Sugar and dietary fibre composition influence, by different hormonal response, the satiating capacity of a fruit-based and a β-glucan-enriched beverage
Roberta
Barone Lumaga
a,
Danilo
Azzali
b,
Vincenzo
Fogliano
a,
Luca
Scalfi
a and
Paola
Vitaglione
*a
aDepartment of Food Science, University of Naples, via Università 100, 80055, Portici, NA, Italy. E-mail: paola.vitaglione@unina.it; Fax: +39 0817762580; Tel: +39 0812539357
bCentro Ricerche Parmalat S.p.a., via S. Vitale 22, 43038, Sala Baganza, PR, Italy
First published on 4th November 2011
Abstract
In this study the satiating capacity of three beverages containing 3 g barley β-glucan, or 2.5 g dietary fibre (DF) from fruit, or without DF (control) was evaluated. Fourteen healthy volunteers were randomized to have isocaloric breakfasts including one of the beverages in different occasions. Appetite ratings over 3 h post-breakfast and energy intakes at ad libitum lunch, blood glucose, insulin, ghrelin, PYY, GLP-1, GIP, and PP concentrations, and 24 h food intake, were assessed. The bevaerages containing DF increased fullness and satiety over 3 h post-breakfast, but only the β-glucan-enriched vs. the control significantly reduced energy intakes by 18% at lunch and 40% over the rest of the day. Blood ghrelin and PP responses were differently modulated by beverages. The fruit-based and the β-glucan-enriched beverage suppressed by 8.9% and 8.1% ghrelin response over the 3 h and the first hour post-breakfast, respectively, while only the latter increased PP response by 34.6%, compared to the control. A sucrose-sweetened beverage providing 3 g barley β-glucans can control food intake by modulating PP response and it can even reduce 24 h energy intake. Ghrelin suppression by fruit dietary fibre and mixed sugars was not sufficient to significantly reduce food intake compared to the control.
Introduction
Overweight and obesity are a global social burden widely diffused in high-income countries and dramatically on the rise in low- and middle-income countries. WHO estimated that, by 2015, approximately 2.3 billion of adults will be overweight and more than 700 million will be obese.1The consumption of beverages especially among young people is associated to weight gain. The energy density of most beverages and their common effect to determine in consumers energy intake compensation and, in many cases, overconsumption at subsequent meals play a major role in this dangerous process.2–7
Cognitive factors linked to predictive power of foods and dependent on many physiological factors determine the different satiating capacity of solid and liquid foods.8,9 Specifically, the quicker consumption, the absence of chewing, the more rapid transit time, and the lower energy density and osmotic pressure of fluids than solids differently influence satiety cues, starting from cephalic phase, gastric filling and emptying, up to activation of gastrointestinal nutrient receptors and osmoreceptors.10–14 In this context different types of carbohydrate and sweetener formulation (glucose, fructose, high fructose corn syrup) may have a specific impact thus possibly eliciting different satiety responses.15 No conclusive evidence on the influence of beverage carbohydrate composition on satiety is available, as different sugars have not been systematically studied using an unique protocol.15 In the case of differently sweetened beverages, only one study16 evaluated the overall picture of gastro-intestinal hormone response, beside insulin, underlying the satiety effect.
On the other hand, several studies reported that the presence of dietary fibre (DF) in beverages can influence appetite perception and/or energy intake in the short-term when compared to control beverages without DF.17–22 Viscosity of beverages played a major role in influencing perceived satiety20,21 while it did not influence energy intakes 90 min from preload.23 An inverse correlation of viscosity with both gastric emptying and anorexygenic hormone secretion was found.23 Notwithstanding the blood gastrointestinal hormone response following a β-glucan-enriched beverage compared to a control beverage without DF was never assessed.
Similarly, literature focused on fruit-based beverages is lacking in the influence of gastro-intestinal hormone response in eliciting the higher satiating effect of fruit purees than the relative fruit juices (without DF).17–19
In this study we aimed to investigate the satiating effect of three beverages with different sugar and dietary fibre composition. They were a commercially available 100% fruit-based beverage, a barley β-glucan-enriched beverage and a control beverage without DF. The appetite perceptions and the blood profile secretion of six gastrointestinal hormones in the short term as well as the overall 24 h energy intakes following consumption of each beverage were monitored. The study design showed for the first time, a detailed picture of some mechanisms underlying satiety process in the short term and allowed us to discuss on the role played on satiety by sugar and DF composition of beverages.
Subjects and methods
Subjects
Fourteen volunteers (8M/6F; mean age 27.8 ± 4.9, range 24–39 years and with normal body mass index BMI 20.2–24.6 kg m−2) were selected to participate in this study. The enrolment was performed among the students of the Faculty of Agriculture, University of Naples, who were asked to fill a questionnaire on their medical status, subjective eating habits and food preferences (scores from 1 to 9) for 100 items. The selected subjects were healthy, not undergoing any medication or drug therapy, they were not on a restrictive diet, they usually had breakfast and they had not eating behaviour disease on the basis of scores obtained filling the Three Factor Eating Questionnaire (TFEQ).24The eligible subjects signed an informed written consent before entering the study. They were advised not to vary their physical activity during all the period of the study, always avoiding it the day before each test day.
Foods
The fruit-based beverage was a commercially available product prepared as a mix of different fruit purees and juices with a typical tropical taste. Its ingredients were: apple puree (40.5%), white grape juice (37.3%), pineapple juice (6.0%), banana puree (5.8%), orange juice (5%), mango puree (4.5%), apple and pumpkin extract, dried coconut milk (0.1%) and natural aromas. Per 250 mL portion it contained 34.3 g sugars, of which 51% fructose, 35% glucose and 14% sucrose, and 2.5 g DF from fruits, i.e. mainly pectins.
The β-glucan-enriched beverage was developed as a tropical fruit-flavoured beverage. It was constituted by: water, sucrose (13.8%), barley β-glucans (1.2%), citric acid as acidifier, apple and pumpkin extract, dried coconut milk (0.1%) and natural aromas. The β-glucan-enrichment (3 g per a 250 mL portion) was feasible through inclusion in the beverage recipe of Glucagel™, a powder containing ≥75% barley β-glucans concentrate, purchased from DKSH Italia s.r.l. (Milano, Italy).
The control was a tropical fruit-flavoured beverage and differed from the β-glucan-enriched beverage only for the content of sucrose (14.9% vs. 13.8%, respectively) and of β-glucans, that were absent (no DF).
Thus the sugar content of the β-glucan-enriched and the control beverage, per 250 mL portion was 34.5 g and 37.3 g respectively, and was 100% sucrose.
Additionally, the 3 beverages contained citric acid and tropical taste flavoring.
The chemical composition of the three beverages is summarized in Table 1. The β-glucan-enriched and the control beverage were formulated on the basis of the fruit-based beverage characteristics in order to have three beverages with the same energy density and similar nutrient content, except for sugar types and the amount and type of DF. The negligible differences of beverages in such a minor amount of proteins (≤0.4%) and fats (≤0.1%) could not influence satiety cues.
| β-glucan beverage | Fruit-based beverage | Control beverage | |
|---|---|---|---|
| Portion volume (mL) | 250 | 250 | 250 |
| Carbohydrates (g) | 34.5 | 34.3 | 37.3 |
| fructose | — | 17.5 | — |
| glucose | — | 12.0 | — |
| sucrose | 34.5 | 4.80 | 37.3 |
| Proteins (g) | 0.2 | 1.0 | — |
| Fats (g) | 0.3 | 0.3 | — |
| Total dietary fibre (g) | 3.00 | 2.5 | — |
| β-glucans | 3.0 | — | — |
| pectins | — | 2.5 | — |
| Energy density (kcal, kJ) | 147.5 (617.1) | 148.9 (623.0) | 149.2 (624.2) |
All beverages were served at room temperature (∼25 °C). The taste perception and hedonics of beverages were also assessed on anchored 100 mm VAS during screening. For each beverage subjects were asked to complete the following scales: how sweet, sour, bitter, or salty the beverage was; how fatty, creamy, and fresh the flavor of the beverage was; and how pleasant the drink was in the mouth.
No difference was found among beverages as regards the perception of sweet, sour, bitter and salty. On the contrary, slight differences were found as regards perception of fatness, creaminess and freshness. The rating order was: for fatness β-glucan-enriched ≥ fruit-based > control beverage; for creaminess β-glucan-enriched ≥ fruit-based > control beverage; for freshness control beverage > β-glucan-enriched ≥ fruit-based beverage. These differences did not influence the total pleasantness in the mouth that was rated as sufficient and not significantly different among all beverages.
| Proteins | Carbohydrates (CHO) | Sugars (g) | Starch | Dietary fibre | Fats | Energy value kcal (kJ) | |
|---|---|---|---|---|---|---|---|
| Bread | 8.6 | 66.9 | 1.9 | 59.1 | 3.2 | 0.4 | 315.2 (1317.5) |
| Pasta with tomato sauce | 2.87 | 16.35 | 1.85 | 13.2 | 1.36 | 10.33 | 167.4 (699.7) |
| Pasta with zucchini | 3.78 | 19.2 | 1.74 | 15.9 | 1.34 | 10.36 | 181.6 (759.1) |
| Beef meat with tomato | 21.02 | 0.75 | 0.75 | — | 0.38 | 6.03 | 142.1 (594) |
| Fish | 29.8 | — | — | — | — | 5.8 | 171.4 (716.5) |
| Green salad | 1.7 | 2.1 | 2.1 | — | 1.42 | 5.38 | 66.5 (277.8) |
| Chips | 3.9 | 29.9 | 0.6 | 26.6 | 2.2 | 6.7 | 189.1 (790.4) |
| Apple | 0.3 | 13.7 | 13.7 | — | 2 | 0.1 | 60.9 (254.6) |
Study design
The study design and protocols were approved by the Ethics Committee of the University of Naples. The study was performed in two separate trials as previously described.25 The first trial was planned in order to evaluate appetite ratings and energy intakes following beverage consumption, while the second one was focused on monitoring gastrointestinal hormones and glucose metabolism biomarkers over the time.Both protocols had a cross-over, single blind, randomized design, they were characterized by three treatments per each subject that were conducted on separate days with a 1-week wash-out period from each other. All in all each subject participated in six tests.
The subjects were instructed to consume a standardized dinner on the evening before each test, always before the 2200 h and were required to be fasting when they reached the research centre at 0830 h to have breakfast. Before starting each session, volunteers filled out a questionnaire on their current general well-being. Test was postponed in case of menstruation or any kind of physical/psychological discomfort. The treatments consisted in the consumption (at 0900 h) of an isocaloric breakfast (∼536.3 kcal, 2245.6 kJ) including 4 slices of toasted bread and a 250 mL portion of the control beverage (meal 1) or the β-glucan-enriched beverage (meal 2) or the fruit-based beverage (meal 3). The breakfast had to be entirely consumed always within 15 min.
At 1200 h subjects were invited to have an ad libitum lunch. They were called individually and left free to compose their lunch tray based on their current desire to eat. Subjects were asked to consume the test meal until they felt “comfortably full”. During the lunch test subjects sat separately and they were not allowed to see or talk to each other. The water consumption in the time interval between breakfast and lunch was measured and food intakes at lunch were calculated as the difference in the weight of the dishes before and after lunch.
After the ad libitum lunch the subjects left the research centre, but they were asked to fill out a food diary until 0900 h of the day after. Energy and nutrients intakes at ad libitum lunch and at the following hours over the treatment days were calculated based on the amount consumed and nutritional composition of each food.
Blood analyses
Blood samples were collected into serum tubes for gel separation and immediately added with protease inhibitors, such as dipeptidylpeptidase IV (DPPIV) inhibitor (Millipore's DPPIV inhibitor; St Charles, MO, USA) and phenylmethanesulfonyl fluoride (PMSF, Sigma, St. Louis, MO, USA). They were centrifuged at 2400 g per 10 min at 4 °C, and the supernatants were stored at −40 °C until the analysis.Gut hormone concentrations (for active ghrelin, active GLP-1, total GIP, total PYY, PP, and insulin) were simultaneously quantified in 25 μl of serum by using a human gut hormones multiplex kit (Millipore, St Charles, MO, USA) and by using Luminex Technology (Bio-Plex; Bio-Rad, Milano, Italy). Each sample was analyzed in triplicate. The sensitivity levels of the assay (in pg mL−1) correspond to the following values: ghrelin, 1.8; GIP, 0.2; GLP-1, 5.2; PP, 2.4; insulin, 44.5; and PYY, 8.4. The inter-assay variation (%CV) was 19%, and the intra-assay variation (%CV) was 11%.
Collected blood samples and serum samples ready for the analysis were accurately handled according to multiplex kit manufacturer instructions.
Glycaemia by finger pricking and using a bedside glucometer (OneTouch Sure Step; Life Scan Inc., Milpitas, CA, USA) was measured immediately before the blood draw.
Accuracy of the glucometer was evaluated by the manufacturer using least-squares linear regression analysis and found to be 97% “clinically accurate” when compared with reference (YSI2700) results.
Statistical analysis
The number of subjects was based on power calculations derived from our previous study.25 We calculated that, at α = 0.05 with a power of 80%, 12 subjects would allow us to detect a 19% difference in energy intake at ad libitum lunch and 8 subjects would allow to detect a 23% and 16% difference in AUC0–180 of ghrelin and PYY, respectively.Statistical analyses were performed using the statistical package SPSS for Windows (version15). The results of both appetite scores and of biochemical analyses were analyzed and expressed as the absolute changes from the baseline to reduce possible effects of inter-subject fasting variability. The total area under the curves (AUC) for desire to eat, fullness and satiety ratings (from baseline over 3 h from breakfast consumption) as well as for glucose and gut hormones blood concentrations were also estimated using the linear trapezoidal rule. By the analysis of variance (ANOVA) for repeated measures the subjective appetite sensations recorded after the consumption of the three types of beverage and the response curves of glucose, insulin, ghrelin, GLP-1, GIP, PYY and PP were compared and tested for the effect of treatment and of time as factors. For all the tests, following a significant main effect in the ANOVA, individual means were compared using the Bonferroni test (p < 0.05). Results were considered significant at p < 0.05. All values were reported as means ± SEM.
Results
Appetite perceptions
Perceptions of hunger, fullness and satiety over the three hours following meal 1 or meal 2 or meal 3 were reported in Fig. 1.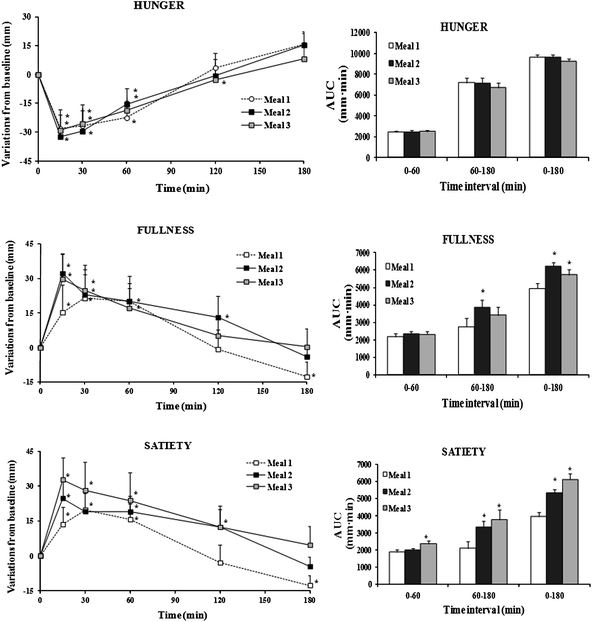 | ||
| Fig. 1 Appetite rating-time curves and AUC of perceived sensations over specific time intervals (0–60 min, 60–180 min and 0–180 min) following the consumption of each beverage. In time-curves graphs *: p < 0.05 vs. baseline value. In AUC graphs *: p < 0.05 vs. meal 1. | ||
All sensations peaked 15 min after consumption of the three beverages. Hunger was significantly reduced up to 60 min and returned to baseline values at 120 min. On the contrary, both fullness and satiety significantly increased over the first hour post-breakfasts. At 120 min fullness was still significantly higher than baseline only with meal 2 (containing the β-glucan-enriched beverage) while satiety was still perceived higher than baseline, after consumption of both meal 2 and meal 3 (containing the β-glucan-enriched beverage and the fruit-based beverage, respectively).
Analysis of total (0–180 min) and partial (0–60 min and 60–180 min) AUC for all sensations (Fig. 1) showed that there was no significant difference among the three beverages as far as hunger sensation. On the contrary AUC0-180 of fullness and satiety were significantly higher after consumption of meal 2 (by 25.3% and 34.8%) and meal 3 (by 15.8% and 54.6%) than after meal 1. Looking at partial AUC of fullness, no difference was recorded among the three beverages over the first hour post-breakfast while, over the following two hours, fullness caused by meal 2 consumption was 39.4% (p < 0.05) higher than that caused by meal 1. Partial AUCs of satiety after meal 3 were higher by 25.7% (AUC0–60) and 80.5% (AUC60–180) than those recorded after meal 1, while satiety after meal 2 was 21.3% (p < 0.05) higher than that relative to meal 1 only after the first hour from consumption.
Energy intakes (EI)
The 24 h EI following the consumption of each beverage and the contribution of ad libitum lunch and of meals consumed over the post-lunch time until the morning after, are reported in Fig. 2. When subjects consumed meal 2 compared to meal 1, significant 18% (−168 ± 16.4 kcal) and 40% (−502 kcal ± 55.5 kcal) reductions of EI at ad libitum lunch and over the post-lunch time period, respectively, were recorded. Thus over the 24 h meal 2 consumption reduced by 30% EI (−670 kcal ± 71.9 kcal, p < 0.05) vs. meal 1. On the other hand EI found after meal 3 were not statistically different either from meal 1 and meal 2. No difference in water consumption over the three hours after each meal was found.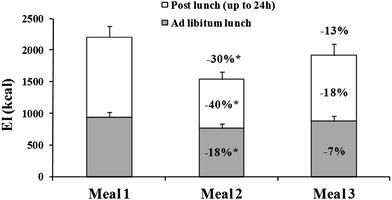 | ||
| Fig. 2 Energy intakes (kcal) measured at ad libitum lunch, post-lunch and over the all experiment day upon consumption of each beverage. *: p < 0.05 vs. meal 1. | ||
The food preferences of subjects, as well as the mean nutrients and DF composition of ad libitum lunch (Table 3), did not vary upon different experimental conditions. Thus, nutrient composition of lunches after meal 1, meal 2 or meal 3 could not have differently affected the EI recorded over the post-lunch period.
| Proteins | Carbohydrates | Fats | Dietary fibre | EI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| g | % EI | g | % EI | g | % EI | g | % EI | kcal | kJ | |
| a p < 0.05 for meal 2 vs. meal 1. | ||||||||||
| Meal 1 | 45.8 ± 7.0 | 19.3 ± 1.3 | 125.7 ± 9.6 | 54.2 ± 1.7 | 25.6 ± 2.3 | 24.7 ± 1.9 | 8.2 ± 0.6 | 1.8 ± 0.1 | 932.8 ± 82.3 | 3905.7 ± 344.7 |
| Meal 2 | 38.0 ± 6.3 | 19.9 ± 2.6 | 97.1 ± 7.8 | 50.7 ± 1.6 | 23.9 ± 2.9 | 27.8 ± 1.9 | 6.0 ± 0.4 | 1.6 ± 0.1 | 767.5 ± 65.8a | 3213.7 ± 275.6a |
| Meal 3 | 44.2 ± 10.4 | 19.1 ± 3.1 | 113.0 ± 11.0 | 52.6 ± 3.9 | 25.1 ± 3.5 | 26.3 ± 2.9 | 8.3 ± 0.5 | 1.9 ± 0.1 | 871.2 ± 79.3 | 3647.9 ± 331.8 |
Biochemical analysis
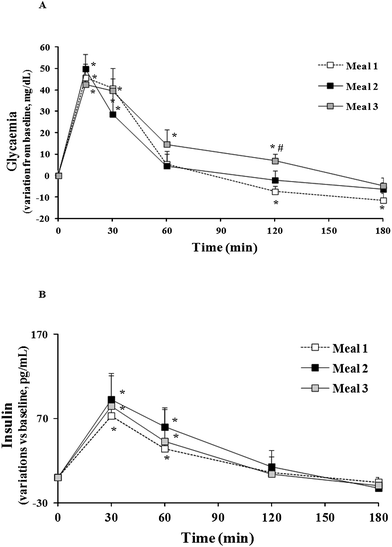 | ||
| Fig. 3 Glycaemia and insulin response over 3 h following consumption of each beverage. *: p < 0.05 vs. respective baseline value; #: p < 0.05 vs. meal 1. | ||
Fig. 3B showed that serum insulin concentrations varied from relative baseline values over the first 60 min after meal consumption and returned to baseline at 120 min. No difference among the meals was found at each time point and over the three hours monitored (AUC0–180 being 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 001.68 ± 1980.00 pg min mL−1 following meal 1, 29
001.68 ± 1980.00 pg min mL−1 following meal 1, 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 681.34 ± 2329.09 pg min mL−1 following meal 2 and 29
681.34 ± 2329.09 pg min mL−1 following meal 2 and 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 098.16 ± 3683.15 pg min mL−1 following meal 3).
098.16 ± 3683.15 pg min mL−1 following meal 3).
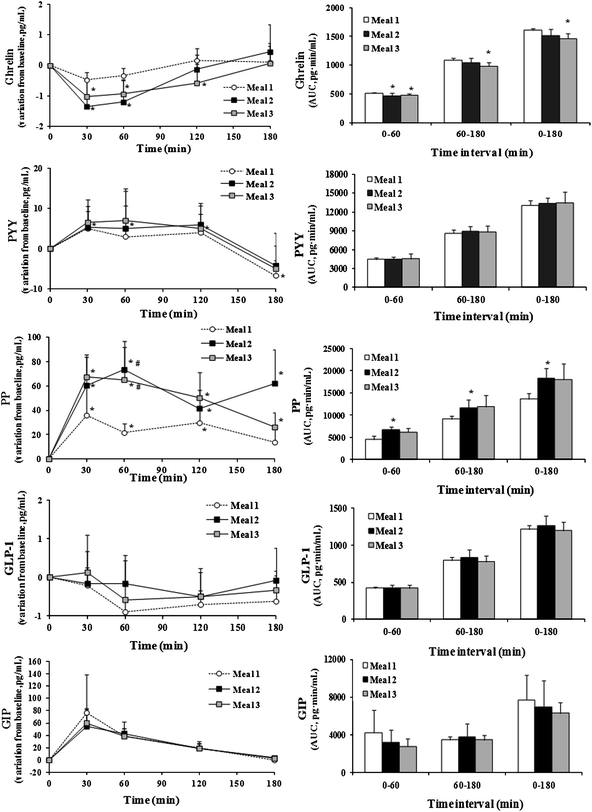 | ||
| Fig. 4 Gastro-intestinal hormone response (time-concentration curves and AUC) following consumption of each beverage. In time-concentration curves *: p < 0.05 vs. baseline value; #: p < 0.05 vs. meal 1. In AUC graphs *: p < 0.05 vs. meal 1. | ||
Ghrelin concentrations were reduced from baseline over the first hour following both meal 2 and meal 3 consumption. Accordingly, over this time interval, the AUC of the hormone was significantly lower after meal 2 and meal 3 than after meal 1 (AUC0–60 being 471.79 ± 30.00 pg min mL−1 and 481.09 ± 22.80 pg min mL−1vs. 513.44 ± 9.38 pg min mL−1, respectively). Only meal 3 induced, in comparison with meal 1, a reduced plasma ghrelin response over the next two hours (AUC60–180 being 983.56 ± 64.42 pg min mL−1vs. 1093.81 ± 31.57 pg min mL−1, respectively, p < 0.05). No difference between meal 2 and meal 3 was found.
PYY concentrations over the three hours post-meal 2 and meal 3 did not vary, while at 180 min following meal 1, PYY was significantly reduced from baseline (60.3 ± 18.3 pg mL−1vs. 64.5 ± 18.6 pg mL−1). No effect of beverages on AUCs of the hormone secretion was found.
PP plasma concentrations peaked between 30 min and 60 min after consumption of all beverages and at 180 min returned to baseline value only after meal 1. Moreover at 60 min after consumption of meal 2 and meal 3 variations from baseline of plasma PP concentration were higher than after meal 1: relative concentrations from baseline ranging between 53.22 ± 23.83 pg mL−1 and 111.61 ± 39.33 pg mL−1 after meal 2, between 60.84 ± 36.12 pg mL−1 and 126.00 ± 56.02 pg mL−1 after meal 3, and between 58.52 ± 27.14 pg mL−1 and 83.49 ± 33.91 pg mL−1 after meal 1. However, according to AUC0–180, only meal 2 caused a blood PP response significantly higher than meal 1 (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 385.74 ± 2220.62 pg min mL−1vs. 13
385.74 ± 2220.62 pg min mL−1vs. 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 661.72 ± 1270.69 pg min mL−1, respectively).
661.72 ± 1270.69 pg min mL−1, respectively).
GLP-1 concentrations did not significantly vary from baseline over the three hours post-breakfast with meal 2 or meal 3. A decrease from baseline was found only at 60 and 120 min following consumption of control beverage. However, no significant difference was found among beverages at each time points and as regards to AUC values.
GIP peaked at 30 min and was significantly higher than baseline until 120 min post-meals. No difference among AUC of the hormone following the consumption of any meal was recorded.
Discussion
The experimental protocol of this work was designed to evaluate the ability of a fruit-based and a barley β-glucan-enriched beverage to modulate appetite and energy intakes, in healthy subjects, over 24 h after isocaloric breakfasts.Data showed that both beverages containing DF were able to increase perceived fullness and satiety (Fig. 1). However, only after consumption of the β-glucan-enriched beverage subjects significantly reduced their energy intakes at subsequent ad libitum lunch and over the 24 h after breakfast, compared to the control beverage (Fig. 2).
The increase of fullness and satiety upon consumption of the fruit-based beverage vs. the control one was consistent with literature studies reporting the same effects after consumption of either fruit puree vs. fruit juice17,18 or orange juices added with pectins (from 5 to 20 g) vs. the same juices without DF.27 Similarly, findings on meal 2 vs. meal 1 were consistent with the studies from Lyly and co-workers.20,21 In the most recent one, the authors reported that oat DF-enriched beverages (2.5 or 5.1 g of β-glucans) increased post-meal satiety compared to the control beverage without DF: this effect being dependent to the type of DF and beverage viscosities, and independent from the amount of DF and beverage energy content.
In the present study neither different sugars nor DF composition of the fruit-based and β-glucan-enriched beverage significantly influenced the appetite perceptions after beverage consumption. Accordingly, previous works showed that beverages sweetened with a mixture of fructose and glucose or with sucrose have no different effects on appetite sensations.16,28 To the best of our knowledge, no previous study compared satiating capacity of barley β-glucans and fruit DF. In this respect our data indicated that the different amount of DF (2.5 g pectinsvs. 3 g β-glucans), as well as the different viscosity of the two beverages (90 mPa·s for fruit-based beverage vs. 55 mPa·s for the β-glucan-enriched beverage) were not sufficient to modify subjects appetite perceptions.
Food matrix and type of DF influenced glycaemia recorded after consumption of fruit-based or β-glucan-enriched beverage compared to the control. Blood glucose concentrations after meal 3 were always higher than baseline over the two hours post-ingestion and appeared blunted compared to those measured after meal 1. This phenomenon might be due to fruit pectins present in the fruit-based beverage and playing a role in glucose homeostasis. Haber & coworkers showed that plasma-glucose rapidly fall under baseline levels, during the second hour after apple juice consumption, and that this fall was blunted after apple puree and, even more, after whole apple consumption.19 Similarly, in the present study, the control beverage caused a higher reduction of blood glucose at 120 min, than the other beverages. This result might be connected with the higher food intake at lunch, that was higher after the meal 1 than after the meal 2 or 3.29,30
Data in Fig. 3 also showed that meal 2 did not reduce glycaemia compared to meal 1. Literature reporting hypoglycaemic effect of β-glucans mainly refers to studies testing food products naturally containing β-glucans, such as whole oat/barley or their bran or flour added to foods or meals.31–38 Furthermore it has been reported that the hypoglycaemic effect of β-glucans from these foods was found when 1 g β-glucans was consumed together with 50 g carbohydrates.34 In our previous work we reported that 3 g of β-glucans extracted from barley, included in conventional bread and consumed as a part of breakfast totally providing 57.4 g digestible carbohydrates (5.2% β-glucans by carbohydrates) showed an hypoglycaemic effect, in healthy subjects, compared to a control bread, without β-glucans. The discrepancy of finding might depend from two factors: (i) the low dosage of concentrated β-glucans tested here (2.3% β-glucans by carbohydrates) compared to our previous study, and (ii) the presence in the β-glucan-enriched beverage of sucrose that is more easily absorbed in the short term compared to the starch, constituting the major carbohydrate in bread or whole cereals/meals tested in the previous studies.
Although the appetite ratings were in accordance to significantly lower EI at ad libitum lunch only for the meal 2 (p < 0.05) vs. meal 1, a trend to reduced EI also after meal 3 vs. meal 1 (p = n.s.) was found.
Looking at blood gastro-intestinal hormone response following the consumption of the three meals, only two hormones by six monitored, namely ghrelin and PP, were differently modulated by the beverages, while the other four (GLP-1, GIP, PYY and insulin) were not influenced by the type of beverage. Over the three hours post-meal, ghrelin concentration (the orexygenic hormone that regulates food intakes in the short term) was reduced only when meal included the fruit-based beverage compared to the control one (the reduction following meal 2 vs. meal 1 occurred only upon the first hour after consumption); on the contrary PP concentration (anorexygenic hormone) was significantly higher when the meal included the β-glucan-enriched beverage compared to the control one. The effect of the fruit-based beverage on food intakes appeared to be regulated by ghrelin suppression and it might be sustained by a prolonged increase in intestinal osmolarity with this beverage compared to the control one;39 this might be consistent, in turn, with the more regular blood glucose time-concentration curve after the fruit-based beverage than the control (see Fig. 3, meal 3 vs. meal 1). On the other hand, differential ghrelin suppression by meal 3 vs. meal 1 did not correlate with insulin or with GIP and GLP-1 secretion; thus suggesting that differences in gastric emptying rate between the fruit-based and the control beverage were not likely to be implicated in this process.40
The significant increase of PP following meal 2 vs. meal 1 was correlated with increased satiety and reduced EI at ad libitum lunch after meal 2 consumption, and might indicate a reduced gastric emptying following this beverage compared to the control one.41,42 Previous literature generally indicated no effect of DF on this hormone,43 however this is the first study evaluating the effect of β-glucans on postprandial PP. On the other hand, the role of PP in reducing gastric emptying rate was previously shown44 and it was consistent, in the present study, with the higher viscosity of the β-glucan-enriched than the control beverage (55 mPa·s vs. 1.5 mPa·s, respectively).20,23 In this respect, we hypothesized that the fruit-based beverage failed to elicit a significant effect on PP secretion (despite its even slightly higher viscosity than the β-glucan-enriched beverage) because of the different type of DF in the two beverages. It may be that since β-glucans were added in the beverage in pure form, they might stimulate gastro-intestinal receptors more efficiently than fruit pectins, which were well-structured in the original food matrix.
As hormone response was monitored only in the short-term (over 3 h post-breakfast), the dramatic reduction of EI over all the experiment day following meal 2 vs. meal 1 (−40% kcal consumed post-lunch) could not be correlated with the hormonal status. Anyway, it may be hypothesized that the reduced EI over the period post-lunch might be due to the fermentation of β-glucans by intestinal microflora and the consequent production of short chain fatty acids45 which might, in turn, regulate secretion of gastro-intestinal anorexygenic peptides.46
The possibility that gastro-intestinal discomforts might have reduced food intakes at both ad libitum lunch and over all the experiment day following meal 2 and meal 3 was negligible as subjects reported no gastro-intestinal symptoms related to their consumption.
Taken together the data reported in this study demonstrated that a sucrose-sweetened beverage providing 3 g barley β-glucans can control appetite and food intake by modulating PP response, and it can also reduce 24 h energy intakes. On the other hand, a fruit-based beverage, providing 2.5 g fruit dietary fibre (mainly pectins) and sweetened with mixed sugars, can control appetite and suppress ghrelin response in the short-term, but it is not able to reduce food intake compared to a control beverage.
Acknowledgements
P. V. thanks GraceLinc Limited (Christchurch, New Zealand) for contributing in funding this research that was done in the frame of an agreement with the University of Naples “Federico II”; Rita Schettino and Ivana Galluccio for their practical help during some test days; Patrizia Scognamiglio (the nurse) and volunteers for their kindness.References
- World Health Organisation, Fact sheet: obesity and overweight. Available at: http://www.who.int/mediacentre/factsheets/fs311/en. Accessed 10 October 2010.
- L. Harnack, J. Stang and M. Story, Soft drink consumption among US children and adolescents: nutritional consequences, J. Am. Diet. Assoc., 1999, 99, 436–441 CrossRef CAS.
- D. S. Ludwig, K. E. Peterson and S. L. Gortmaker, Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis, Lancet, 2001, 357, 505–508 CrossRef CAS.
- G. Mrdjenovic and D. A. Levitsky, Nutritional and energetic consequences of sweetened drink consumption in 6- to 13-year-old children, J. Pediatr., 2003, 142, 604–610 CrossRef.
- C. S. Berkey, H. R. Rockett, A. E. Field, M. W. Gillman and G. A. Colditz, Sugar-added beverages and adolescent weight change, Obesity, 2004, 12, 778–788 CrossRef.
- T. H. Raben, A. C. Vasilaras and A. Moller, Astrup, Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects, Am. J. Clin. Nutr., 2002, 76, 721–729 Search PubMed.
- M. B. Schulze, J. E. Manson, D. S. Ludwig, G. A. Colditz, M. J. Stampfer, W. C. Willett and F. B. Hu, Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women, JAMA, J. Am. Med. Assoc., 2004, 292, 927–934 CrossRef CAS.
- R. D. Mattes, Dietary compensation by humans for supplemental energy provided as ethanol or carbohydrate in fluids, Physiol. Behav., 1996, 59, 179–187 CrossRef CAS.
- D. P. Di Meglio and R. D. Mattes, Liquid versus solid carbohydrate: effects on food intake and body weight, Int. J. Obes., 2000, 24, 794–800 CrossRef CAS.
- H. R. Kissileff, G. Klingsberg and T. Van Itallie, Universal eating monitor for continuous recording of solid or liquid consumption in man, Am. J. Physiol., 1980, 238, 14–22 Search PubMed.
- J. R. Malagelada, V. L. W. Go and W. H. J. Summerskill, Differential gastric, pancreatic, and biliary responses to solid-liquid or homogeneous meals, Dig. Dis. Sci., 1979, 24, 101–110 CrossRef CAS.
- K. A. Houpt, Gastrointestinal factors in hunger and satiety, Neurosci. Biobehav. Rev., 1982, 6, 145–164 CrossRef CAS.
- K. Teff, Cephalic phase insulin release in humans: Mechanism and function, in Appetite and body weight regulation: Sugar, fat, and macronutrient substitutes, ed. J. D. Fernstrom and G. D. Miller, Boca Raton, FL, CRC Press, 1994, pp. 37–50 Search PubMed.
- J. G. Brand, R. H. Cagan and M. Naim, Chemical senses in the release of gastric and pancreatic secretions, Annu. Rev. Nutr., 1982, 2, 249–276 CrossRef CAS.
- T. H. Moran, Fructose and Satiety, J. Nutr., 2009, 139, 1253–1256 CrossRef.
- S. Soenen and M. S. Westerterp-Plantenga, No differences in satiety or energy intake after high-fructose corn syrup, sucrose, or milk preloads, Am. J. Clin. Nutr., 2007, 86, 1586–1594 CAS.
- J. E. Flood-Obbagy and B. J. Rolls, The effect of fruit in different forms on energy intake and satiety at a meal, Appetite, 2009, 52, 416–422 CrossRef.
- R. D. Mattes and W. W. Campbell, Effects of food form and timing of ingestion on appetite and energy intake in lean young adults and in young adults with obesity, J. Am. Diet. Assoc., 2009, 109, 430–437 CrossRef.
- G. B. Haber, K. W. Heaton, D. Murphy and L. F. Burroughs, Depletion and disruption of dietary fibre. Effects on satiety, plasma-glucose, and serum-insulin, Lancet, 1977, 310, 679–682 CrossRef.
- M. Lyly, K. H. Liukkonen, M. Salmenkallio-Marttila, L. Karhunen, K. Poutanen and L. Lähteenmäki, Fibre in beverages can enhance perceived satiety, Eur. J. Nutr., 2009, 48, 251–258 CrossRef CAS.
- M. Lyly, N. Ohls, L. Lähteenmäki, M. Salmenkallio-Marttila, K. H. Liukkonen, L. Karhunen and K. Poutanen, The effect of fibre amount, energy level and viscosity of beverages containing oat fibre supplement on perceived satiety, Food Nutr. Res., 2010, 54 DOI:10.3402/fnr.v54i0.2149.
- P. Monsivais, B. E. Carter, M. Christiansen, M. M. Perrigue and A. Drewnowski, Soluble fiber dextrin enhances the satiating power of beverages, Appetite, 2011, 56, 9–14 CrossRef CAS.
- K. R. Juvonen, A. K. Purhonen, M. Salmenkallio-Marttila, L. Lähteenmäki, D. E. Laaksonen, K. H. Herzig, M. I. Uusitupa, K. S. Poutanen and L. J. Karhunen, Viscosity of oat bran-enriched beverages influences gastrointestinal hormonal responses in healthy humans, J. Nutr., 2009, 139, 461–466 CrossRef CAS.
- J. Stunkard and S. Messick, The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger, J. Psychosom. Res., 1985, 29, 71–83 CrossRef.
- P. Vitaglione, R. Barone Lumaga, A. Stanzione, L. Scalfi and V. Fogliano, β-Glucan-enriched bread reduces energy intake and modifies plasma ghrelin and peptide YY concentrations in the short term, Appetite, 2009, 53, 338–344 CrossRef CAS.
- S. M. Green, H. J. Delargy, D. Joanes and J. E. Blundell, A satiety quotient: a formulation to assess the satiating effect of food, Appetite, 1997, 29, 291–304 CrossRef CAS.
- C. M. Tiwary, J. A. Ward and B. A. Jackson, Effect of pectin on satiety in healthy US Army adults, J. Am. Coll. Nutr., 1997, 16, 423–428 CAS.
- P. Monsivais, M. M. Perrigue and A. Drewnowski, Sugars and satiety: does the type of sweetener make a difference?, Am. J. Clin Nutr., 2007, 86, 116–123 CAS.
- F. R. J. Bornet, A. E. Jardy-Gennetier, N. Jacquet and J. Stowell, Glycaemic response to foods: Impact on satiety and long-term weight regulation, Appetite, 2007, 49, 535–553 CrossRef CAS.
- B. Sloth and A. Astrup, Low glycemic index diets and body weight, Int. J. Obes., 2006, 30, 47–51 CrossRef.
- D. J. Jenkins, T. M. Wolever, R. H. Taylor, H. Barker, H. Fielden, J. M. Baldwin, A. C. Bowling, H. C. Newman, A. L. Jenkins and D. V. Goff, Glycemic index of foods: a physiological basis for carbohydrate exchange, Am. J. Clin. Nutr., 1981, 34, 362–366 CAS.
- D. J. A. Jenkins, T. M. S. Wolever, A. L. Jenkins, M. J. Thorne, R. Lee, J. Kalmusky, R. Reichert and G. S. Wong, The glycaemic index of foods tested in diabetic patients: A new basis for carbohydrate exchange favouring the use of legumes, Diabetologia, 1983, 24, 257–264 CrossRef CAS.
- D. J. A. Jenkins, T. M. S. Wolever, A. L. Jenkins, R. G. Josse and G. S. Wong, The glycaemic response to carbohydrate foods, Lancet, 1984, 324(8399), 388–391 CrossRef.
- L. Jenkins, D. J. Jenkins, U. Zdravkovic, P. Würsch and V. Vuksan, Depression of the glycemic index by high levels of beta-glucan fiber in two functional foods tested in type 2 diabetes, Eur. J. Clin. Nutr., 2002, 56, 622–628 CrossRef.
- P. A. Krezowski, F. Q. Nuttall, M. C. Gannon, C. J. Billington and S. Parker, Insulin and glucose responses to various starch-containing foods in type II diabetic subjects, Diabetes Care, 1987, 10, 205–212 CAS.
- L. Tappy, E. Gügolz and P. Würsch, Effects of breakfast cereals containing various amounts of beta-glucan fibers on plasma glucose and insulin responses in NIDDM subjects, Diabetes Care, 1996, 19, 831–834 CAS.
- T. M. Wolever and D. J. Jenkins, Application of glycaemic index to mixed meals, Lancet, 1985, 326(8461), 944 CrossRef.
- T. M. Wolever, P. M. Nguyen, J. L. Chiasson, J. A. Hunt, R. G. Josse, C. Palmason, N. W. Rodger, S. A. Ross, E. A. Ryan and M. H. Tan, Determinants of diet glycemic index calculated retrospectively from diet records of 342 individuals with non-insulin-dependent diabetes mellitus, Am. J. Clin. Nutr., 1994, 59, 1265–1269 CAS.
- D. E. Cummings, Ghrelin and the short- and long-term regulation of appetite and body weight, Physiol. Behav., 2006, 89, 71–84 CrossRef CAS.
- W. A. Blom, A. Lluch, S. Vinoy, A. Stafleu, R. Van den Berg, J. J. Holst, F. J. Kok and H. F. Hendriks, Effects of gastric emptying on the postprandial ghrelin response, Am. J. Physiol.: Endocrinol. Metab., 2005, 290, 389–395 CrossRef.
- S. Kojima, N. Ueno, A. Asakawa, K. Sagiyama, T. Naruo, S. Mizuno and A. Inui, A role for pancreatic polypeptide in feeding and body weight regulation, Peptides, 2007, 28, 459–463 CrossRef CAS.
- G. Katsuura, A. Asakawa and A. Inui, Roles of pancreatic polypeptide in regulation of food intake, Peptides, 2002, 23, 323–329 CrossRef CAS.
- L. J. Karhunen, K. R. Juvonen, A. Huotari, A. K. Purhonen and K. H. Herzig, Effect of protein, fat, carbohydrate and fibre on gastrointestinal peptide release in humans, Regul. Pept., 2008, 149, 70–78 CrossRef CAS.
- P. T. Schmidt, E. Näslund, P. Grybäck, H. Jacobsson, J. J. Holst, L. Hilsted and P. M. Hellström, A Role for Pancreatic Polypeptide in the Regulation of Gastric Emptying and Short-Term Metabolic Control, J. Clin. Endocrinol. Metab., 2005, 90, 5241–5246 CrossRef CAS.
- K. M. Queenan, M. L. Stewart, K. N. Smith, W. Thomas, R. G. Fulcher and J. L. Slavin, Concentrated oat beta-glucan, a fermentable fiber, lowers serum cholesterol in hypercholesterolemic adults in a randomized controlled trial, Nutr. J., 2007, 6, 6 CrossRef.
- P. D. Cani, E. Lecourt, E. M. Dewulf, F. M. Sohet, B. D. Pachikian, D. Naslain, F. De Backer, A. M. Neyrinck and N. M. Delzenne, Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal, Am. J. Clin. Nutr., 2009, 90, 1236–1243 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
