spectra of the blank SiO2@Fe3O4 shows that the peak at 580 cm−1 could be due to the Fe–O vibration. Meanwhile, the Si–O–Si stretching modes of the silica shell can be observed as a strong peak at 1097 cm−1. The IR curve of free AIL shows typical bands at 1563 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibrations of the imidazole ring),29 3143 cm−1 (sp2 C–H stretching vibration of imidazole moiety), 2948, 2872 and 1456 cm−1 (alkyl chain stretching and deformation vibrations), 1167 and 1041 cm−1 (S
C vibrations of the imidazole ring),29 3143 cm−1 (sp2 C–H stretching vibration of imidazole moiety), 2948, 2872 and 1456 cm−1 (alkyl chain stretching and deformation vibrations), 1167 and 1041 cm−1 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration).30 While in the spectrum of AIL@MNP, the characteristic peaks are at the same wavenumbers, with a small shift due to the interaction with the support. However, all of these significant features cannot be observed in the blank MNP. Thus, the above results indicate that the acidic IL was successfully grafted onto the SiO2@Fe3O4.
O stretching vibration).30 While in the spectrum of AIL@MNP, the characteristic peaks are at the same wavenumbers, with a small shift due to the interaction with the support. However, all of these significant features cannot be observed in the blank MNP. Thus, the above results indicate that the acidic IL was successfully grafted onto the SiO2@Fe3O4.
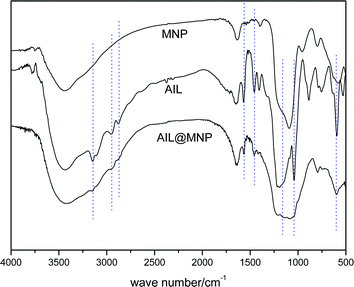 | ||
| Fig. 3 FT-IR spectra of SiO2@Fe3O4 (MNP), precursor 2 (AIL) and AIL@MNP. | ||
The stability of the AIL@MNP was determined by the thermogravimetric (TG) analysis (Fig. 4). The TG curve indicates an initial weight loss of 2.4% up to 150 °C, which is due to the adsorbed water on the support. Complete loss of the grafted IL was seen in the temperature range from 250 °C to 530 °C, and the amount of organic moiety was about 22.5% against the total solid catalyst. Meanwhile, the DTG curve shows that the decomposition of the organic structure mainly occurred in one step, from 320 °C to 500 °C, which is related to main weight loss of 19.4%. The peak in the DTG curve shows that the fastest loss of the IL occurred at 390 °C. Therefore, the AIL@MNP is stable around or below 250 °C.
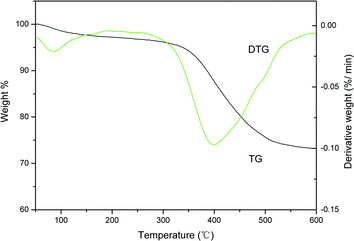 | ||
| Fig. 4 TG–DTG analysis for AIL@MNP. | ||
The magnetic properties of the AIL@MNP and the neat SiO2@Fe3O4 were investigated at room temperature (Fig. 5). The magnetization of samples could be completely saturated at high fields of up to 1.0 T and the saturation magnetization of samples changed from 12.5 to 5.6 emu g−1, because of the functionalization by precursor 2. Moreover, the room temperature magnetization curves of the magnetic nanoparticle, before and after functionalization, exhibit no hysteresis which demonstrates its superparamagnetic characteristics.26 The strong magnetization of the nanoparticle was also revealed by simple attraction with an external magnet.
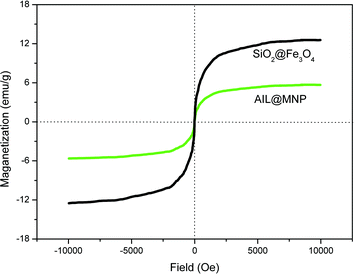 | ||
| Fig. 5 Magnetic curves of SiO2@Fe3O4 and AIL@MNP. | ||
Synthesis of benzoxanthenes by AIL@MNP
The activity of the novel catalyst was tested using a one-pot three-component condensation of dimedone (2.4 mmol) with 4-chlorobenzaldehyde (2 mmol) and 2-naphthol (2 mmol) as model substrates at 90 °C under solvent-free conditions.| Entry | Catalyst (mol%) | Time (min) | Yield (%)a |
|---|---|---|---|
| a Isolated yield. b The loading was 0.70 mmol g−1. c Second run. d 75 min. | |||
| 1 | — | 180 | trace |
| 2 | SiO2@Fe3O4 (55 mg) | 180 | 21 |
| 3 | AIL@MNP (0.5) | 90 | 54 |
| 4 | AIL@MNP (1.0) | 60 | 85 |
| 5 | AIL@MNP (1.5) | 40 | 91 |
| 6 | AIL@MNP (2) | 40 | 89 |
| 7 | AIL@SiO2 (1.5)b | 40 | 63 |
| 8 | 20 wt% H2SO4@MNP (1.5) | 40 | 75 (34)c |
| 9 | [MIMPSA][HSO4] (1.5) | 40 | 76 (85)d |
| 10 | p-Toluenesulfonic acid (TSA) (1.5) | 40 | 62 |
Without the addition of catalyst, only trace amounts of product were detected (Table 1, entry 1). The support SiO2@Fe3O4 and a typical acidic IL [MIMPSA][HSO4]12 produced the product with 21 and 76% yields, respectively (Table 1, entries 2 and 9). Yet, their analogous combination in the form of AIL@MNP increased the yield up to 91% in a shorter reaction time (Table 1, entry 5). This was presumed to occur due to the adsorption of reactants on the surface of the nano-support, increasing the local concentration of reactants around the active sites of the AIL@MNPs and promotes the reaction effectively. In addition, this novel catalyst showed a higher yield in comparison with AIL@SiO2 (Table 1, entry 7),22 which indicated that the smaller particle mitigated more diffusion effects and then accelerated the reaction. Although 20 wt% H2SO4@MNP also gave a good yield, which was even better than p-TSA under the same conditions, the recyclability of the H2SO4@MNP was bad (Table 1, entries 8 and 10). To investigate the effect of catalyst loading, the model reaction was carried out in the presence of different amounts of catalyst. The best result was obtained in the presence of just 1.5 mol% of AIL@MNP (Table 1, entries 3–6).
In order to study the generality of this procedure, a series of aldehydes and cyclic 1,3-dicarbonyl compounds were applied. As seen from Table 2, the procedure was highly effective for the preparation of benzoxanthenes. A variety of aromatic aldehydes with either electron-donating or electron-withdrawing groups were converted to benzoxanthenes in good to excellent yields (80–94%). Both dimedone and 1,3-cyclohexanedione could give good results. Thus, the AIL@MNP acts as a highly efficient catalyst for the synthesis of benzoxanthenes.
| Entry | R1 | R2 | Time (min) | Product | Yield (%)a | Mp (°C) |
|---|---|---|---|---|---|---|
| a Isolated yield. | ||||||
| 1 | Ph | Me | 45 |
|
89 | 149–151 |
| 2 | 4-Me–C6H4 | Me | 45 |
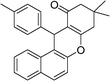
|
86 | 176–177 |
| 3 | 4-MeO–C6H4 | Me | 60 |
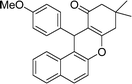
|
84 | 203–205 |
| 4 | 4-Cl–C6H4 | Me | 40 |
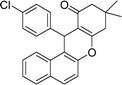
|
91 | 175–176 |
| 5 | 3-Cl–C6H4 | Me | 45 |
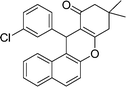
|
89 | 160–162 |
| 6 | 2,4-Cl2–C6H3 | Me | 35 |
|
94 | 184–186 |
| 7 | 3,4-Cl2–C6H3 | Me | 50 |
|
89 | 189–191 |
| 8 | 4-Br–C6H4 | Me | 45 |
|
90 | 179–181 |
| 9 | 3-Br–C6H4 | Me | 50 |
|
88 | 165–167 |
| 10 | 4-NO2–C6H4 | Me | 30 |
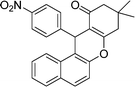
|
93 | 176–178 |
| 11 | 3-NO2–C6H4 | Me | 40 |
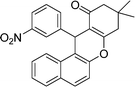
|
90 | 168–169 |
| 12 | 2-NO2–C6H4 | Me | 60 |
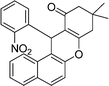
|
85 | 218–220 |
| 13 | 4-OH–C6H4 | Me | 50 |
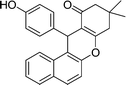
|
87 | 214–216 |
| 14 | 3-MeO-4-OH–C6H3 | Me | 65 |
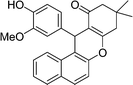
|
80 | 167–168 |
| 15 | 2-OH–C6H4 | Me | 50 |
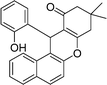
|
88 | 229–230 |
| 16 | Ph | H | 45 |
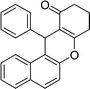
|
87 | 187–189 |
| 17 | 4-Me–C6H4 | H | 45 |
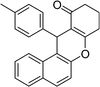
|
85 | 204–205 |
| 18 | 4-MeO–C6H4 | H | 55 |
|
84 | 180–182 |
| 19 | 4-Cl–C6H4 | H | 40 |
|
90 | 204–206 |
| 20 | 3-NO2–C6H4 | H | 40 |
|
91 | 233–235 |
A plausible mechanism for the formation of benzoxanthenes catalyzed by AIL@MNP is shown in Scheme 3. We propose that the reaction proceeds via the initial formation of ortho-quinone methides (o-QMs),10 which are formed by the nucleophilic addition of 2-naphthol to the aldehyde, assisted by AIL@MNP. The o-QMs react with dimedone via a Michael addition and then eliminate one molecule of H2O to afford the target products, which is also promoted by AIL@MNP. It is worth noting that the AIL@MNP had a better dispersibility of the nanoparticles due to the presence of the supported IL, compared with those without the IL support, which made it more suitable for the neat reaction conditions. As its active sites were unrestricted and had a good “solubility” in the reaction system due to soft imidazolium chains. All of these novel features make the AIL@MNP become a “quasi-homogeneous” catalyst which can facilitate the reaction efficiently.
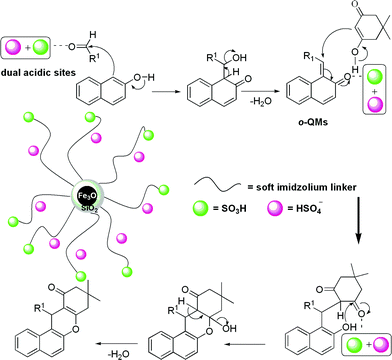 | ||
| Scheme 3 A plausible mechanism for the synthesis of benzoxanthenes using AIL@MNP. | ||
Reuse of AIL@MNP and the hot filtration test
The recovery and reuse of a catalyst is highly preferable for a greener process. Thus, the recyclability of AIL@MNP was investigated by using 4-chlorobenzaldehyde, 2-naphthol and dimedone as model substrates. The catalyst was easily separated by attaching an external magnet after the reaction and washed with ethyl acetate, dried under vacuum and reused in a subsequent reaction. Nearly quantitative catalyst (up to 98%) could be recovered from each run. In a test of six cycles, the catalyst could be reused without any significant loss of catalytic activity (Fig. 6).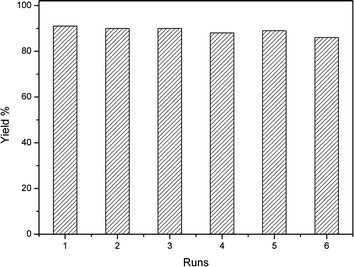 | ||
| Fig. 6 Recycling experiment of AIL@MNP. | ||
The recovered catalyst after six runs had no obvious change in structure, referring to the FT-IR spectra in comparison with the fresh one (Fig. 7). A TEM observation of the recovered catalyst was also made, and there were no obvious changes in morphology and size (Fig. 2c). Moreover, according to elemental analysis, the loading amount of IL was still 0.52 mmol g−1. These results revealed that the catalyst was very stable and could endure these reaction conditions.
 | ||
| Fig. 7 FT-IR spectrum for the comparison of the fresh catalyst and the six-times reused catalyst. | ||
When using a supported catalyst, there is a possibility that the active species might migrate from the solid support to the liquid phase, since the leached active ones become responsible for the good catalytic activity. To explore the catalyst leaching, the reaction of 4-chlorobenzaldehyde (2 mmol), 2-naphthol (2 mmol) and dimedone (2.4 mmol) catalyzed by AIL@MNP (55 mg) was carried out at 90 °C under solvent-free conditions. When the reaction time reached 15 min, hot ethyl acetate (20 mL) was added and the AIL@MNP was easily removed using a magnetic force. The solution was averagely divided into two parts (P1 and P2). The corresponding product of P1 was obtained with a 42% yield. Moreover, the neat P2 was reacted under the above conditions for another 25 min to afford the product in 45% yield, which was similar to P1 and less than normal (91%; Table 1, entry 5). Therefore, these above results convinced us that the leaching of AIL@MNP was negligible in the catalytic process.
Conclusions
In conclusion, we developed a novel MNP supported acidic IL catalyst for the first time and used it in a one-pot three-component condensation of dimedone with aldehyde and 2-naphthol to prepare benzoxanthenes in excellent yields (up to 94%). Owing to the combination of nano-support features and soft imidazolium linkers, the active sites of the supported catalyst are more free and have a good “solubility” in the reaction system, just like a “quasi-homogeneous” catalyst, to facilitate the condensation effectively. Moreover, the catalyst could be readily separated by use of a magnetic force and reused without any significant loss of catalytic activity after six runs. Besides the synthesis of benzoxanthenes, such an environmentally benign catalyst should find a wider application in various acid-catalyzed reactions, which is an ongoing project.Experimental Section
General information
Melting points were determined on a Perkin–Elmer differential scanning calorimeter and was uncorrected. The IR spectra were run on a Nicolete spectrometer (KBr). 1H NMR spectra were recorded on Bruker DRX500 (500 MHz) and 13C NMR spectra on Bruker DRX500 (125 MHz) spectrometer. Mass spectra were obtained with an automated FININIGAN TSQ Advantage mass spectrometer. Elemental analysis was performed on a Elementar Vario MICRO spectrometer. Thermogravimetric analysis was carried out under nitrogen using a Shimadzu TGA-50 spectrometer. X-Ray diffraction (XRD) images were obtained from a Bruker XRD D8 Advance instrument with Cu Kα radiation. Transmission electron microscope (TEM) images were obtained from a JEOL JEM-2010 instrument. The magnetization curve was obtained by a vibrating sample magnetometer (JDM-13T, China). All the solvents used were strictly dried according to standard operation and stored on 4 Å molecular sieves. All other chemicals (AR grade) were commercially available and used without further purification.Preparation of the magnetic nanoparticle supported dual acidic ionic liquid catalyst (AIL@MNP)
1H NMR (500 MHz, CDCl3): δ 7.53 (s, 1H), 7.07 (s, 1H), 6.93 (s, 1H), 3.96 (t, J = 7.5 Hz, 2H), 3.82 (q, J = 7.0 Hz, 6H), 1.90 (m, 2H), 1.23 (t, J = 7.0 Hz, 9H), 0.57 (t, J = 8.0 Hz, 2H); 13C NMR (125 MHz, CDCl3): δ 7.2, 18.1, 24.8, 48.9, 58.2, 118.6, 129.0, 137.0.
1,4-Butane sultone (1.42 g, 10.5 mmol) was added dropwise into a solution of 1 (2.72 g, 10 mmol) in toluene (30 mL) over 30 min. The mixture was then stirred for 8 h and evaporated under reduced pressure. Conc. H2SO4 (0.54 mL, 10 mmol) was added dropwise into the solution of the above residue in ethanol (30 mL) over 30 min. The final mixture was stirred at 50 °C for another 8 h and evaporated under reduced pressure to give precursor 2 as a viscous yellow liquid in 97% yield.
1H NMR (500 MHz, DMSO-d6): δ 9.19 (s, 1H), 7.80 (s, 2H), 4.18 (t, J = 7.5 Hz, 2H), 4.13 (t, J = 7.5 Hz, 2H), 3.75 (q, J = 7.0 Hz, 6H), 2.44 (t, J = 7.5 Hz, 2H), 1.89–1.83 (m, 4H), 1.54 (m, 2H), 1.15 (t, J = 7.0 Hz, 9H), 0.51 (t, J = 8.0 Hz, 2H); 13C NMR (125 MHz, DMSO-d6): δ 7.3, 18.6, 22.1, 24.5, 29.0, 49.0, 50.9, 51.6, 58.3, 122.8, 123.0, 136.6.
General procedure for the synthesis of benzoxanthenes
A mixture of aldehyde (2 mmol), 2-naphthol (2 mmol), dimedone (2.4 mmol) and AIL@MNP (55 mg) was stirred at 90 °C in an oil bath for 35–65 min, as indicated by TLC for a complete reaction. Ethyl acetate was added and the catalyst was separated magnetically from the product solution, washed with ethyl acetate, and used for subsequent cycles after drying under vacuum. Pure amidoalkyl naphthols were afforded by evaporation of the solvent, followed by recrystallization from ethanol or by column chromatography on silica gel using ethyl acetate/hexane as the eluent. The naphthols were characterized by the use of spectral data and comparison of their physical data with the literature. Spectral data for 12-(4-hydroxy-3-methoxy-phenyl)-9,9-dimethyl-8,9,10,12-tetrahydrobenzo[a]xanthen-11-one (Table 2, entry 14): 1H NMR (500 MHz, CDCl3) δ 8.01 (d, J = 8.4 Hz, 1H), 7.79 (dd, J = 13.8, 8.4 Hz, 2H), 7.48–7.44 (m, 1H), 7.42–7.39 (m, 1H), 7.34 (d, J = 8.9 Hz, 1H), 7.03 (s, 1H), 6.68 (m, 2H), 5.66 (s, 1H), 5.46 (s, 1H), 3.85 (s, 3H), 2.58 (s, 2H), 2.35–2.27 (m, 2H), 1.14 (s, 3H), 1.00 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.1, 163.8, 147.8, 146.1, 143.8, 137.0, 131.5, 131.4, 128.8, 128.4, 127.0, 124.9, 123.7, 121.0, 117.8, 117.0, 114.5, 114.1, 111.3, 55.9, 50.9, 41.4, 34.2, 32.3, 29.3, 27.2; ESI-MS: m/z = 401 [M + H]; Anal. calc. for C26H24O4: C, 77.98; H, 6.04. Found: C, 78.06; H, 6.10.Acknowledgements
We are grateful for the financial support from the Natural Science Foundation of Jiangsu Province of China (No. BK2010485), the Doctoral Fund of Ministry of Education of China for New Teacher (No. 20082881029), and the Science and Technology Development Fund of Nanjing University of Science and Technology (No. XKF09066).Notes and references
- H. N. Hafez, M. I. Hegab, I. S. Ahmed-Farag and A. B. A. El-Gazzar, Bioorg. Med. Chem. Lett., 2008, 18, 4538 CrossRef CAS.
- R. W. Lambert, J. A. Martin, J. H. Merrett, K. E. B. Parkes and G. J. Thomas, CT Int. Appl. WO9706178, 1997.
- T. Hideo and J. Teruomi, (Sankyo Co.), Jpn. Patent, 56005480, 1981.
- J. P. Poupelin, G. Saint-Ruf, O. Foussard-Blanpin, G. Narcisse, G. Uchida-Ernouf and R. Lacroix, Eur. J. Med. Chem., 1978, 13, 67 CAS.
- G. Saint-Ruf, A. De and H. T. Hieu, Bull. Chim. Ther., 1972, 7, 83 Search PubMed.
- R. M Ion, Prog. Catal., 1997, 2, 55 Search PubMed.
- S. M. Menchen, S. C. Benson, J. Y. L. Lam, W. Zhen, D. Sun, B. B. Rosenblum, S. H. Khan and M. Taing, U.S. Patent, 6583168, 2003.
- M. Ahmad, T. A. King, D.-K. Ko, B. H. Cha and J. J. Lee, J. Phys. D: Appl. Phys., 2002, 35, 1473 CrossRef CAS.
- C. G. Knight and T. Stephens, Biochem. J., 1989, 258, 683 CAS.
- A. Kumar, S. Sharma, R. A. Maurya and J. Sarkar, J. Comb. Chem., 2010, 12, 20 CrossRef CAS; G. C. Nandi, S. Samai, R. Kumar and M. S. Singh, Tetrahedron, 2009, 65, 7129 CrossRef; B. Das, K. Laxminarayana, M. Krishnaiah and Y. Srinivas, Synlett, 2007, 20, 3107 CrossRef; Z. H. Zhang, H. J. Wang, X. Q. Ren and Y. Y. Zhang, Monatsh. Chem., 2009, 140, 1481 CrossRef; J. J. Li, W. Y. Tang, L. M. Lu and W. K. Su, Tetrahedron Lett., 2008, 49, 7117 CrossRef; R. Z. Wang, L. F. Zhang and Z. S. Cui, Synth. Commun., 2009, 39, 2101 CrossRef; J. M. Khurana and D. Magoo, Tetrahedron Lett., 2009, 50, 4777 CrossRef; N. Karimi, H. A. Oskooie, M. M. Heravi and L Tahershamsi, Synth. Commun., 2011, 41, 307 CrossRef; J. J. Li, L. M. Lu and W. K. Su, Tetrahedron Lett., 2010, 51, 2434 CrossRef.
- P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3772 CrossRef CAS; V. I. Pârvulescu and C. Hardacre, Chem. Rev., 2007, 107, 2615 CrossRef; R. Giernoth, Angew. Chem., Int. Ed., 2010, 49, 2 CrossRef.
- X. M. Liu, M. Liu, X. W. Guo and J. X. Zhou, Catal. Commun., 2008, 9, 1 CrossRef CAS; Y. Y. Wang, X. Gong, Z. Wang and L. Y. Dai, J. Mol. Catal. A: Chem., 2010, 322, 7 CrossRef; D. Fang, J. Chen, Z. Fei, K. Gong and Z. L. Liu, Catal. Commun., 2008, 9, 1924 CrossRef.
- A. C. Cole, J. L. Jensen, I. Ntai, T. Tran, K. J. Weaver, D. C. Forbes and J. H. Davis Jr, J. Am. Chem. Soc., 2002, 124, 962 Search PubMed; D. Fang, X. Zhou, Z. Ye and Z. Liu, Ind. Eng. Chem. Res., 2006, 45, 7982 CrossRef CAS.
- D. Zhao, Y. Liao and Z. Zhang, Clean, 2007, 35, 42 CAS.
- C. P. Mehnert, R. A. Cook, N. C. Dispenziere and M. Afeworki, J. Am. Chem. Soc., 2002, 124, 12932 CrossRef CAS; C. P. Mehnert, E. J. Mozeleski and R. A. Cook, Chem. Commun., 2002, 3010 RSC; C. DeCastro, E. Sauvage, M. H. Valkenberg and W. F. Hölderich, J. Catal., 2000, 196, 86 CrossRef; J. P. T. Mikkola, P. P. Virtanen, K. Kordás, H. Karhu and T. O. Salmi, Appl. Catal., A, 2007, 328, 68 CrossRef; H. Hagiwara, T. Nakamura, T. Hoshi and T. Suzuki, Green Chem., 2011, 13, 1133 RSC; K. B. Sidhpuria, A. L. Daniel-da-Silva, T. Trindade and J. A. P. Coutinho, Green Chem., 2011, 13, 340 RSC; J. L. Bideau, L. Viau and A. Vioux, Chem. Soc. Rev., 2011, 40, 907 RSC.
- W. Chen, Y. Y. Zhang, L. B. Zhu, J. Lan, R. Xie and J. S. You, J. Am. Chem. Soc., 2007, 129, 13879 CrossRef CAS; D. W. Kim and D. Y. Chi, Angew. Chem., Int. Ed., 2004, 43, 483 CrossRef; Z. J. Xu, H. Wan, J. M. Miao, M. J. Han, C. Yang and G. F. Guan, J. Mol. Catal. A: Chem., 2010, 332, 152 CrossRef.
- B. Gadenne, P Hesemann and J. J. E. Moreau, Chem. Commun., 2004, 1768 RSC; R. Ciriminna, P. Hesemann, J. J. E. Moreau, M. Carraro, S. Campestrini and M. Pagliaro, Chem.–Eur. J., 2006, 12, 5220 CrossRef CAS; K. Yamaguchi, C. Yoshida, S. Uchida and N. Mizuno, J. Am. Chem. Soc., 2005, 127, 530 CrossRef; B. Karmi, D. Elhamifar, J. H. Clark and A. J. Hunt, Chem. Eur. J., 2010, 16, 8047 Search PubMed.
- K. Qiao, H. Hagiwara and C. Yokoyama, J. Mol. Catal. A: Chem., 2006, 246, 65 CrossRef CAS.
- A. Chrobok, S. Baj, W. Pudło and A. Jarzębski, Appl. Catal., A, 2009, 366, 22 CrossRef CAS.
- R. Sugimura, K. Qiao, D. Tomida and C. Yokoyama, Catal. Commun., 2007, 8, 770 CrossRef CAS.
- A. S. Amarasekara and O. S. Owereh, Catal. Commun., 2010, 11, 1072 CrossRef CAS.
- Q. Zhang, J Luo and Y. Y. Wei, Green Chem., 2010, 12, 2246 RSC.
- V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara and J. M. Basset, Chem. Rev., 2011, 111, 3036 CrossRef CAS; S. Shylesh, V. Schunemann and W. R. Thiel, Angew. Chem., Int. Ed., 2010, 49, 3428 CrossRef.
- R. Abu-Reziq, H. Alper, D. S. Wang and M. L. Post, J. Am. Chem. Soc., 2006, 128, 5279 CrossRef CAS; G. Chouhan, D. S. Wang and H. Alper, Chem. Commun., 2007, 4809 RSC; T. Hara, T. Kaneta, K. Mori, T. Mitsudome, T. Mizugaki, K. Ebitani and K. Kaneda, Green Chem., 2007, 9, 1246 RSC; M. J. Jin and D. H. Lee, Angew. Chem., Int. Ed., 2010, 49, 1119 CrossRef; H. Q. Yang, Y. Wang, Y. Qin, Y. Chong, Q. Yang, G. Li, L. Zhang and W. Li, Green Chem., 2011, 13, 1352 RSC.
- E. Rafiee and S. Eavani, Green Chem., 2011, 13, 2116 RSC; C. Ó. Dálaigh, S. A. Corr, Y. Gun'ko and S. J. Connon, Angew. Chem., Int. Ed., 2007, 46, 4329 CrossRef CAS; D. M. Lai, L. Deng, J. Li, B. Liao, Q. X. Guo and Y. Fu, ChemSusChem, 2011, 4, 55 CrossRef; Y. Zhang and C. G. Xia, Appl. Catal., A, 2009, 366, 141 CrossRef; X. X. Zheng, S. Z. Luo, L. Zhang and J. P. Cheng, Green Chem., 2009, 11, 455 RSC.
- X. Wang, P. P. Dou, P. Zhao, C. M. Zhao, Y. Ding and P. Xu, ChemSusChem, 2009, 2, 947 CrossRef CAS; J. Lee, Y. Lee, J. K. Youn, H. B. Na, T. Yu, H. Kim, S. M. Lee, Y. M. Koo, J. H. Kwak, H. G. Park, H. N. Chang, M. Hwang, J. G. Park, J. Kim and T. Hyeon, Small, 2008, 4, 143 CrossRef.
- H. Z. Zhi, C. X. Lü, Q. Zhang and J. Luo, Chem. Commun., 2009, 2880 Search PubMed; J. Luo and Q. Zhang, Monatsh. Chem., 2011, 142, 923 CrossRef CAS.
- A. Bee, R. Massart and S. Neveu, J. Magn. Magn. Mater., 1995, 149, 6 CrossRef CAS.
- A. Bordoloi, S. Sahoo, F. Lefebvre and S. B. Halligudi, J. Catal., 2008, 259, 232 CrossRef CAS.
- K. Miyatake, H. Iyotani, K. Yamamoto and E. Tsuchida, Macromolecules, 1996, 29, 6969 CrossRef CAS.
- A. M. Lazarin, Y. Gushikem and S. C. de Castro, J. Mater. Chem., 2000, 10, 2526 RSC.
| This journal is © The Royal Society of Chemistry 2012 |
