DOI:
10.1039/C1FO10166H
(Paper)
Food Funct., 2012,
3, 76-82
(−)-Secoisolariciresinol attenuates high-fat diet-induced obesity in C57BL/6 mice
Received
19th August 2011
, Accepted 8th October 2011
First published on 27th October 2011
Abstract
Flaxseed lignan, secoisolariciresinol has been reported to possess health benefits. We previously synthesized each stereoisomer of secoisolariciresinol and found that (−)-secoisolariciresinol reduces lipid accumulation and induces adiponectin production in 3T3-L1 adipocytes. Here we show the effects of (−)-secoisolariciresinol on high-fat diet-induced obesity in C57BL/6 male mice. Oral administration of (−)-secoisolariciresinol for 28 consecutive days significantly suppressed the gain of body weight. Increased serum adiponectin level and decreased gene expression of fatty acid synthase and sterol regulatory element-binding protein-1c in liver, which are related to fatty acid synthesis, were observed in the mice orally administered with (−)-secoisolariciresinol. In addition, subcutaneous injection of (−)-secoisolariciresinol also significantly suppressed the gain of body weight. Serum leptin levels were significantly increased by treating with (−)-secoisolariciresinol or (−)-enterolactone. Subcutaneous injection of (−)-secoisolariciresinol, (−)-enterolactone, or (−)-enterodiol promoted gene expression of acyl-CoA oxidase, carnitine palmitoyl transferase-1, and peroxisome proliferator-activated receptor α, which are related to β-oxidation. Overall results suggest that (−)-secoisolariciresinol exerts a suppressive effect on the gain of body weight of mice fed a high-fat diet by inducing gene expression of adiponectin, resulting in the altered expression of various genes related to the synthesis and β-oxidation of fatty acids.
Introduction
Obesity is a medical condition in which body fat has accumulated to the extent that it may have an adverse effect on health, leading to reduced life expectancy and/or increased health problems. It is well known that there is a strong positive correlation between obesity and both type 2 diabetes and cardiovascular disease, including hypertension.1 Further, obesity increases the incidence of various diseases, particularly metabolic disorders, such as hyperlipidemia and non-alcoholic fatty liver disease.2,3 Although some cases of obesity are primarily caused by genetic and/or environmental factors, a combination of excessive food energy intake and a lack of physical activity is commonly thought to explain most cases of obesity. Thus, improving daily diet by taking lower fat or sugars instead of high fat foods is one of the main treatments for obesity, along with physical exercise.
Flax (Linum usitatissimum) is often cultivated in North America.4 Flaxseed has attracted increased attention due to the potential health benefits of its components, such as α-linolenic acid and secoisolariciresinol (SECO) diglycoside. Flaxseed is known as the richest dietary source of SECO diglycoside, and some fruits and vegetables also contain SECO diglycoside.5 In experimental animals, intake of SECO diglycoside exhibits positive effects on hypercholesterolemic atherosclerosis6 and on inhibiting the development of type 1 and 2 diabetes.7,8 Recent studies have indicated that oral administration of SECO diglycoside reduces plasma cholesterol levels in hypercholesterolemic subjects.9,10 These results suggest that SECO diglycoside could be a potentially useful dietary source for prevention and improvement of lifestyle-related diseases, such as obesity, diabetes, and dyslipidemia. Furthermore, our recent results showed that (−)-SECO, a synthetically prepared pure stereoisomer of SECO, has potent effects on suppressing triglyceride accumulation and stimulating adiponectin production in 3T3-L1 adipocytes,11 suggesting that (−)-SECO is useful to improve obesity by reducing fat accumulation. In the present study, we investigated the effects of sterically pure (−)-SECO on the suppression of fat accumulation in high-fat diet-induced obesity in C57BL/6 mice. This study shows that administration of (−)-SECO to the obese mice suppresses body weight gain in a dose-dependent manner by regulating the expression of various genes related to fatty acid synthesis and β-oxidation.
Experimental
Chemicals
(2R,3R)-2,3-Bis(4-hydroxy-3-methoxybenzyl)-1,4-butanediol ((−)-SECO), (3R,4R)-3,4-bis[(3-hydroxyphenyl)methyl]oxolan-2-one ((−)-ENL), and (2R,3R)-2,3-bis[(3-hydroxyphenyl)methyl]butane-1,4-diol ((−)-END), as shown in Fig. 1, were synthesized as previously reported.12,13 All chemicals used herein were purchased from Wako Pure Chemical Industries (Osaka, Japan) or Sigma (St. Louis, MO, USA) unless otherwise noted.
 |
| | Fig. 1 Chemical structures of (−)-SECO, (−)-ENL, and (−)-END. | |
Mice and diets
Male 7-week old C57BL/6 mice were obtained from Japan SLC (Shizuoka, Japan) and kept in a specific pathogen-free facility. They were given free access to food and water, and the animal room was maintained under controlled conditions of temperature at 24 ± 1 °C, humidity at 55 ± 5%, and 12-h light/12-h dark cycle. After mice were acclimatized to their housing environment for 1 week, they were fed either a standard or high-fat diet for the oral administration experiment. For the subcutaneous administration experiment, all mice were fed a high-fat diet. The standard diet consisted of (w/w) 33.1% sucrose, 29.9% dextrin, 19.0% casein, 4.74% powdered cellulose, 3.32% maltodextrin, 2.37% soybran oil, 1.90% lard, 1.56% potassium citrate tribasic monohydrate, 0.95% DIO mineral mix, 0.95% AIN-76A vitamin mix, 0.52% calcium carbonate, 0.28% L-cystine, 0.19% choline bitartrate, and 0.05% yellow dye (Japan SLC). The high-fat diet consisted of 31.7% lard, 25.8% casein, 16.2% maltodextrin, 8.89% sucrose, 6.46% powdered cellulose, 3.23% soybean oil, 2.13% potassium citrate tribasic monohydrate, 1.68% dicalcium phosphate, 1.29% DIO mineral mix, 1.29% AIN-76A vitamin mix, 0.71% calcium carbonate, 0.39% L-cystine, 0.26% choline bitartrate, and 0.006% blue dye (Japan SLC).
Administration
For oral administration, (−)-SECO dissolved in DMSO was diluted 10 times in mineral oil, and 20 μl of the solution was administered at 0, 0.058, 0.58, or 5.8 mg kg−1 body weight per day for 28 consecutive days. Food consumption and body weight were measured weekly for 4 weeks. For subcutaneous administration, (−)-SECO, (−)-ENL, or (−)-END dissolved in DMSO was diluted 10 times in mineral oil. Mice were given daily subcutaneous injections with 50 μl of 0.15 or 1.5 mg kg−1 body weight/day of (−)-SECO, 0.12 or 1.2 mg kg−1 body weight/day of (−)-ENL, 0.12 or 1.2 mg kg−1 body weight/day of (−)-END, or DMSO alone for 28 consecutive days. Food consumption and body weight were measured weekly for 4 weeks. All animal experiments were carried out in accordance with protocols approved by Ehime University Animal Care and Use Committee and were performed in accordance with applicable guidelines and regulations.
Tissue collection
The day following the consecutive 28 days administration, mice were sacrificed by cervical dislocation, and serum, liver, and epididymal white adipose tissue (WAT) were collected. Epididymal WAT was weighed to calculate an adiposity index.
Measurement of serum leptin and adiponectin levels
The leptin level in serum was determined by in-house-developed enzyme-linked immunosorbent assay (ELISA). One hundred μl of anti-mouse leptin antibody (R&D systems, Minneapolis, MN, USA) diluted 1000 times in 50 mM sodium carbonate-bicarbonate buffer (pH 9.6) was added to each well of a 96-well microtiter plate (Nunc, Roskilde, Denmark) and incubated at 4 °C overnight. After washing with phosphate-buffered saline (PBS) containing 0.05% Tween 20 (T-PBS) 3 times, each well was blocked with 5% skim milk in PBS for 2 h. After washing with T-PBS 3 times, each well was treated with 50 μl of a standard solution of recombinant mouse leptin (R&D systems) or mouse serum and incubated for 1 h at room temperature. After washing with T-PBS 3 times, each well was treated with 100 μl of biotinylated anti-mouse leptin antibody (R&D systems) diluted in 5% skim milk–PBS and incubated for 1 h at room temperature. After washing with T-PBS 3 times, streptavidin–horseradish peroxidase conjugate (Invitrogen, Carlsbad, CA, USA) diluted 2000 times for 1 h at room temperature. After washing with T-PBS 3 times, 0.6 mg ml−1 of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt dissolved in a 0.03% H2O2-0.05 M citrate buffer (pH 4.0) was added at 100 μl well−1, and the absorbance at 415 nm was measured after adding 100 μl well−1 of 1.5% oxalic acid for terminating the coloring reaction. Adiponectin level was determined by a commercially available ELISA kit from CycLex (Nagano, Japan).
Real-time reverse transcription-polymerase chain reaction (RT-PCR) analysis
Total RNA was isolated from liver by using Sepasol RNA-I Super (Nacalai Tesque, Kyoto, Japan) and from WAT with RNeasy Lipid Tissue Mini kit (Qiagen, Hilden, Germany) according to the manufacturer's instruction and used as a template for a cDNA synthesis reaction performed using MMLV-reverse transcriptase (Promega, Madison, WI, USA) with an oligo-(dT)20 primer. A 20-μl real-time PCR mixture consisted of Fast SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA), 1 μM forward primer, 1 μM reverse primer, and 0.1 μg of a cDNA sample. Thermal cycling condition was as follows: 20 s at 95 °C, and 40 cycles of 3 s at 95 °C and 30 s at 60 °C. PCR products were measured with a StepOnePlus Real-Time PCR System (Applied Biosystems), and relative gene expression was calculated using the comparative CT method by StepOne Software v2.1 (Applied Biosystems). Mouse β-actin cDNA was amplified as an internal control for normalization. The specific PCR primer sequences for each gene were shown in Table 1.
Table 1 Sequences of primers
| Primer |
Sequence (5′ to 3′) |
| Adiponectin |
5′-GGCTCTGTGCTGCTCCATCT-3′ (sense) |
|
5′-AGAGTCGTTGATGTTATCTGCATAG-3′ (antisense) |
|
Leptin
|
5′-CCGCCAAGCAGAGGGTCAC-3′ (sense) |
|
5′-GCATTCAGGGCTAACATCCAACT-3′ (antisense) |
| FAS |
5′-AGCACTGCCTTCGGTTCAGTC-3′ (sense) |
| 5′-AAGAGCTGTGGAGGCCACTTG-3′ (antisense) |
|
SREBP-1
|
5′-ATCGGCGGGGAAGCTGTCGGGGTAGCGTC-3′ (sense) |
| 5′-ACTGTCTTGGTTGTTGATGAGCTGGAGCAT-3′ (antisense) |
|
ACOX
|
5′-CTTGTTCGCGCAAGTGAGG-3′ (sense) |
|
5′-CAGGATCCGACTGTTTACC-3′ (antisense) |
|
CPT-1
|
5′-CGCACGGAAGGAAAATGG-3′ (sense) |
|
5′-TGTGCCCAATATTCCTGG-3′ (antisense) |
|
PPARα
|
5′-AGGCAGATGACCTGGAAAGTC-3′ (sense) |
| 5′-ATGCGTGAACTCCGTAGTGG-3′ (antisense) |
|
β-Actin
|
5′-CATCCGTAAAGACCTCTATGCCAAC-3′ (sense) |
|
5′-ATGGAGCCACCGATCCACA-3′ (antisense) |
Statistical analysis
Results are expressed as mean ± standard deviation (SD). When significant F values were found, the differences among groups were examined by Tukey's multiple range test. *p < 0.05, **p < 0.01, and ***p < 0.001 vs. control.
Results
Effects of (−)-SECO on body weight gain, food intake, and epididymal WAT weight in C57BL/6 mice
After feeding a high-fat diet for 28 days, mice significantly developed obesity in the control group by increasing their body weight by 111% as compared with the standard diet group (Fig. 2). When mice were treated with (−)-SECO at 5.8 mg kg−1 day−1 for 4 weeks, gain of body weight significantly reduced by 31% as compared with the control group. Although no statistical significances were found, gain of body weight was slightly suppressed in the groups treated with (−)-SECO at 0.058 mg kg−1 day−1 and 0.58 mg kg−1 day−1. Development of obesity was also confirmed by increase of epididymal WAT weight. The adiposity index of the control group was increased by 148% as compared with the standard diet group (Table 2). Treatment of (−)-SECO at 5.8 mg kg−1 day−1 for 4 weeks decreased the adiposity index by 32% as compared with the control group with no statistical significance. The increased or decreased rate of WAT was very close to the rate of the gain of body weight, as shown in Fig. 2. Slight differences between the control and (−)-SECO-treated groups were found in food intake and energy intake, when the mice were administered at 5.8 mg kg−1 day−1; however, there was no statistical significance (Table 2). On the other hand, no effects were observed in the groups treated with (−)-SECO at 0.058 mg kg−1 day−1 or 0.58 mg kg−1 day−1 on the adiposity index, food intake, or energy intake as compared with the control group. Interestingly, the energy intake was similar among all groups (Table 2).
 |
| | Fig. 2 Body weight gain of mice fed a standard or high-fat diet and orally administered with (−)-SECO. Open circle; control group, closed circle; standard diet group, open square; (−)-SECO treatment at 0.058 mg kg−1 day−1, open triangle; (−)-SECO treatment at 0.58 mg kg−1 day−1, open lozenge; (−)-SECO treatment at 5.8 mg kg−1 day−1. Data are expressed as mean ± SD (n = 4). *p < 0.05 vs. control. | |
Table 2 Effects of orally administered (−)-SECO on C57BL/6 mice fed a standard or high-fat dieta
| |
Standard diet |
Control |
High-fat diet + (−)-SECO (mg kg−1 day−1) |
| 0.058 |
0.58 |
5.8 |
|
Adiposity index (%) was calculated as WAT weight (g)/body weight (g) × 100. Data are expressed as mean ± SD (n = 4).
p < 0.001 vs. control.
|
|
Food intake (g mouse−1 day−1) |
2.90 ± 0.27b |
2.24 ± 0.39 |
2.18 ± 0.38 |
2.22 ± 0.34 |
2.07 ± 0.34 |
| Energy intake (kJ mouse−1 day−1) |
45.75 ± 4.33 |
48.36 ± 8.36 |
47.15 ± 7.31 |
47.89 ± 7.32 |
44.66 ± 8.18 |
| Adiposity index (%) |
1.63 ± 0.32b |
4.04 ± 1.14 |
3.85 ± 1.03 |
4.11 ± 0.56 |
2.74 ± 0.54 |
Effect of (−)-SECO on the adiponectin level in serum
Adiponectin levels in mouse sera were examined. The result exhibited that adiponectin secretion into serum increased in a dose-dependent manner by administrating (−)-SECO (Fig. 3). Especially, the adiponectin production in mice treated with (−)-SECO at 5.8 mg kg−1 day−1 significantly increased compared with the control group. The result indicates that oral administration of (−)-SECO stimulates adiponectin production in mice fed a high-fat diet.
 |
| | Fig. 3 Adiponectin level in serum from mice fed a high-fat diet administered with (−)-SECO. Data are expressed as mean ± SD (n = 4). C represents the control. Each sample was measured in triplicate. *p < 0.05 vs. control. | |
Effects of (−)-SECO on the gene expression of lipid metabolism-related proteins in liver
Effects of (−)-SECO on the expression of lipid metabolism-related genes, such as sterol regulatory element-binding protein (SREBP)-1c and fatty acid synthase (FAS), in liver were examined by real-time RT-PCR. As shown in Fig. 4, the gene expression levels of both SREBP-1c (Fig. 4A) and FAS (Fig. 4B) were decreased by administration with (−)-SECO. In both cases, significant differences were observed between the control group and the highest-dose group. These results indicate that oral administration of (−)-SECO inhibits the gene expression of lipid metabolism-related proteins, such as SREBP-1c and FAS, in mice fed a high-fat diet.
 |
| | Fig. 4
Gene expression of lipid metabolism-related proteins in mice fed a standard or high-fat diet and administered with (−)-SECO. (A) SREBP-1c, (B) FAS. C represents the control. Total RNA was extracted from liver and relative gene expression was evaluated by real-time RT-PCR. β-Actin mRNA was analyzed as an internal control. Data are expressed as mean ± SD (n = 4). Each sample was measured in triplicate. ***p < 0.001 vs. control. | |
Effects of (−)-SECO, (−)-ENL, or (−)-END on body weight gain, food intake, and energy intake in C57BL/6 mice
After ingestion, (−)-SECO was metabolized into (−)-enterodiol ((−)-END) and (−)-enterolactone ((−)-ENL) by bacteria in mammalian intestines. Thus, it is of great significance to examine the effects of (−)-END and (−)-ENL on obesity profiles as well as (−)-SECO. To find out whether body weight gain is inhibited by (−)-SECO itself or by its metabolites, we next examined the effect of (−)-SECO, (−)-END, or (−)-ENL on C57BL/6 mice fed a high-fat diet. These chemicals were administered for 28 days by subcutaneous injection to avoid metabolism by enteric bacteria. The result shows that the groups treated with (−)-END or (−)-ENL decreased gain of body weight compared with the control group; however, the differences were not statistically significant (Fig. 5). In contrast, the mice treated with (−)-SECO showed significant decrease in the gain of body weight when administered for 2 and 3 weeks at 0.15 mg kg−1 day−1 and for 3 and 4 weeks at 1.5 mg kg−1 day−1. Food intake and energy intake of each group were also measured. It was found that both food intake and energy intake of the groups treated with (−)-SECO at 0.15 mg kg−1 day−1 and with (−)-END at 1.2 mg kg−1 day−1 were obviously reduced compared with the control group (Table 3), indicating that the appetite was decreased by treating with (−)-SECO or (−)-END. There was no significant difference in adiposity indexes among all groups.
 |
| | Fig. 5 Body weight gain of mice fed a high-fat diet and administered with (−)-SECO, (−)-ENL, or (−)-END by subcutaneous injection. Closed square; control, open circle; (−)-SECO treatment at 0.15 mg kg−1 day−1, closed circle; (−)-SECO treatment at 1.5 mg kg−1 day−1, open lozenge; (−)-ENL treatment at 0.12 mg kg−1 day−1, cross; (−)-ENL treatment at 1.2 mg kg−1 day−1, open square; (−)-END treatment at 0.12 mg kg−1 day−1, open triangle; (−)-END treatment at 1.2 mg kg−1 day−1. Data are expressed as mean ± SD (n = 4). Each sample was measured in triplicate. *, **p < 0.05, 0.01 vs. control. | |
Table 3 Effects of subcutaneously administered (−)-SECO, (−)-ENL, and (−)-END on C57BL/6 mice fed a high-fat diet.a
| |
Control |
(−)-SECO (mg kg−1) |
(−)-ENL (mg kg−1) |
(−)-END (mg kg−1) |
| 0.15 |
1.5 |
0.12 |
1.2 |
0.12 |
1.2 |
|
Adiposity index (%) was calculated as epididymal WAT (g)/body weight (g) × 100. Data are expressed as mean ± SD (n = 4).
p < 0.05 vs. control.
|
|
Food intake (g mouse−1 day−1) |
2.27 ± 0.37 |
1.97 ± 0.36b |
2.13 ± 0.37 |
2.23 ± 0.28 |
2.10 ± 0.43 |
2.13 ± 0.28 |
2.03 ± 0.38b |
| Energy intake (kJ mouse−1 day−1) |
48.96 ± 7.94 |
42.56 ± 7.82b |
45.98 ± 8.04 |
48.08 ± 6.10 |
45.32 ± 9.37 |
45.94 ± 6.02 |
43.79 ± 8.27b |
| Adiposity index (%) |
2.43 ± 0.53 |
2.59 ± 0.23 |
2.71 ± 0.48 |
2.64 ± 1.01 |
3.02 ± 0.52 |
2.50 ± 0.72 |
3.07 ± 0.95 |
Effect of (−)-SECO, (−)-ENL, and (−)-END on leptin production
To clarify the appetite-suppressive effect of (−)-SECO and (−)-END as shown above, we measured leptin levels in serum. As shown in Fig. 6A, the serum leptin levels significantly increased by treating with (−)-SECO or (−)-ENL at both low and high doses compared with the control group. Gene expression of leptin was monitored by real-time RT-PCR using a total RNA extracted from epididymal WAT. The result shows that (−)-ENL treatment at 1.2 mg kg−1 day−1 stimulated the leptin expression in epididymal WAT, although there was no statistical significance (Fig. 6B).
 |
| | Fig. 6
Leptin production in mice fed a high-fat diet and administered with (−)-SECO, (−)-ENL, or (−)-END by subcutaneous injection. (A) Leptin level in serum was measured by ELISA. (B) Total RNA was extracted from liver and relative gene expression was evaluated by real-time RT-PCR. C represents the control. β-Actin mRNA was analyzed as an internal control. Data are expressed as mean ± SD (n = 4). Each sample was measured in triplicate. *, **p < 0.05, 0.01 vs. control. | |
Effects of (−)-SECO, (−)-ENL, and (−)-END on the expression of β-oxidation-related genes in liver
To clarify the mechanism of the suppressive effect on body weight gain, mRNA transcriptional levels of β-oxidation-related genes in liver were examined by real-time RT-PCR. Increased gene expression levels of peroxisome proliferator-activated receptor (PPAR) α, acyl-CoA oxidase (ACOX), and carnitine palmitoyl transferase (CPT)-1 were found in all groups compared with the control group (Fig. 7A–C). Although there was no statistical significance in PPARα and ACOX mRNA levels, CPT-1 was significantly increased by treating with (−)-SECO at 0.15 mg kg−1 day−1, (−)-ENL at 1.2 mg kg−1 day−1, and (−)-END at both low and high doses.
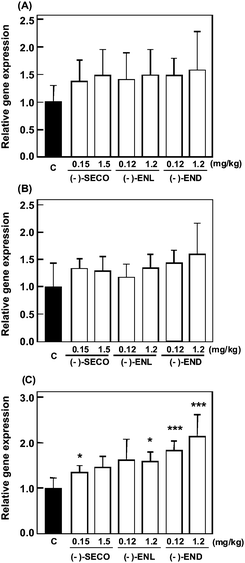 |
| | Fig. 7
Gene expression of β-oxidation-related proteins in mice fed a high-fat diet and administered with (−)-SECO, (−)-ENL, or (−)-END by subcutaneous injection. (A) PPARα, (B) ACOX, (C) CPT-1. C represents the control. Total RNA was extracted from liver and relative gene expression was evaluated by real-time RT-PCR. β-Actin mRNA was analyzed as an internal control. Data are expressed as mean ± SD (n = 4). Each sample was measured in triplicate. *, ***p < 0.05, 0.001 vs. control. | |
Discussion
In the previous study, we have examined the effect of each stereoisomer of SECO on adipogenesis using 3T3-L1 adipocytes and found that (−)-SECO most reduced the formation of lipid droplets in adipocytes and that only (−)-SECO accelerated the adiponectin gene expression at both mRNA and protein levels, while (+)- and meso-SECO isomers did not.11 Therefore, we focused on (−)-SECO to examine its in vivo effect in this study. At first, effects of (−)-SECO on C57BL/6 male mice were examined by oral administration. Only male mice were used because any influence of ovarian hormones and cycling on insulin secretion are excluded and the phenotype of the males is more severe in many animal models of obesity and insulin resistance.14 Recent papers have demonstrated that a dietary flaxseed lignan extract reduces plasma cholesterol and glucose concentrations in a dose-dependent manner,9 and that SECO diglycoside reduces fat accumulation and induces adiponectin expression in C57BL/6 mice.15 In this study, we administered the synthesized (−)-SECO compound to evaluate its effect on body weight gain in mice fed a high-fat diet for 28 days. The result revealed that oral administration of (−)-SECO at 5.8 mg kg−1 day−1 in mice fed a high-fat diet significantly decreases their body weight gain (Fig. 2) and reduces food intake and adiposity index with no statistical significance (Table 2), indicating that (−)-SECO has an effect to regulate the body weight gain. Because flaxseed contains SECO diglycoside at 2.93 μmol g−1 according to Sung et al.,16 5.8 mg of SECO would be comparable to 5.5 g of flaxseed. When (−)-SECO was orally administered to mice fed a standard diet, such an effect to decrease the body weight gain was not observed (data not shown). In our previous study, (−)-SECO had an effect to accelerate adiponectin production by 3T3-L1 adipocytes.11 Thus, we examined the effect of orally administered (−)-SECO on adiponectin levels in serum from mice fed a high-fat diet. The result showed that serum adiponectin levels were promoted by administering (−)-SECO in a dose-dependent manner (Fig. 3). Because genetic, pharmacological, and clinical studies have demonstrated potent insulin-sensitizing effects of adiponectin,17 the result indicates that (−)-SECO might be useful for the prevention of lifestyle-related diseases for high-fat diet-induced obesity. There is a paper reporting that feeding SECO diglycoside significantly increased the mRNA level of adiponectin in mice fed a high-fat diet,15 supporting that (−)-SECO is valuable to accelerate adiponectin gene expression for high-fat diet-induced obesity. The work also showed that ENL and END may be ligands of PPARγ, resulting in increased gene expression of adiponectin, a target of PPARγ. We also observed accelerated gene expression of PPARγ by (−)-SECO (data not shown), suggesting that (−)-SECO may act as a PPARγ ligand or stimulator of PPARγ gene expression. On the other hand, gene expression levels of adiponectin receptor adipoR1 in skeletal muscle and adipoR2 in liver were also examined; however, no statistical significance was observed as compared with the control group (data not shown).
Because the mice treated with a high dose of (−)-SECO at 5.8 mg kg−1 day−1 were observed to suppress their body weight gain, we attempted to explore its mechanism. We found that the expression of SREBP-1c and FAS genes were significantly down-regulated in liver from the group treated with (−)-SECO at 5.8 mg kg−1 day−1 (Fig. 4). Because adiponectin was reported to induce the suppression of serum triglyceride amount through inhibiting the gene expression of SREBP-1c and FAS in liver and skeletal muscle,18 it is suggested that the suppression of body weight gain in mice administered with (−)-SECO at 5.8 mg kg−1 day−1 resulted from the down-regulation of lipid metabolism-related genes, such as SREBP-1c and FAS, in liver by an increased adiponectin level triggered by (−)-SECO treatment. On the other hand, the expression of these genes was not significantly different from the control group in skeletal muscle (data not shown).
When mammals ingest (−)-SECO, it is supposed to be metabolized into (−)-END and (−)-ENL in the intestine and colon by gut flora.19,20 Thus, orally administered (−)-SECO would be modified in the gut before being absorbed into the body. To see the effect of (−)-SECOin vivo without being metabolized by intestinal bacterial flora, mice were next administered by subcutaneous injection. Not only (−)-SECO but also (−)-END and (−)-ENL were used to examine their effects in vivo. The gain of body weight was again inhibited by (−)-SECO treatment at both low and high doses with statistical significance (Fig. 5). Because (−)-END and (−)-ENL showed no statistically significant differences from the control group, the suppressive effect on body weight gain seems to be caused by (−)-SECO. Reduced food intake and energy intake were found in the groups administered with (−)-SECO at 0.15 mg kg−1 day−1 and with (−)-END at 1.2 mg kg−1 day−1 (Table 3). We measured leptin levels in serum from mice fed a high-fat diet, because leptin, the product of the obgene in mice, is a hormone produced by adipose tissue and known to indicate appetite suppression when its secretion was increased.21 We found that (−)-SECO and (−)-END seems to be able to stimulate the leptin production (Fig. 6A, B), indicating that decreased food intake and energy intake results from the appetite suppression caused by increased secretion of leptin. (−)-ENL also increased the leptin production, but there was no statistical significance.
We examined the expression of β-oxidation-related genes, such as PPARα, CPT-1, and ACOX, in liver and found that the expression of all three genes studied here was up-regulated in all groups treated with (−)-SECO, (−)-END, or (−)-END (Fig. 7A–C). Especially, CPT-1 gene expression significantly increased. It was reported that adiponectin induces the suppression of serum triglyceride levels through increasing the β-oxidation-related genes, such as PPARα, CPT-1, and ACOX, in liver and skeletal muscle.22 Therefore, our overall results suggest that (−)-SECO reduces fatty acid synthesis by suppressing the expression of SREBP-1c and FAS genes in liver and promotes β-oxidation of fatty acids in liver by inducing the expression of β-oxidation-related genes, such as PPARα, CPT-1, and ACOX.
Conclusions
(−)-SECO suppressed the gain of body weight in C57BL/6 male mice fed a high-fat diet by oral administration and by subcutaneous injection. The effect seems to result from reduced fatty acid synthesis by suppressing the expression of SREBP-1c and FAS genes in liver and from accelerated β-oxidation in liver by stimulating the expression of β-oxidation-related genes, such as PPARα, CPT-1, and ACOX. Increased adiponectin production triggered by (−)-SECO treatment was assumed to regulate the gene expression of these genes. (−)-SECO treatment also increased the leptin production in serum from mice fed a high-fat diet, resulting in decreased food intake. Not only (−)-SECO but also (−)-ENL and (−)-END showed an effect to decrease the gain of body weight; however, (−)-SECO showed the highest activity among them.
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (21580142).
Notes and references
- S. Rössner, Int. J. Obes., 2002, 26, S2–S4 CrossRef.
- C. Couillard, P. Mauriège, P. Imbeault, D. Prud'homme, A. Nadeau, A. Tremblay, C. Bouchard and J. P. Després, Int. J. Obes., 2000, 24, 782–788 CrossRef CAS.
- A. Ørgaard and L. Jensen, Exp. Biol. Med., 2008, 233, 1066–1080 CrossRef.
- J. T. Moraghan, Plant Soil, 1993, 150, 61–68 CrossRef CAS.
- S. J. Bhathena and M. T. Velasquez, Am. J. Clin. Nutr., 2002, 76, 1191–1201 CAS.
- K. Prasad, Circulation, 1999, 99, 1355–1362 CAS.
- K. Prasad, S. V. Mantha, A. D. Muir and N. D. Westcott, Mol. Cell. Biochem., 2000, 206, 141–150 CrossRef CAS.
- K. Prasad, J. Lab. Clin. Med., 2001, 138, 32–39 CrossRef CAS.
- W. Zhang, X. Wang, Y. Liu, H. Tian, B. Flickinger, M. W. Empie and S. Z. Sun, Br. J. Nutr., 2008, 99, 1301–1309 CAS.
- S. Fukumitsu, K. Aida, H. Shimizu and K. Toyoda, Nutr. Res., 2010, 30, 441–446 CrossRef CAS.
- S. Tominaga, T. Sugahara, S. Nishimoto, M. Yamawaki, Y. Nakashima, T. Kishida, K. Akiyama, M. Maruyama and S. Yamauchi, Biosci., Biotechnol., Biochem., 2009, 73, 35–39 CrossRef CAS.
- S. Yamauchi, T. Masuda, T. Sugahara, Y. Kawaguchi, M. Ohuchi, T. Someya, J. Akiyama, S. Tominaga, M. Yamawaki, T. Kishida, K. Akiyama and M. Maruyama, Biosci., Biotechnol., Biochem., 2008, 72, 2981–2986 CrossRef CAS.
- P. Eklund, A. Lindholm, J.-P. Mikkola, A. Smeds, R. Lehtilä and R. Sjöholm, Org. Lett., 2003, 5, 491–493 CrossRef CAS.
- S. Andrikopoulos, C. M. Massa, K. Aston-Mourney, A. Funkat, B. C. Fam, R. L. Hull, S. E. Kahn and J. Proietto, J. Endocrinol., 2005, 187, 45–53 CrossRef CAS.
- S. Fukumitsu, K. Aida, N. Ueno, S. Ozawa, Y. Takahashi and M. Kobori, Br. J. Nutr., 2008, 100, 669–676 CrossRef CAS.
- M. K. Sung, M. Lautens and L. U. Thompson, Anticancer Res., 1998, 18, 1405–1408 CAS.
- M. Bouskila, U. B. Pajvani and P. E. Scherer, Int. J. Obes., 2005, 29, S17–S23 CrossRef CAS.
- H.-J. Kim, M. Takahashi and O. Ezaki, J. Biol. Chem., 1999, 274, 25892–25898 CrossRef CAS.
- M. Axelson, J. Sjövall, B. E. Gustafsson and K. D. R. Setchell, Nature, 1982, 298, 659–660 CrossRef CAS.
- K. D. R. Setchell, S. P. Borriello, H. Gordon, A. M. Lawson, R. Harkness, D. M. L. Morgan, D. N. Kirk, H. Adlercreutz, L. C. Anderson and M. Axelson, Lancet, 1981, 318, 4–7 CrossRef.
- G. Fantuzzi, J. Allergy Clin. Immunol., 2005, 115, 911–919 CrossRef CAS.
- N. F. Brown, J. K. Hill, V. Esser, J. L. Kirkland, B. E. Corkey, D. W. Foster and J. D. McGarry, Biochem. J., 1997, 327, 225–231 CAS.
|
| This journal is © The Royal Society of Chemistry 2012 |
Click here to see how this site uses Cookies. View our privacy policy here. 






