Aqueous and biphasic nitrile hydration catalyzed by a recyclable Ru(II) complex under atmospheric conditions†
Wei-Chih
Lee
and
Brian J.
Frost
*
Department of Chemistry, MS 216, University of Nevada, Reno, Nevada 89557, USA. E-mail: frost@unr.edu
First published on 3rd November 2011
Abstract
[RuCl2(PTA)4] (PTA = 1,3,5-triaza-7-phosphaadamantane) was found to be a highly active catalyst for aqueous phase nitrile hydration at 100 °C in air. Near quantitative conversion of aromatic, alkyl, and vinyl nitriles to their corresponding amides was observed. The reaction tolerated ether, hydroxyl, nitro, bromo, formyl, pyridyl, benzyl, alkyl, and olefinic functional groups. Some amides were isolated by simple decantation from the aqueous phase catalyst. Catalyst loading down to 0.001 mol% was examined with turnover numbers as high as 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 observed. The catalyst was stable for weeks in solution and could be reused more than five times without significant loss of activity.
000 observed. The catalyst was stable for weeks in solution and could be reused more than five times without significant loss of activity.
Amides are versatile and important synthetic intermediates used in the production of a variety of plastics, detergents, lubricants, and polymers.1,2 Traditional methods of hydrating nitriles to amides involve the use of strong bases or acids under harsh conditions, often inducing undesired hydrolysis to carboxylic acids.1–5 During neutralization salt formation can lead to contamination, separation, and pollution problems.1–3 Moreover, poor tolerance for sensitive functional groups is often observed under such conditions.1–3 A variety of transition metal complexes have been developed as efficient catalysts for nitrile hydration in organic media often with air-sensitive or non-recyclable catalysts.1–7Water, with its unique properties, has seen increased use as a solvent in stoichiometric and catalytic reactions.8 Recently several aqueous homogeneous9–11 and heterogeneous12–15nitrile hydration reactions have been reported under inert atmosphere.
One common mechanistic pathway proposed for the hydration of nitriles involves nitrile coordination to a metal followed by nucleophilic attack by water on the nitrile carbon.1 Recent studies have shown that nucleophilic attack can be promoted by activating waterviahydrogen bonding to a functionalized ligand.9–12,16–18 The concept of bifunctional catalysis19 has been applied in transfer hydrogenation of carbonyl groups,20isomerization of alkenes,21,22 and hydration of alkynes23–26 and nitriles.9–12,16–18 Recently, Cadierno and co-workers reported a series of Ru(II) and Ru(IV) complexes with nitrogen-containing phosphines for nitrile hydration in water.9–12,27 The superior activity of [RuCl2(η6-benzene)(PTA)], where PTA = 1,3,5-triaza-7-phosphaadamantane, over [RuCl2(η6-benzene)(PPh3)], was attributed to the cooperative effect of the PTA amine functionality activating waterviahydrogen bonding. Cadierno's work prompted us to investigate the catalytic activity of [RuCl2(PTA)4] for nitrile hydration compared with the inactive [RuCl2(PPh3)3] analogue. Darensbourg, Joó, and co-workers28 first synthesized the air-stable and water-soluble [RuCl2(PTA)4] and utilized it as a catalyst for the hydrogenation of aldehydes,28olefins,28 and CO229 in aqueous or biphasic media. Herein we describe the hydration of various nitriles to their corresponding amides in aqueous and biphasic media by the air-stable, water-soluble, efficient, and recyclable catalyst: [RuCl2(PTA)4].
The catalytic activity of 5 mol% [RuCl2(PTA)4] toward nitrile hydration was evaluated in aqueous solution at 100 °C with 1 mmol nitrile in a culture tube under air (Scheme 1). Under the conditions described here RuCl3 (5 mol%) provided a 54% conversion of benzonitrile to benzamide in 24 h. Benzonitrile hydration by 2 mol% RuCl3 was previously reported to yield 28% benzamide after 3 h at 130 °C.15 No hydration was observed in the absence of a catalyst, or with PTA, [RuCl2(η6-toluene)]2, or [RuCl2(PPh3)3] as catalysts. Benzonitrile hydration by [RuCl2(PTA)4] did not occur at 50 °C and provided only 23% conversion after 24 h at 75 °C. The hydration of benzonitrile catalyzed by [RuCl2(PTA)4] showed a >99% conversion to benzamide at 100 °C after 7 h, in contrast to the inactive [RuCl2(PPh3)3],30 potentially demonstrating a cooperative effect of the nitrogen-containing PTAversusPPh3.31 For comparison, nitrile hydration catalyzed by 5 mol% [RuCl2(η6-arene)(PTA)] (η6-arene = benzene, p-cymene, 1,3,5-trimethylbenzene, and hexamethylbenzene), showed >98% conversions in 4–9 h for aqueous benzonitrile hydration under N2 at 100 °C.9
![[RuCl2(PTA)4]-catalyzed nitrile hydration.](/image/article/2012/GC/c1gc15950j/c1gc15950j-s1.gif) | ||
| Scheme 1 [RuCl2(PTA)4]-catalyzed nitrile hydration. | ||
The conversion of various nitriles to the corresponding amides was explored with results summarized in Table 1. All nitriles were efficiently converted to amides with 67–99% conversion in 7 h and >99% conversions by 24 h, with the exception of 2-cyanopyridine (81% after 24h). After completion, the reactions were cooled to 0 °C and, in most cases, the product amides crystallized out as white needles and were easily isolated in 67–81% yield by decantation (Fig. 1).32 The identity of the isolated amides was confirmed by GC-MS and NMR spectroscopy.
| Entry | Substrate | Conversionb (%) | Isolated yield (%) |
|---|---|---|---|
| a Conditions: nitrile (1 mmol), [RuCl2(PTA)4] (5 mol%), H2O (3 mL), 100 °C, in air. b GC yields after 7 h (24 h yield in parentheses). c Isolated by column chromatography. d Isolated by decantation. | |||
| 1 |

|
99 | 91c |
| 2 |

|
83(99) | 67d |
| 3 |

|
96(99) | 84c |
| 4 |

|
97(99) | 77d |
| 5 |

|
90(99) | 78d |
| 6 |

|
89(99) | 90c |
| 7 |
|
99 | 86c |
| 8 |

|
99 | 73d |
| 9 |
|
99 | 87c |
| 10 |
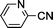
|
43(81) | 72c |
| 11 |

|
99 | 81d |
| 12 |

|
72(99) | 88c |
| 13 |

|
67(99) | 85c |
| 14 |

|
99 | 87c |
 | ||
| Fig. 1 Hydration of p-anisonitrile to p-anisamide. (Left) p-Anisamide crystals appear after cooling the reaction. (Right) Isolated p-anisamide crystals by filtration. | ||
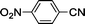
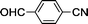
Substituted benzonitriles bearing electron-withdrawing groups (Table 1, entries 7–9) exhibited slightly more efficient conversions to amides than those with electron-donating groups (entries 2–6). Presumably, the presence of the electron-withdrawing group makes the nitrile carbon more susceptible to nucleophilic attack by an activated water molecule. This is in agreement with other catalyst systems12,33 although there have been exceptions reported.15,34 As previously reported for ortho-substituted benzonitriles,18,33–35o-tolunitrile exhibited lower conversion relative to m- and p-tolunitriles (Table 1, entries 2–4), which is attributed to steric hindrance of the o-tolunitriles. This indicates nitrile activation by coordinating to ruthenium may be necessary. Hydration of 4-cyanobenzaldehyde led to 4-formylbenzamide in a 99% conversion in 7 h with an intact formyl moiety (entry 9). The coordinating ability of the pyridyl functionality reduced catalytic activity as hydration of 2-cyanopyridine to picolinamide resulted in only 81% conversion after 24 h (entry 10).
[RuCl2(PTA)4] was also effective as a hydration catalyst for the less reactive aliphatic nitriles (Table 1, entries 11–13). 4-Methylbenzyl cyanide was transformed with 99% conversion in 7 h (entry 11) into the amide. Hydration of the sterically bulky pivalonitrile to pivalamide proceeded with a 99% conversion in 24 h although a modest conversion of 67% was observed after 7 h (entry 13). The resistance of tertiary nitriles toward hydrolysis has been noted.36 The industrially important acrylonitrile was almost quantitatively converted into acrylamide in 7 h without observation of polymerization or hydrolysis byproducts (Table 1, entry 14). For all the nitrile hydrations studied, the corresponding amides were the only product observed (no carboxylic acids were detected by GC-MS). Thus, the catalytic conditions described here are compatible with ether (entry 5), hydroxyl (entry 6), nitro (entry 7), bromo (entry 8), formyl (entry 9), pyridyl (entry 10), benzyl (entry 11), alkyl (entries 12–13), and olefinic (entry 14) functional groups, which establishes a wide synthetic scope.
Little or no induction period was observed in a kinetics plot of the hydration of benzonitrile by [RuCl2(PTA)4] in water (Fig. 2). Free PTA was observed in solution while monitoring the reaction by GC-MS. This suggests that formation of the catalytically active species may involve PTA dissociation, presumably followed by nitrile coordination. Dissociation of PTA from [RuCl2(PTA)4] to allow coordination of unsaturated aldehydes has been discussed for the hydrogenation of aldehydes.28,2931P{1H} NMR spectroscopy was obtained on the reaction mixture after 2 h in attempt to identify active species in water.32 The NMR spectrum was consistent with a series of substitutional isomers [Ru(PTA)x(H2O)y(PhCN)6−x−y]2+ similar to the results of Joó and coworkers from reaction of [Ru(H2O)6]2+ with PTA37 and cis/transisomerization of [RuCl2(PTA)4] by our group.38
![Kinetics for the hydration of benzonitrile using [RuCl2(PTA)4]. Conversions were determined by GC-MS with each data point a separate reaction under identical conditions: benzonitrile (1 mmol), [RuCl2(PTA)4] (5 mol%), H2O (3 mL), 100 °C, in air.](/image/article/2012/GC/c1gc15950j/c1gc15950j-f2.gif) | ||
| Fig. 2 Kinetics for the hydration of benzonitrile using [RuCl2(PTA)4]. Conversions were determined by GC-MS with each data point a separate reaction under identical conditions: benzonitrile (1 mmol), [RuCl2(PTA)4] (5 mol%), H2O (3 mL), 100 °C, in air. | ||
The durability of [RuCl2(PTA)4] was evaluated by reducing the catalyst loading (Table 2). We examined catalyst loading down to 0.001 mol% for the hydration of benzonitrile. Turnover numbers (TONs) up to 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (entry 9, Table 2) were obtained, which are among the highest reported to date.1 Preparative scale reactions were also examined for the hydration of benzonitrile (Table 2, entries 5, 7, 8). For example, 2.02 g of benzonitrile was hydrated at 0.1 mol% catalyst loading to give a 2.25 g (93%) isolated benzamide with a TON of 930 and a TOF of 30 h−1 (Table 2, entry 5). The TOF increases with a decrease in catalyst loading or increase in [nitrile]. For example in Table 2 entries 4 and 5 the benzonitrile concentration is increased from 0.33 M to 6.66 M at 0.1 mol% catalyst loading and the TOF increases from 14.1 to 30 h−1. Faster reactions with higher concentration of the nitrile have been observed in other ruthenium catalytic systems.15,16,39 It should be noted that in the preparative scale reactions (2 mL benzonitrile, 3 mL water) two phases were observed during hydration followed by formation of a homogeneous solution, indicating hydration was complete. The rate of hydration was also found to increase with an increase in the volume of water (i.e. dilution) possibly indicative of substrate/product inhibition at higher concentration, Table 3. Better catalytic performance (higher TOF) at lower concentration of the catalyst has also been reported for other ruthenium complexes.16,40 Addition of 1 mmol (100 mol%, 0.33 M) NaCl to the hydration of benzonitrile resulted in a slight decrease in conversion from 99 to 93% after 7 h.41 This is in contrast to a Pd nanoparticle system in which addition of 5 mol% chloride ion (0.02 M) reduces nitrile hydration conversion from 87 to 2.7%.42
000 (entry 9, Table 2) were obtained, which are among the highest reported to date.1 Preparative scale reactions were also examined for the hydration of benzonitrile (Table 2, entries 5, 7, 8). For example, 2.02 g of benzonitrile was hydrated at 0.1 mol% catalyst loading to give a 2.25 g (93%) isolated benzamide with a TON of 930 and a TOF of 30 h−1 (Table 2, entry 5). The TOF increases with a decrease in catalyst loading or increase in [nitrile]. For example in Table 2 entries 4 and 5 the benzonitrile concentration is increased from 0.33 M to 6.66 M at 0.1 mol% catalyst loading and the TOF increases from 14.1 to 30 h−1. Faster reactions with higher concentration of the nitrile have been observed in other ruthenium catalytic systems.15,16,39 It should be noted that in the preparative scale reactions (2 mL benzonitrile, 3 mL water) two phases were observed during hydration followed by formation of a homogeneous solution, indicating hydration was complete. The rate of hydration was also found to increase with an increase in the volume of water (i.e. dilution) possibly indicative of substrate/product inhibition at higher concentration, Table 3. Better catalytic performance (higher TOF) at lower concentration of the catalyst has also been reported for other ruthenium complexes.16,40 Addition of 1 mmol (100 mol%, 0.33 M) NaCl to the hydration of benzonitrile resulted in a slight decrease in conversion from 99 to 93% after 7 h.41 This is in contrast to a Pd nanoparticle system in which addition of 5 mol% chloride ion (0.02 M) reduces nitrile hydration conversion from 87 to 2.7%.42
| Entry | Catalyst (mol%) | Time (h) | Conv.b (%) | TONc | TOF d (h−1) |
|---|---|---|---|---|---|
| a Conditions: nitrile (1 mmol, 0.33 M), H2O (3 mL), 100 °C, in air. b Determined by GC (isolated yields in parentheses). c TON (mol product)/(mol catalyst). d TOF (mol product)/(mol catalyst) h¬1. e Nitrile (20 mmol, 6.66 M in water), H2O (3 mL). f Isolated yield by recrystallization from water (2.12 g, 88%) and purification of the filtrate by chromatography providing an additional 0.13 g (5%) benzamide. g Based on isolated yield. h Isolated yield by column chromatography. | |||||
| 1 | 5 | 7 | 99 | 19.8 | 2.8 |
| 2 | 1 | 7 | 87 | 87 | 12.4 |
| 3 | 1 | 24 | 99 | 99 | 4.1 |
| 4 | 0.1 | 70 | 99 | 990 | 14.1 |
| 5e | 0.1 | 31 | 98(93)f | 930g | 30g |
| 6 | 0.01 | 504 | 66 | 6600 | 13.1 |
| 7e | 0.01 | 528 | (83)h | 8300g | 15.7g |
| 8e | 0.001 | 864 | (12)h | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g 000g |
13.9g |
| 9 | 0.001 | 2328 | (22)h | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g 000g |
9.5g |
Further evidence for the robust nature of the catalyst was obtained upon storing an aqueous solution of [RuCl2(PTA)4] under air for four weeks at ∼0 °C. Hydration of benzonitrile performed using the stored aqueous solution of [RuCl2(PTA)4] (1 mol% loading) resulted in a 83% conversion after 7 h compared to 87% by freshly-prepared catalyst in water; both reactions reached >99% conversion by 24 h.
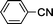
The ability to store aqueous solutions of [RuCl2(PTA)4] prompted us to explore reusability of the catalyst through recycling experiments with benzonitrile and 4-methylbenzyl cyanide, Table 4. After 7 h the reaction was cooled overnight and the aqueous supernatant containing the catalyst was carefully transferred to another reaction tube followed by addition of fresh nitrile. The precipitated amide product was collected and washed with cold water. Further purification was unnecessary as determined by GC-MS, 1H and 13C{1H} NMR spectroscopy. The isolated yields in Table 4 were obtained by decantation; maximized isolated yields (93%) can be obtained by extraction with an organic solvent (Table 4, run 1, see footnote c). Recycling was carried out at least seven times without significant loss of the catalytic activity or selectivity in the case of benzonitrile. With 4-methylbenzyl cyanide a decrease in activity was observed after the fifth recycling experiment. We attribute this to incomplete catalyst recovery during transfer of the aqueous supernatant indicated by a faint yellow color observed in the product.32,43
| Recycling experimentb | |||||||
|---|---|---|---|---|---|---|---|
| Substrate | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| a Conditions: nitrile (1 mmol), [RuCl2(PTA)4] (5 mol%), H2O (3 mL), 100 °C, 7 h, in air. b Conversions are determined by GC (isolated yields by decantation are given in parentheses). c A 93% isolated yield can be obtained by extraction with CH2Cl2 (3 mL × 5). d See ref. 32. | |||||||
|
99 | 99 | 99 | 99 | 99 | 99 | 99 |
| (18)c | (53) | (67) | (55) | (61) | (52) | (66) | |

|
99 | 99 | 99 | 96 | 95 | 85 | 38d |
| (69) | (80) | (92) | (85) | (89) | (87) | ||
The isolated yields in the aqueous phase recycling experiments with benzonitrile were modest as decantation left some benzamide dispersed in water. Aqueous/organic biphasic hydration of benzonitrile was thus studied, Table 5. Due to the mixing efficiency of two phases aqueous biphasic hydration of benzonitrile to benzamide reached 84% conversion after 7 h compared to 99% conversion in water; therefore, 24 h was used in the biphasic reactions. After 24 h the organic phase (tert-amyl alcohol) was decanted off and benzamide isolated. Fresh benzonitrile and tert-amyl alcohol were added to the aqueous phase for the next reaction. The catalyst could be recycled five times without significant loss of catalytic activity. A slight decrease in catalytic activity was observed after the fifth cycle attributed to catalyst leaching into the organic layer (slight yellow color observed over time in the organic layer). Ruthenium leaching into the organic layer was indeed observed by ICP-AES with the [Ru] increasing with each cycle: recycling experiment 1 (2.9 ppm Ru), 4 (24.5 ppm Ru), and 7 (77.2 ppm Ru).32
Conclusions
In conclusion, we have described an efficient and recyclable catalytic system to convert nitriles to amides in aqueous environments with tolerance of air and a variety of functional groups. Advantages of the catalytic system discussed here include easy catalyst preparation, simple reaction setup, and the use of green solvent (water). The catalyst is robust and highly recyclable under atmospheric conditions (no inert atmosphere required). Isolation of many amides by decantation from water largely decreases or circumvents the use of organic solvents, even in the workup steps. The gram-scale amide synthesis by hydration of nitriles using [RuCl2(PTA)4] in water is simple, practical and environmentally friendly.Experimental section
General procedure for the catalytic nitrile hydration
Under air, 1 mmol nitrile, 3 mL water, and 5 mol% [RuCl2(PTA)4] (40 mg) were added to a Telfon-sealed screw-cap culture tube and stirred at 100 °C for 7 h. The GC yields were obtained by taking a small aliquot (∼50 μL) from the hot solution and extracting with CH2Cl2 (2 mL × 3) and analysing by GC-MS. Isolated yields were obtained by either decanting the aqueous layer from the product crystals or by evaporation of the solvent followed by column chromatography over silica gel (eluent: ethyl acetate).32Acknowledgements
Support of this work by the National Science Foundation CAREER program (CHE-0645365) is gratefully acknowledged. Support from NSF is also acknowledged for the NMR facilities (CHE-0521191).Notes and references
- T. J. Ahmed, S. M. M. Knapp and D. R. Tyler, Coord. Chem. Rev., 2010, 255, 949–974.
- V. Y. Kukushkin and A. J. L. Pombeiro, Inorg. Chim. Acta, 2005, 358, 1–21 CAS.
- V. Y. Kukushkin and A. J. L. Pombeiro, Chem. Rev., 2002, 102, 1771–1802 CrossRef CAS.
- J. L. Eglin, Comments Inorg. Chem., 2002, 23, 23–43 CrossRef CAS.
- A. W. Parkins, Platinum Met. Rev., 1996, 40, 169–174 CAS.
- R. A. Michelin, M. Mozzon and R. Bertani, Coord. Chem. Rev., 1996, 147, 299–338 CrossRef CAS.
- S.-I. Murahashi and H. Takaya, Acc. Chem. Res., 2000, 33, 225–233 CrossRef CAS.
- For recent reviews see: (a) K. H. Shaughnessy, Chem. Rev., 2009, 109, 643–710 CrossRef CAS; (b) N. Pinault and D. W. Bruce, Coord. Chem. Rev., 2003, 241, 1–25 CrossRef CAS; (c) F. Joó, Aqueous Organometallic Catalysis, Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001 Search PubMed; (d) R. A. Sheldon, Green Chem., 2005, 7, 267–278 RSC.
- V. Cadierno, J. Francos and J. Gimeno, Chem.–Eur. J., 2008, 14, 6601–6605 CrossRef CAS.
- R. García-Álvarez, J. Díez, P. Crochet and V. Cadierno, Organometallics, 2010, 29, 3955–3965 CrossRef CAS.
- V. Cadierno, J. Díez, J. Francos and J. Gimeno, Chem.–Eur. J., 2010, 16, 9808–9817 CrossRef CAS.
- S. E. García-Garrido, J. Francos, V. Cadierno, J.-M. Basset and V. Polshettiwar, ChemSusChem, 2011, 4, 104–111 CrossRef CAS.
- T. Mitsudome, Y. Mikami, H. Mori, S. Arita, T. Mizugaki, K. Jitsukawa and K. Kaneda, Chem. Commun., 2009, 3258–3260 RSC.
- V. Polshettiwar and R. S. Varma, Chem.–Eur. J., 2009, 15, 1582–1586 CrossRef CAS.
- K. Yamaguchi, M. Matsushita and N. Mizuno, Angew. Chem., Int. Ed., 2004, 43, 1576–1580 CrossRef CAS.
- T. Oshiki, H. Yamashita, K. Sawada, M. Utsunomiya, K. Takahashi and K. Takai, Organometallics, 2005, 24, 6287–6290 CrossRef CAS.
- T. Šmejkal and B. Breit, Organometallics, 2007, 26, 2461–2464 CrossRef CAS.
- M. Muranaka, I. Hyodo, W. Okumura and T. Oshiki, Catal. Today, 2011, 164, 552–555 CrossRef CAS.
- For reviews, see: (a) D. B. Grotjahn, Pure Appl. Chem., 2010, 82, 635–647 CrossRef CAS; (b) D. B. Grotjahn, Dalton Trans., 2008, 6497–6508 RSC; (c) D. B. Grotjahn, Chem.–Eur. J., 2005, 11, 7146–7153 CrossRef CAS; (d) D. B. Grotjahn, Chem. Lett., 2010, 39, 908–914 CrossRef CAS.
- T. Ikariya and A. J. Blacker, Acc. Chem. Res., 2007, 40, 1300–1308 CrossRef.
- D. B. Grotjahn, C. R. Larsen, J. L. Gustafson, R. Nair and A. Sharma, J. Am. Chem. Soc., 2007, 129, 9592–9593 CrossRef CAS.
- G. Erdogan and D. B. Grotjahn, J. Am. Chem. Soc., 2009, 131, 10354–10355 CrossRef CAS.
- D. B. Grotjahn and D. A. Lev, J. Am. Chem. Soc., 2004, 126, 12232–12233 CrossRef CAS.
- D. B. Grotjahn, C. D. Incarvito and A. L. Rheingold, Angew. Chem., Int. Ed., 2001, 40, 3884–3887 CrossRef CAS.
- F. Boeck, T. Kribber, L. Xiao and L. Hintermann, J. Am. Chem. Soc., 2011, 133, 8138–8141 CrossRef CAS.
- L. Hintermann, T. T. Dang, A. Labonne, T. Kribber, L. Xiao and P. Naumov, Chem.–Eur. J., 2009, 15, 7167–7179 CrossRef CAS.
- R. García-Álvarez, J. Francos, P. Crochet and V. Cadierno, Tetrahedron Lett., 2011, 52, 4218–4220 CrossRef CAS.
- (a) D. J. Darensbourg, F. Joó, M. Kannisto, A. Katho, J. H. Reibenspies and D. J. Daigle, Inorg. Chem., 1994, 33, 200–208 CrossRef CAS; (b) D. J. Darensbourg, F. Joó, M. Kannisto, A. Katho and J. H. Reibenspies, Organometallics, 1992, 11, 1990–1993 CrossRef CAS.
- (a) G. Laurenczy, F. Joó and L. Nádasdi, Inorg. Chem., 2000, 39, 5083–5088 CrossRef CAS; (b) F. Joó, G. Laurenczy, P. Karády, J. Elek, L. Nádasdi and R. Roulet, Appl. Organomet. Chem., 2000, 14, 857–859 CrossRef CAS; (c) F. Joó, G. Laurenczy, L. Nádasdi and J. Elek, Chem. Commun., 1999, 971–972 RSC.
- It is unlikely that the activity of [RuCl2(PTA)4] is due to increased [OH−] as aqueous solutions of [RuCl2(PTA)4] are slightly acidic (pH ∼ 5). Our efforts exploring the effect of pH on ruthenium catalyzed nitrile hydration will be the subject of a future manuscript.
- While inactive for nitrile hydration [RuCl2(PPh3)3] is active for isomerization reactions in aqueous solution: (a) C.-J. Li, D. Wang and D.-L. Chen, J. Am. Chem. Soc., 1995, 117, 12867–12868 CrossRef CAS; (b) D. Wang, D. Chen, J. X. Habelman and D.-L. Chen, Tetrahedron, 1998, 54, 5129–5142 CrossRef CAS . The inactivity for nitrile hydration may be a consequence of [RuCl2(PPh3)3] being insoluble in water.
- See ESI†for details.
- R. Ramón, N. Marion and S. P. Nolan, Chem.–Eur. J., 2009, 15, 8695–8697 CrossRef CAS.
- A. Y. Kim, H. S. Bae, S. Park, S. Park and K. H. Park, Catal. Lett., 2011, 141, 685–690 CrossRef CAS.
- A. Goto, K. Endo and S. Saito, Angew. Chem., Int. Ed., 2008, 47, 3607–3609 CrossRef CAS.
- X.-B. Jiang, A. J. Minnaard, B. L. Feringa and J. G. de Vries, J. Org. Chem., 2004, 69, 2327–2331 CrossRef CAS.
- J. Kovács, F. Joó, A. C. Bényei and G. Laurenczy, Dalton Trans., 2004, 2336–2340 RSC.
- C. A. Mebi and B. J. Frost, Inorg. Chem., 2007, 46, 7115–7120 CrossRef CAS.
- C. S. Yi, T. N. Zeczycki and S. V. Lindeman, Organometallics, 2008, 27, 2030–2035 CrossRef CAS.
- A. Cavarzan, A. Scarso and G. Strukul, Green Chem., 2010, 12, 790–794 RSC.
- Mass spectrometry and NMR spectroscopic data (see ESI†) are consistent with facile chloride dissociation from [RuCl2(PTA)4] in water thus addition of NaCl has only a slight negative effect on catalysis.
- A. Ishizuka, Y. Nakazaki and T. Oshiki, Chem. Lett., 2009, 38, 360–361 CrossRef CAS.
-
PTA and O
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PTA were observed by ESI-MS and 31P{1H} NMR spectroscopy of the reaction mixtures which
may also be responsible for the loss of activity during recycling.
PTA were observed by ESI-MS and 31P{1H} NMR spectroscopy of the reaction mixtures which
may also be responsible for the loss of activity during recycling.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details including nitrile hydration and catalyst recycling procedures, 31P{1H} NMR spectrum of the reaction mixture, and GC chromatogram,1H and 13C{1H} NMR spectra of isolated amides. See DOI: 10.1039/c1gc15950j |
| This journal is © The Royal Society of Chemistry 2012 |

