Entrapment of Lactobacillus acidophilus into alginate beads for the effective treatment of cold restraint stress induced gastric ulcer
Pramod Kumar
Singh
,
Parneet Kaur
Deol
and
Indu Pal
Kaur
*
University Institute of Pharmaceutical Sciences, UGC Centre of Advanced Study, Panjab University, Chandigarh, 160 014, India. E-mail: indupalkaur@yahoo.com; Fax: +91-172-2541142; Tel: +91-172-2534191
First published on 31st October 2011
Abstract
Lactobacillus acidophilus (LAB) loaded alginate floating beads (FBs) were developed with an intent to (i) preserve their viability during manufacture and upon exposure to adverse physiological conditions existing in the stomach, (ii) achieve an increased stay of the system in the stomach for improved pharmacodynamics and to provide for their effective establishment within the gastric mucosa. In vitro characterization of developed beads was performed in terms of entrapment efficiency, buoyancy, and surface as well as cross sectional morphology and viability studies of LAB in a gastric environment. The developed system was evaluated and was found to be significantly better in an experimental model of cold restraint stress (CRS) induced gastric ulcer model in terms of ulcer index, hemorrhagic streak length, histopathological and biochemical markers and their cross talk with reactive oxygen/nitrogen species. The present study emphasizes the advantages and future potential of probiotic loaded FBs in gastric disorders.
1. Introduction
Probiotics, meaning “life” are rapidly becoming a popular and important tool for preserving our natural health. They have been defined in several ways, depending on our understanding of the mechanism(s) of action of their effects on the health and well-being of humans. The most commonly used definition is that of Fuller:1 “Probiotics are live microbial feed supplements that beneficially affect the host by improving its intestinal microbial balance.” Lactobacillus and Bifidobacterium species constitute a significant proportion of probiotic cultures used in developed countries that may undergo antagonistic interactions with pathogenic bacteria.2Mucosal inflammations and alterations in gut microbiota are often refractive to conventional treatments involving the employment of anti-inflammatory and immunosuppressant drugs and this has led to a search for alternative therapies based on the use of probiotics.3,4
A mechanistic study suggests that probiotics influence the protein expression in the stomach wall cells, leading to an increase in the formation of new blood cells and increased healing of the ulcer.5 In addition, probiotics form short chain fatty acids which serve as important nutrients for the mucosal cells and also help to improve the blood supply to the gut wall.6Lactobacillus also stimulates certain cells of the immune system, which improve the body defense mechanism. Epidemiologic and experimental studies suggest that the consumption of fermented milk products and lactic acid bacteria (LAB) decrease the incidence of various gastrointestinal disorders. In the last few decades the use of probiotic bacteria has gained considerable attention as a safe and accessible form of treatment for gastrointestinal diseases.7,8
Use of probiotics in clinical or supplemental therapy is however limited, because:
1. Most probiotics are fastidious, noncompetitive, and sensitive to their environment, hence repetitive large doses of probiotic are required9 to elicit physiological effect; and the effect is observed only for the time for which the probiotic is being administered.
2. Orally administered probiotics during their passage from the mouth to the intestine face adverse physiological conditions, like acidic pH, mechanical stresses, digestive enzymes, and bile acids, limiting their establishment in the gut mucosa.
3. Stability of the probiotic is another parameter of concern during their manufacture and storage.
To act as probiotics, the bacteria must arrive in the intestine alive and in sufficient number. Further, a local therapeutic action with a long duration is desirable.10 The significance of survival of probiotics in the GI-tract, their translocation and colonization and the fate of probiotic derived active components indicate a need and scope of packaging them into a suitable delivery system to increase the viability of the probiotics, both outside and inside the body.11
A floating drug delivery system (FDDS) forms the most promising delivery system for local gastric effects. It ensures a prolonged and continuous release of the probiotic in the stomach allowing sufficient time for its adhesion and establishment onto the gastric mucosa.12 In the light of these considerations, the main objective of our study was to encapsulate the LAB in FBs by orifice ionic gelation method and then to evaluate the survival upon encapsulation and of encapsulated cells under gastrointestinal condition (pH 1.2). Developed system was evaluated in experimental model of cold restraint stress induced gastric ulcer in terms of ulcer index; hemorrhagic streak length; oxido-nitrosative stress; mucus content and histopathological examination.
2. Materials and methods
2.1 Materials
Probiotic (Lactobacillus acidophilus) was procured as a gift sample from Ranbaxy Gurgaon, India (NLT 200 Billion CFU g−1). All other chemicals or reagents used in the study were of AR or GR grade.2.2 Establishment of cell count of the probiotics used
An exactly weighed quantity of probiotic (10 mg) was suspended in 0.1% peptone water and hydrated for 30 min. The suspension was vortexed and serially diluted with peptone water to obtain 2000–4000 colony forming units (CFU) ml−1. Accurately measured 0.1 ml of this dilution was mixed with 30 ml of sterile De Man Rogosa Sharpe (MRS-agar) media and plated by the pour plate method (n = 8). The plates were incubated anaerobically at 37 °C for 48 h and the colonies thus formed were counted.The extent of viability of the probiotic after 6 h of anaerobic incubation in (i) simulated gastric fluid (SGF; pH 1.2); (ii) triple distilled water and (iii) peptone water (0.1% v/v) was also confirmed, as explained above.
2.3 Preparation of Lactobacillus acidophilus loaded calcium alginate floating beads
Calcium alginate beads were prepared by the orifice ionic gelation method.13 The measured amount (20 mg probiotic) of the probiotic was suspended in water. The solution was dispersed in sodium alginate solution (3% w/v) containing HPMC (alginate: HPMC = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w). The gas forming agent calcium carbonate was added to the solution in weight ratio ranging from 0.25
1 w/w). The gas forming agent calcium carbonate was added to the solution in weight ratio ranging from 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 0.75
1 to 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (gas forming agent: alginate w/w). The mixture was degassed under bath sonicator (20–30 min) to remove any entrapped air. The resulting solution was dropped through a 26 G syringe needle into 1% w/v calcium chloride solution containing 10% v/v acetic acid. The solution containing suspended beads was allowed to stir for 1 h to improve mechanical strength and for completion of the reaction to produce gas at room temperature. Carbonate salts are insoluble at neutral pH while its cation (calcium ion) is released in the presence of acid. The beads were prepared aseptically using sterile solutions, vehicles and container. The formed beads were separated and freeze-dried overnight using freeze dryer maintained at a temperature of −40 °C. Product was lyophilized further for 6 h at −70 °C. Final weight of the formed beads was noted and 2.11 ± 1.23 g of beads were formed when 20 mg of free probiotic was loaded in to these beads (n = 6).
1 (gas forming agent: alginate w/w). The mixture was degassed under bath sonicator (20–30 min) to remove any entrapped air. The resulting solution was dropped through a 26 G syringe needle into 1% w/v calcium chloride solution containing 10% v/v acetic acid. The solution containing suspended beads was allowed to stir for 1 h to improve mechanical strength and for completion of the reaction to produce gas at room temperature. Carbonate salts are insoluble at neutral pH while its cation (calcium ion) is released in the presence of acid. The beads were prepared aseptically using sterile solutions, vehicles and container. The formed beads were separated and freeze-dried overnight using freeze dryer maintained at a temperature of −40 °C. Product was lyophilized further for 6 h at −70 °C. Final weight of the formed beads was noted and 2.11 ± 1.23 g of beads were formed when 20 mg of free probiotic was loaded in to these beads (n = 6).
2.4 Characterization and evaluation of floating beads
V b = Bulk volume of the beads = 10 ml, Vp = True/Tap volume of the beads, V = v = Vb − Vp = void volume of the particles (spaces between the particles)
The internal morphology of the beads was examined by cutting them in half with a steel blade.
2.5 In vivo studies
Further, the animals were divided into groups II–IX (Table 1). Groups II and III constituted CRS control groups, Groups IV and V were the CRS group that received free probiotic (107 CFU per oral, suspended in 1% carboxy methyl cellulose), groups VI and VII were the CRS group that received cimetidine (10 mg kg−1) orally, and groups VIII and IX constituted of CRS groups receiving probiotic FBs (equivalent to 107 CFU per oral, suspended in 1% carboxy methyl cellulose). All animals were sacrificed by cervical dislocation, their stomach was isolated and cut along the longitudinal axis, washed with ice cold saline and assessed for ulcers and hemorrhagic streaks. Suitable portions of stomach were preserved in formalin solution for histopathological examination and the remaining portion were used for mucus content and oxido-nitrosative stress determination. Ulcer index was calculated by adding the total number of ulcers plus severity of ulcers. Severity of ulceration was judged based on the scale17 at two different time points of 2 h and 10 h post CRS and administration of a suitable treatment. The sum of the respective lengths of various hemorrhagic streaks (l) was also measured and used as another parameter for assessing the extent of ulcers. Groups II, IV, VI and VIII constituted the 2 h data points while groups III, V, VII and IX were sacrificed 10 h after CRS induction and suitable treatment. Isolated stomach from the sacrificed animals were cut along the longitudinal axis, washed with ice cold saline and observed for ulcers and hemorrhagic streaks length (l).
| Time of sacrifice | Group No. | Treatment received |
|---|---|---|
| 2 h | I | Naive Control |
| II | CRS | |
| IV | CRS + Free Probiotic (107 CFU) | |
| VI | CRS + Cimetidine (10 mg kg−1) | |
| VIII | CRS + FBs-PB (Equivalent to 107 CFU) | |
| 10 h | III | CRS |
| V | CRS + Free Probiotic (107 CFU) | |
| VII | CRS + Cimetidine (10 mg kg−1) | |
| IX | CRS + FBs-PB (Equivalent to 107 CFU) |
2.6 Statistical analysis
Raw data obtained from in vitro studies is expressed as mean ± S.D (standard deviation).The in vivo results are expressed as mean ± SEM (standard error of mean). The intergroup variation was measured by one-way analysis of variance (ANOVA) followed by Tukey's test. Statistical significance was considered at p < 0.05.
3. Results
3.1 Establishment of cell count of the probiotics used
Cell count of the probiotic used was confirmed by pour plate method and the results were compared with the labeled values of the received sample. Viable count determinations were repeated eight times (n = 8) and the mean viable count was found to be (133 ± 12) ×106CFU mg−1. The clamed count was 200 × 106CFU mg−1.3.2 Viability of probiotic in different media after 6 h incubation
Purpose of the experiment was to observe the adverse effect of the gastric pH on the viability of the probiotic bacteria used in the study. After 6 h of an anaerobic incubation viable count in peptone water was (701 × 105 CFU mg−1) more than that in triple distilled water (629 × 105 CFU mg−1) and SGF. A log 2 times reduction of viable count was observed in SGF (358 × 103CFU mg−1), as compared to the peptone water, due to the highly acidic nature of the medium, which can kill the probiotic bacteria.3.3 Characterization and evaluation of floating beads
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 0.75
1 to 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 2). Increasing the amount of gas forming agent increases the production and entrapment of CO2 within the beads thus increasing their tap volume.
1 (Table 2). Increasing the amount of gas forming agent increases the production and entrapment of CO2 within the beads thus increasing their tap volume.
 | ||
| Fig. 1 (a) Surface picture of probiotic loaded beads; (b) Closer views (higher magnification) of probiotic loaded FBs showed rod shaped LAB; (c) a cross sectional picture of probiotic loaded beads. | ||
| Time (h) | K value | |
|---|---|---|
| Free probiotic | Probiotic floating beads | |
| a All values are significantly (p < 0.05) different at each time point. | ||
| 2 | −0.1135 | −0.2147 |
| 4 | 0.1072 | −0.0857 |
| 6 | 0.3766 | 0.0033 |
 | ||
| Fig. 2 (a) % Survival of bacteria after exposure to SGF at 37 °C at different time intervals (n = 4) and (b) Viable counts of probiotic loaded FBs in SGF at 37 °C at different time intervals (n = 4). | ||
Fig. 2b shows the release pattern of probiotic from the developed FBs.
3.4 In vivo studies
Studies were performed for two time points of 2 h and 10 h, representing the normal gastric transit time of 1.5 to 2 h, which will coincide for free probiotic, while the 10 h time point signifies a prolonged stay being achieved with FBs. Opening the stomach at different time points post administration of probiotic FBs, it could be observed that significant beads (22%) were retained with in the stomach even at 10 h. | ||
| Fig. 3 Effect of free probiotic, cimetidine and probiotic loaded FBs in CRS model of gastric ulcer in rat. (a) ulcer index and (b) hemorrhagic streak length. ap < 0.05 as compared to group I, bp < 0.05 as compared to group II and III, cp < 0.05 as compared to group V, dp < 0.05 as compared to group VII. | ||
 | ||
| Fig. 4 Histological micrographs of rat stomachs at the end of 10 h study in CRS model of gastric ulcer in rat. (a) Naive control, (b) CRS, (c) free probiotic treated, (d) cimetidine treated, (e) probiotic loaded FBs treated. | ||
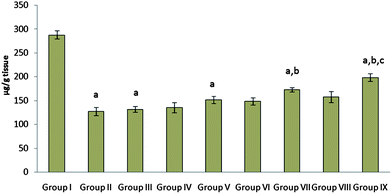 | ||
| Fig. 5 The effect of free probiotic, cimetidine and probiotic loaded FBs on mucosal content in a CRS model of gastric ulcer in rat. Group I: Naive control; Group II and Group III: CRS (sacrificed 2 h and 10 h after CRS induction); Group IV and Group V: CRS induced groups received free probiotic (107 CFU) (sacrificed 2 h and 10 h after CRS induction); Group VI and Group VII: CRS induced groups received cimetidine (10 mg kg−1 body weight) (sacrificed 2 h and 10 h after CRS induction); Group VIII and Group IX: CRS induced groups received probiotic loaded FBs (equivalent to 107 CFU) (sacrificed 2 h and 10 h after CRS induction).ap < 0.05 as compared to group I, bp < 0.05 as compared to group II and III, cp < 0.05 as compared to group V, dp < 0.05 as compared to group VII. | ||
The elevated TBARS levels were attenuated upon treatment with probiotic, cimetidine, and also with the FBs of probiotic. Very interesting observation was a lack of significanct difference between naive and FBs treated group i.e LPO levels return to the normal naive level (Fig. 6a) upon treatment with probiotic FBs.
 | ||
| Fig. 6 The effect of free probiotic, cimetidine and probiotic loaded FBs on lipid peroxides (a), superoxide dismutase (b), catalase levels (c) and nitrite levels (d) in CRS model of gastric ulcer in rat. Group I: Naive control; Group II and Group III: CRS (sacrificed 2 h and 10 h after CRS induction); Group IV and Group V: CRS induced groups received free probiotic (107 CFU) (sacrificed 2 h and 10 h after CRS induction); Group VI and Group VII: CRS induced groups received cimetidine (10 mg kg−1 body weight) (sacrificed 2 h and 10 h after CRS induction); Group VIII and Group IX: CRS induced groups received probiotic loaded FBs (equivalent to 107 CFU) (sacrificed 2 h and 10 h after CRS induction).ap < 0.05 as compared to group I, bp < 0.05 as compared to group II and III, cp < 0.05 as compared to group V, dp < 0.05 as compared to group VII. | ||
Similarly, probiotic loaded beads were significantly (p < 0.05) more effective than cimetidine and free probiotic in restoring the SOD activity (Fig. 6b). A significant (p < 0.05) decrease in catalase activity was observed in the CRS treated group as compared to naive control. Administration of cimetidine, probiotic and probiotic loaded FBs significantly increased the catalase levels (Fig. 6c). A significant increase in nitrite levels was, however, observed in the CRS group as compared to the naive control. Significantly higher nitrite levels in CRS induced group suggest their involvement in the pathogenesis of stress induced ulcers. A similar increase in the serum nitrite levels has been reported by Demirbilek et al. in CRS rats.24 The administration of free probiotic and cimetidine lower nitrite levels but probiotic loaded FBs produced a significant effect (Fig. 6d).
4. Discussion
The present study emphasizes the advantages and future potential of probiotic loaded beads in the treatment of gastrointestinal disorders. We developed probiotic loaded FBs for post-induction protective effect against CRS induced gastric ulcers and to assess the effects of formulation variables on bead characteristics, entrapment and viability in SGF. The study indicated that 0.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w ratio of calcium carbonate and sodium alginate yields beads with a significantly better DEE as compared to the other two formulations (B1 and B3). Internal ionotropic gelation effect of divalent Ca2+ of calcium carbonate on alginate results in stronger gels, such that the developed beads show significant entrapment.20 As the concentration of gas forming agent (calcium carbonate) increases, the entrapment efficiency increases; however, a high proportion of gas-forming agent (B3 batch) can make the beads highly porous and fragile13 due to which the beads are unable to retain the drug efficiently. Thus, when concentration of calcium carbonate was increased from 0.50
1 w/w ratio of calcium carbonate and sodium alginate yields beads with a significantly better DEE as compared to the other two formulations (B1 and B3). Internal ionotropic gelation effect of divalent Ca2+ of calcium carbonate on alginate results in stronger gels, such that the developed beads show significant entrapment.20 As the concentration of gas forming agent (calcium carbonate) increases, the entrapment efficiency increases; however, a high proportion of gas-forming agent (B3 batch) can make the beads highly porous and fragile13 due to which the beads are unable to retain the drug efficiently. Thus, when concentration of calcium carbonate was increased from 0.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00 to 0.75
1.00 to 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00 (calcium carbonate
1.00 (calcium carbonate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) alginate), a decrease in entrapment efficiency was observed (Table 2).
alginate), a decrease in entrapment efficiency was observed (Table 2).
The porosity and floating properties of the beads were increased with increase in the gas content of the polymer matrix. This could be due to the increasing quantity of the gas forming agent (CaCO3) used in their formulation, which would result in an increase in pore size as well as in the number of pores/area of the formulated FBs, as is apparent from scanning electron microscope pictures of the cross sections of respective FBs. Chemical reaction between calcium carbonate and acetic acid results in the release of CO2. During the formation of beads calcium carbonate effervesces, releasing carbon dioxide, which is entrapped in the gel network (HPMC-alginate), producing a formulation that remains buoyant for prolonged periods. So the higher the calcium carbonate quantity, the more CO2 that will be produced, such that more and/or larger pores will be formed. As expected, the % viability of free probiotic was significantly less compared to that loaded into FBs (Fig. 2a), thus confirming that encapsulation of probiotic bacteria within FBs protects them from the harsh acidic conditions in gastric fluids. The viability of probiotics is reported to be influenced strongly by their physiological and chemical environment.25,26 Even though LAB is reported to tolerate the acidic pH of the stomach,27 yet there was a considerable loss of viability in SGF at 6 h (based on the fed state of stomach the transit time can be up to 6 h).
CRS induces gastric mucosal damages and the possible reasons assigned for the same are:
(i) lipid peroxidation, oxidation of some critical cellular proteins, and depletion of antioxidants, indicating production of ROS during gastric ulceration; (ii) activation of superoxide dismutase (SOD), which in turn favors endogenous accumulation of hydrogen peroxide; (iii) generation of oxygen ion at an enhanced rate during stress, as evidenced by increased SOD activity; (iv) transition metal ions play an important role in the generation of stress-ulcer; and (v) the hydroxyl ion is generated at a higher rate from an oxygen ion and hydrogen peroxide through the metal-catalyzed Haber–Weiss reaction and accounts for the major oxidative damage in stress-induced gastric ulceration.28 Cold stress leads to a significant decrease in mucus content29 and an increase in the prostaglandin levels30 of rat stomach. Gastric mucus is an important protective factor for the gastric mucosa. Moreover, mucus is capable of acting as antioxidant and thus can reduce the mucosal damage mediated by oxygen free radicals. Mucosal damage increases gut permeability to macromolecules and facilitates the translocation of noxious materials such as carcinogens, endotoxin and other bacterial toxins to the bloodstream.31
The changes in LPO, catalase, SOD, nitrite, mucus content levels, and ulcer index, as well as hemorrhagic streaks induced by stress, were attenuated to normal values by probiotic loaded FBs. The beads seem to be adhering to the gastric mucosa, which may be due to the use of HPMC/sodium alginate in the preparation of FBs; both of these agents are reported to be mucoadhesive.32 A sustained release of probiotic from FBs close to the gastric mucosa for prolonged times may allow its adherence to the gastric mucosa, giving probiotics enough time and space to colonize, thus showing a significantly better effect. Histopathological studies also indicate that most of the mucosa regains its identity and its architecture is also maintained upon treatment with probiotic FBs, confirming the better anti-ulcerative effect of the latter.
5. Conclusion
The developed FBs not only efficiently protect the entrapped probiotic cells, but also help effectively deliver and retain viable bacteria in the stomach. This while ensuring a prolonged and continuous release of the probiotic in the gastric mucosa allows them to wade over the adverse gastric conditions. Pharmacodynamic results suggest the involvement of oxidative-nitrosative stress in CRS induced gastric ulcer. Treatments with FBs of probiotic confirm its therapeutic effectiveness against gastric ulcer post induction probably attributed to its antioxidant, regeneration of mucosal cells, and immunological/anti-inflammatory response. The present study emphasizes the advantages and future potential of probiotic loaded beads in the treatment of gastrointestinal disorders, until recently the probiotics have been largely propounded for their prophylactic and preventive effects. The present study establishes the therapeutic efficacy of the developed system against ulcers post-induction, thus suggesting an efficient line of treatment.References
- R. Fuller, Probiotics in man and animals, J. Appl. Bacteriol., 1989, 66, 365–378 CrossRef CAS.
- I. P. Kaur, A. Kuhad, A. Garg and K. Chopra, Probiotics: delineation of prophylactic and therapeutic benefits, J. Med. Food, 2009, 12, 219–235 CrossRef CAS.
- D. M. Brenner, M. J. Moeller, W. D. Chey and P. S. Schoenfeld, The utility of probiotics in the treatment of irritable bowel syndrome: a systematic review, Am. J. Gastroenterol., 2009, 104, 1033–1049 CrossRef CAS.
- E. M. Quigley, Probiotics in functional gastrointestinal disorders: what are the facts?, Curr. Opin. Pharmacol., 2008, 8, 704–708 CrossRef CAS.
- E. K. Lam, L. Yu and H. P. Wong, Probiotic Lactobacillus rhamnosus GG enhances gastric ulcer healing in rats, Eur. J. Pharmacol., 2007, 565, 171–179 CrossRef CAS.
- D. L. Topping, Short-chain fatty acids produced by intestinal bacteria, Asia Pacific J. Clin. Nutr., 1996, 5, 15–19 Search PubMed.
- R. Bibiloni, R. N. Fedorak and G. W. Tannock, Probiotic-Mixture Induces Remission in Patients with Active Ulcerative Colitis, Am. J. Gastroenterol., 2005, 100, 1539–1546 CrossRef.
- E. Isolauri, M. Kaila, H. Mykkanen, W. H. Ling and S. Salminen, Oral bacteriotherapy for viral gastroenteritis, Dig. Dis. Sci., 1994, 39, 2595–2600 CrossRef CAS.
- N. P. Shah, Probiotic Bacteria: Selective Enumeration and Survival in Dairy Foods, J. Dairy Sci., 2000, 83, 894–907 CrossRef CAS.
- I. P. Kaur, K. Chopra and A. Saini, Probiotics: potential pharmaceutical applications, Eur. J. Pharm. Sci., 2002, 15, 1–9 CrossRef CAS.
- K. Kailasapathy, Microencapsulation of probiotic bacteria: technology and potential applications, Curr. Issues Intest. Microbiol., 2002, 3, 39–48 CAS.
- P. K. Singh and I. P. Kaur, Development and evaluation of a gastro-retentive delivery system for improved antiulcer activity of ginger extract (Zingiber officinale), J. Drug Targeting, 2011, 19, 741–751 CrossRef.
- B. Y. Choi, H. J. Park, S. J. Hwangb and J. B. Parkc, Preparation of alginate beads for floating drug delivery system: effects of CO2 gas-forming agents, Int. J. Pharm., 2002, 239, 81–91 CrossRef CAS.
- R. A. H. Ishak, G. A. S. Awad, N. D. Mortada and S. A. K. Nour, Preparation, in vitro and in vivo evaluation of stomach-specific metronidazole-loaded alginate beads as local anti-Helicobacter pylori therapy, J. Controlled Release, 2007, 119, 207–214 CrossRef CAS.
- A. Martin, P. Bustamante, A. H. C. Chun, Micromeritics.Lippincott Williams and Wilkins, Maryland, USA, 2005, pp. 442–443 Search PubMed.
- K. Kasugai, S. J. Watson, R. A. Flavell, R. J. Davis and A. Todisco, Crucial role of c-Jun NH2-terminal kinase 1 (JNK1) in cold-restraint stress-induced gastric lesions in mice, Dig. Dis. Sci., 2007, 52, 1698–1705 CrossRef CAS.
- S. Narayan, R. S. Devi, M. Jainu, K. G. Sabitha and C. S. S. Devi, Protective effect of a polyphenol drug, ambrex in ethanol induced gastric mucosal lesions in experimental rats, Ind. J. Pharmacol., 2004, 36, 34–37 CAS.
- G. H. El-sokkarya, S. Cuzzocreab and R. J. Reiterc, Effect of chronic nicotine administration on the rat lung and liver: Beneficial role of melatonin, Toxicology, 2007, 239, 60–67 CrossRef.
- S. L. Cuppett, S. S. K. Wijeratne and V. Schlegel, Hydrogen peroxide induced oxidative stress damage and antioxidant enzyme response in caco-2 human colon cells, J. Agric. Food Chem., 2005, 53, 8768–8774 CrossRef.
- G. R. Schinella, G. Troiani, V. Davila, P. M. D. Buschiazzo and H. A. Tournier, Antioxidant effects of aqueous extract of Ilex paraguariensis, Biochem. Biophys. Res. Commun., 2000, 269, 357–360 CrossRef CAS.
- A. G. Gornall, C. J. Bardawill and M. M. David, Determination of serum proteins by means of the biuret reaction, J. Biol. Chem.., 1949, 177, 751–766 CAS.
- L. Radenovic, I. Vasiljevic, V. Selakovic and M. Jovanovic, 7-nitroindazole reduces nitrite concentration in rat brain after intrahippocampal kainate-induced seizure, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2003, 135, 443–450 CrossRef.
- H. M. Bilgin, C. Tumer, H. Diken, M. Kelle and A. Sermet, Role of ghrelin in the regulation of gastric acid secretion involving nitrergic mechanisms in rats, Physiol. Res., 2008, 57, 563–568 CAS.
- S. Demirbilek, I. Gurses, N. Sezgin, A. Karaman and N. Gurbuz, Protective Effect of Polyunsaturated Phosphatidylcholine Pretreatment on Stress Ulcer Formation in Rats, J. Pediatr. Surg., 2004, 39, 57–62 CrossRef.
- W. H. Holzapfel, P. Habeerer and R. Geisen, Taxonomy and important features of probiotic microorganisms in food and nutrition, Am. J. Clin. Nutr., 2001, 73, 365–373 Search PubMed.
- W. H. Holzapfel, P. Haberer, J. Snel and U. Schillinger, J.H. Huis in't Veld, Overview of gut flora and probiotics, Int. J. Food Microbiol., 1998, 41, 85–101 CrossRef CAS.
- M. A. Azcarate-Peril, E. Altermann, R. L. Hoover-Fitzula, R. J. Cano and T. R. Klaenhammer, Identification and inactivation of genetic loci involved with Lactobacillus acidophilus acid tolerance, Appl. Environ. Microbiol., 2004, 70, 5315–5322 CrossRef CAS.
- R. K. Banerjee, D. Das, D. Bandyopadhyay and M. Bhattacharjee, Hydroxyl radical is the major causative factor in stress-induced gastric ulceration, Free Radical Biol. Med., 1997, 23, 8–18 CrossRef.
- M. G. Repetto and S. F. Llesuy, Antioxidant properties of natural compounds used in popular medicine for gastric ulcers, Braz. J. Med. Biol. Res., 2002, 35, 523–534 CrossRef CAS.
- Y. N. Ye, H. L. So, E. S. Liu, V. Y. Shin and C. H. Cho, Effect of polysaccharides from Angelica sinensis on gastric ulcer healing, Life Sci., 2003, 72, 925–932 CrossRef CAS.
- C. Bode and J. C. Bode, Effect of alcohol consumption on the gut, Best Pract. Res. Clin. Gastroenterol., 2003, 17, 575–592 CrossRef CAS.
- P. Esposito, I. Colombo and M. Lovrecich, Investigation of surface properties of some polymers by a thermodynamic and mechanical approach: possibility of predicting mucoadhesion and biocompatibility, Biomaterials, 1994, 15, 177–182 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |



