Temporal trend of mercury in polar bears (Ursus maritimus) from Svalbard using teeth as a biomonitoring tissue
Aurore
Aubail
*ab,
Rune
Dietz
*a,
Frank
Rigét
a,
Christian
Sonne
a,
Øystein
Wiig
c and
Florence
Caurant
b
aNational Environmental Research Institute, Aarhus University, Frederiksborgvej 399, P.O. Box 358, DK-4000, Roskilde, Denmark. E-mail: aaubail@yahoo.fr; rdi@dmu.dk; Fax: +45 46301914; Tel: +45 21254035
bLittoral, Environnement et Sociétés (LIENSs), UMR 6250, CNRS—Université de La Rochelle, 2 rue Olympe de Gouges, F-17042, La Rochelle Cedex, France. Fax: +33 (0)546458264; Tel: +33 (0)546507639
cNatural History Museum, University of Oslo, P.O. Box 1172 Blindern, NO-0318, Oslo, Norway
First published on 24th November 2011
Abstract
We examined the use of mercury (Hg) and nitrogen and carbon stable isotopes in teeth of polar bear (Ursus maritimus) from Svalbard as biotracers of temporal changes in Hg pollution exposure between 1964 and 2003. Teeth were regarded as a good matrix of the Hg exposure, and in total 87 teeth of polar bears were analysed. Dental Hg levels ranged from 0.6 to 72.3 ng g−1 dry weight and increased with age during the first 10 years of life. A decreasing time trend in Hg concentrations was observed over the recent four decades while no temporal changes were found in the stable isotope ratios of nitrogen (δ15N) and carbon (δ13C). This suggests that the decrease of Hg concentrations over time was more likely due to a lower environmental Hg exposure in this region rather than a shift in the feeding habits of Svalbard polar bears.
Environmental impactPolar bears are a key species to monitor temporal trends of mercury (Hg) in the Arctic ecosystem. Teeth incorporate pollutants from diet and thus can be used as environmental archives. In this research, Hg concentrations are determined in teeth of Svalbard polar bears from 1964 to 2003. The temporal trends of nitrogen and carbon stable isotopes are investigated over the same period complementarily to the one of Hg to give an insight on potential changes in feeding habits through decades. A decrease in Hg concentrations was detected but no temporal trend for nitrogen or carbon stable isotopes was, indicating that the decrease in Hg concentrations may be linked to a diminution in environmental Hg itself. |
Introduction
Mercury (Hg) is emitted to the atmosphere from both natural and anthropogenic sources around the world. Long-range atmospheric transport represents the major pathway of Hg to the Arctic environment.1,2 However recent evidence show that ocean currents and rivers also contribute significantly to the Arctic Hg pollution.3Mercury is known to accumulate in organisms and biomagnify up the food chains,4 and has therefore been detected in the body tissues of various Arctic organisms including local Inuit populations.5Bioaccumulation processes in the Arctic fauna are still investigated, and various temporal trends of Hg have been provided within the Arctic Monitoring Assessment Programme.5 Due to its long life span and its top position in the marine Arctic food web, the polar bear (Ursus maritimus) accumulates significant amounts of Hg in its tissues. Mercury in this species has been mostly investigated in soft organs such as liver or kidney,6–11 and also in hair6,12–15 and blood.16 However, to our knowledge there is no information available concerning Hg concentrations in polar bear teeth, though this tissue could provide a good material to assess Hg bioaccumulation in this apex predator. Calcified tissues like teeth are indeed considered to be valuable archives, as they record the individual life history, environment and diet, and therefore could be used in different fields of environmental sciences.
Mammalian teeth comprise three anatomically defined tissues: enamel, dentine and cementum. Dentine and cementum are composed of both organic (collagen) and mineral (calcium phosphate, i.e.apatite) fractions that grow throughout life; in marine mammals, these two tissues deposit yearly through layers or Growth Layer Groups (GLGs). Mercury, once ingested, distributes to all internal organs and tissues via the blood stream,17 including to the dentinal increments from blood vessels.18 Contrary to soft tissues, the tooth is not remodelled or very little throughout life, and incorporated elements like Hg are thought not to be remobilised.19,20 Thus, the dental tissue is a valuable material concerning life exposure investigations, and has therefore been previously investigated for Hg in rats,21 marine mammals22–25 and humans.19,20
Since diet is the main pathway for Hg to enter marine mammals, in addition to the general anthropogenic trends a change in feeding habits would also likely result in variations in Hg exposure, and consequently, in the body concentrations of this metal. Moreover, due to the magnification of this element, a change in Hg levels of an organism may only be seen if that organism feeds on different trophic levels than it used to. The naturally occurring carbon (12C and 13C) and nitrogen (14N and 15N) stable isotopes ratios have been proved to be useful tools for characterizing the primary production in marine and terrestrial ecosystems and delineating the trophic position of organisms in food webs, respectively.26 Like trace elements, carbon and nitrogen are incorporated into the dental tissue during its growth. Combined with the analysis of Hg, the measurement of the carbon and nitrogen stable isotopes in the teeth will provide valuable information relative to potential changes in feeding habits or habitats of polar bears. The stable isotopic technique is based on the metabolic discrimination between the heavy and the light isotopes. While 13C/12C values exhibit few variations through successive trophic levels in the food chains,27 the 15N/14N value is significantly and regularly enriched through the food chains with a value found in a consumer's tissue directly related to the one of its prey, providing thus information on one's trophic level.28 Usually, the isotopic measurements are carried out on muscle tissues which give access to the feeding ecology from the last weeks or months, whereas performed on teeth, the isotopic data obtained will represent an average dietary estimate over the animal's lifetime or near to its lifetime,29 erasing any potential shift of diet related to seasonal and physiological stages.
Polar bears are known to prey mainly on ringed seals (Pusa hispida) and to a lesser extent on bearded seals (Erignathus barbatus) across the Arctic.30 However, there is some evidence that the ringed seal dominance in polar bear diet differs for example spatially and temporally in the Arctic.31,32 Polar bears feed also on other species such as harp seals (Phoca groenlandica),33 walrus (Odobenus rosmarus),34 beluga whales (Delphinapterus leucas) and narwhal (Monodon monoceros);35 and diet items like seabirds or even reindeers (Rangifer tarandus) have been shown to be consumed by the polar bears in Svalbard.36 Sea ice is used as a platform by polar bears for seal hunt,30 so that the accessibility of their main prey varies throughout the year due to sea ice extension changes. This close relation to the sea ice makes polar bears vulnerable to a warming climate and a relevant indicator of climate change effects on the ecosystem.37 Global warming has resulted in significant declines in total cover and thickness of sea ice over the last few decades in the Arctic.38 Because of this progressive earlier break-up and later freezing of the Arctic sea ice in some areas, polar bear's access to seals is thus likely to be reduced resulting in longer periods of fasting and searching for alternative food sources. Stable isotopic measurements were therefore used complementarily with the elemental analysis in this study on polar bears to relate temporal variations of tooth Hg to potential changes in feeding habits for this species.
This article presents the Hg concentrations and 15N/14N and 13C/12C values in teeth of polar bears from Svalbard over the recent four decades. The objectives of this study are to assess the influence of the biological factors (sex and age) on Hg concentrations in teeth and to investigate the temporal pattern of Hg concentrations in Svalbard polar bears between the 1960s and the 2000s in order to evaluate whether the tooth is a good biomonitoring tissue.
Materials and methods
Sampling procedure and preparation
Tooth samples were obtained from 87 polar bear skulls archived at the Natural History Museum, University of Oslo (NHM), Norway. The skulls had been collected in the archipelago of Svalbard (Fig. 1), from 1964 to 2003. An overview of the samples used in statistical analysis is given in Table 1. | ||
| Fig. 1 Map of Svalbard (Norway), where polar bear samples have been collected. | ||
| Period | Male | Female | Unknown sex | All | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean age ± sd | n | Mean age ± sd | n | Mean age ± sd | n | Mean age ± sd | |
| 1964–1966 | 32 | 11.0 ± 7.2 | 22 | 9.9 ± 5.0 | 4 | 12.5 ± 15.2 | 58 | 10.7 ± 7.1 |
| 3–10 years old | 19 | 6.6 ± 2.0 | 13 | 6.2 ± 2.6 | 5 | 6.6 ± 1.9 | 37 | 6.5 ± 2.2 |
| All | 42 | 9.5 ± 7.3 | 32 | 8.4 ± 5.3 | 13 | 10.1 ± 10.0 | 87 | 9.2 ± 7.1 |
The first premolar of the lower mandible had been used for age estimation by counting annual layers in the cementum after decalcification, thin sectioning (14 μm), and staining in toluidine blue, as described by Dietz et al.39 The third incisor from the lower right mandible was taken for the Hg and carbon and nitrogen stable isotopes analytical purpose. The extracted tooth was cut into three pieces using a Proxxon diamond saw. The upper third incisor was used for the study of Hg content, while the middle part of the tooth was used for the determination of the stable isotopes, and the lower third incisor was kept for further potential investigations (e.g. additional age determination). It is assumed that in contrast to the root, the upper and middle parts of incisors have the same deposit of layers and have been similarly exposed to Hg.
Prior to analysis, upper and middle parts of the teeth were cleaned of external materials by abrasion, immersed subsequently in 10% nitric acid for 20 s, and rinsed in several ultra-pure Milli-Q water baths. Tooth samples were dried for a minimum of 24 h at room temperature, and subsequently stored in cleaned plastic flasks until analysis.
Analytical procedures and instrumentation for Hg analysis
The Hg measurements were performed at the laboratory of the National Environmental Research Institute in Roskilde (NERI), Denmark, using a solid sample atomic absorption spectrometer AMA-254 (Advanced Mercury Analyser-254 from LECO, Sweden). The use of this instrument does not require a chemical pre-treatment, which reduces considerably contamination risks and the loss of Hg. The analytical process consists of a drying period at 120 °C for 50 seconds, prior to a combustion phase at 750 °C for 250 seconds, which leads to the desorption of Hg from the samples. Subsequently, the Hg vapour produced is carried by an oxygen flow to a gold amalgamator, and trapped on its surface. Thereafter, the collected Hg is released from the amalgamator by a short heat-up to 900 °C, and carried in a pulse through a spectrophotometer, where it is measured by UV absorption. The instrument is described in detail by Hall and Pelchat.40As there is no commercial reference material with a tooth or bone matrix and certified for Hg, a reference material was made from two commercial Standard Reference Materials (SRMs). These were the NIST 1400 Bone ash (National Institute of Standards and Technology, USA) and the DOLT-3 (Dogfish Liver Tissue from the National Research Council of Canada). The Bone ash SRM does not contain any Hg because it has been calcined at high temperatures, but it represents a calcified tissue matrix, while the DOLT-3 contributes to the organic matrix and the certified level of Hg.
In order to validate the use of this customised reference material, an intercalibration between two different techniques (AMA-254 and cold-vapour Atomic Absorption Spectrophotometry on a Perkin Elmer FIMS 100) was carried out at NERI, and moreover, an inter-laboratory comparison was carried out between NERI and CCA (Centre Commun d'Analyses, University of La Rochelle, France) for the AMA-254 analysis. The results showed a good accuracy (i.e. the recovery measured value/theoretical value) of 102.3% and a relative standard deviation of 6.3% (Table 2).
| Hg concentration | ||||
|---|---|---|---|---|
| n | Mean | sd | Relative sd% | |
| a The measured concentration is the average of the Hg concentrations determined by the three sequences of analyses. b The theoretical concentration is based on the DOLT-3 certified concentration of Hg. | ||||
| AMA CCA | 8 | 96.3 | 3.5 | 3.7 |
| AMA NERI | 10 | 105 | 6 | 5.7 |
| FIMS NERI | 5 | 106.3 | 3.6 | 3.4 |
| Measured concentrationa | 23 | 102.3 | 6.4 | 6.3 |
| Theoretical concentrationb | 1 | 100 | 4.1 | 4.1 |
The analytical quality of the Hg measurements by the AMA-254 was ensured by including the customised reference material at the beginning and at the end of the analytical cycle, and by running it for every 10 samples. Results of these measurements (n = 13) showed a good precision with a relative standard deviation of 1.6%, and an accuracy of 106.6% of the assigned concentration.
NERI participates in the international inter-laboratory comparison exercises conducted by the EEC (QUASIMEME), and the latest 2007 analyses by AMA-254 showed satisfactory results (0 < z < 0.5). All data are presented on a dry weight basis (dw) and the detection limit is 0.01 ng.
Analytical procedures and instrumentation for stable isotope measurements
Prior to analysis, tooth samples were crushed into small pieces before being ground into homogeneous powder using a ball mill (Retsch MM2000) for 2 min at the amplitude of 90. Powder was then stored in small glass flasks. The carbonates of the teeth were removed by digesting the teeth with approximately 1 mL of a 4 M hydrochloric acid solution at 45 °C for 48 h. Subsequently, the digested contents were taken up in milli-Q ultrapure quality water, and homogenised before freezing to −20 °C. Thus, the samples were frozen at −80 °C for a short time before freeze drying. Finally, an aliquot of approximately 1.45 mg was taken of each obtained homogenised dried sample, weighed and loaded into tin capsules.Relative abundance of stable isotopes of nitrogen (15N/14N) and carbon (13C/12C) was determined with an Elemental Analyser connected on-line to an Isotope-Ratio Mass Spectrometer (Isoprime, Micromass, UK). Stable isotope results are expressed in delta notation (δ), defined as the part per thousand (‰) deviation from a standard material:
| δ15N or δ13C = [(Rsample/Rstandard) − 1] × 103 |
Data treatment
Prior to the statistical analyses, the Hg data were log-transformed (base e) to reduce skewness and fit parametric requirements of normal distribution and homogeneity of variances. The Shapiro–Wilk test of normality and Bartlett test of homogeneity of variances were applied to test the assumptions of analysis of variance (ANOVA) and linear regression analysis. The assumptions were not fulfilled in few cases due to some high Hg values but ANOVA tests are robust to small deviations of the data from the normal distribution,41 so that the analyses were conducted anyway.Analysis of variance and multiple linear regression analyses were performed to test the influence of sex, age, and year on log-transformed Hg concentrations and stable isotopic values. Only individuals (>4 years old) were used to test the factor sex (n = 49), while only individuals collected from 1964 to 1966 (n = 57) were selected when testing the age effect. The non-parametric test Spearman's rank correlation was used to investigate relationships between log-transformed Hg concentrations, δ13C and δ15N values.
The significance level was set to p = 0.05 and the statistical analyses were performed using the free software R, version 2.1.1.42
Results
Mercury concentrations in relation to age and sex
Dental Hg levels were generally low, exhibiting a mean value ± standard deviation (sd) of 6 ± 8.3 ng g−1 dw (Table 3). The highest concentration was found in a female cub (0 year old) from 1964. It was considered as an outlier due to its very high Hg concentration (72.3 ng g−1 dw), and was consequently excluded from graphs and statistical calculations.| Measurements | Range | Median | Mean ± sd |
|---|---|---|---|
| Hg | 0.6–72.3 | 4.9 | 6.0 ± 8.3 |
| δ 15N | 17.7–21.8 | 19.6 | 19.6 ± 0.7 |
| δ 13C | −17.4 to −14.8 | −15.8 | −15.8 ± 0.4 |
Log-transformed Hg concentrations in polar bear teeth were not influenced by gender (one-way ANOVA, F = 3.00, p = 0.09), so data were pooled across sexes in further data analyses. In contrast, log-transformed Hg concentrations increased significantly with age (Spearman's correlation, ρ = 0.50, p < 0.001). A general increase of Hg concentrations in the teeth of polar bears was observed for the first 10 years of life and followed by a plateau phase (Fig. 2a).
 | ||
| Fig. 2 Age (years) vs.Hg concentrations (in ng g−1 dw) (a), vs. δ15N values (in ‰) (b) and vs. δ13C values (in ‰) (c) in teeth of polar bears from Svalbard, 1964–1966. The smoothing line (robust, locally weighted scatter plot smoothing system based on the LOWESS algorithm) represents the fitted non-linear trend of the values. Note that the y axis is a logarithmic scale in (a). | ||
Stable isotopic values in relation to sex, age and Hg concentrations
The δ15N and δ13C values ranged from 17.7‰ to 21.8‰ and −17.4‰ to −14.8‰, respectively (Table 3). Neither δ15N nor δ13C values in polar bear teeth were influenced by gender (F = 0.01, p = 0.92; F = 0.17, p = 0.68), or correlated with age (ρ = −0.02, p = 0.90, Fig. 2b, and ρ = −0.11, p = 0.42, Fig. 2c, respectively); and no relationship was found between log-transformed Hg concentrations and δ15N or δ13C values (ρ = 0.11, p = 0.29 and ρ = 0.01, p = 0.91, respectively) (Table 4).| Variable | Age | log Hg |
|---|---|---|
| a Spearman's correlations were tested on all individuals excluding the outlier of 72.3 ng g−1 dw (n = 86). Moreover, when the correlation involved the age factor, only individuals from the 1964–1966 period were selected (n = 57). | ||
| log Hg | 0.50 (p < 0.001) | — |
| δ 15N | −0.02 (p = 0.90) | 0.11 (p = 0.29) |
| δ 13C | −0.11 (p = 0.42) | 0.01 (p = 0.91) |
Time trends
Sub-adult and adult individuals with age between 3 and 10 years (n = 37) were selected for the time trend analyses in order to limit the overlap of the periods of life of the animals and in the same time to cover the entire 40 year period. In addition, since polar bear cubs are nursed until their third year of life,43 selecting individuals older than 2 years excludes the animals which have been feeding exclusively on maternal milk. The yearly average age of the sampled individuals (3–10 years) did not follow any time trend (linear regression, F = 0.54, p = 0.47), hence no age normalisation was conducted. However, a significant decreasing trend in Hg concentrations of 2.1% per year was found over the 4 decade period between 1964 and 2003 (F = 9.66, p = 0.004, Fig. 3a), while no significant temporal trend was found for δ15N or δ13C values for the same period (F < 0.01, p = 0.94, Fig. 3b, and F = 0.42, p = 0.52, Fig. 3c, respectively).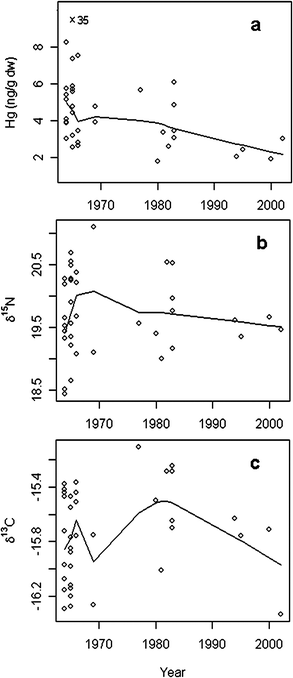 | ||
| Fig. 3 Year vs. dental Hg concentrations (ng g−1 dw) (a), vs. δ15N values (b) and vs. δ13C values (c) in polar bears from Svalbard, aged from 3 to 10 years. Smoothing lines (robust, locally weighted scatter plot smoothing system based on the LOWESS algorithm) represent the fitted non-linear trend of the values. | ||
Discussion
Levels of Hg in the teeth
Diet is the main pathway for Hg to enter into the body of marine mammals.44 Thus, the feeding habits mainly determine the Hg primary load of a species. However, another important factor is the excretion route where hair has been shown to play an important role for pinnipeds or polar bears similar to the one of feathers for birds.45–47 Polar bears, as top predators of the Arctic marine food web, have been shown to accumulate high Hg concentrations (several ppm) in soft tissues7–10 and fur6,12,13 while Hg concentrations in teeth in our study are about 1000 times lower. These levels are consistent with the levels reported in teeth of other Arctic marine mammals. Thus, Outridge et al.23 reported dental Hg concentrations under 2 ng g−1 dw for modern samples of walrus. A geometric mean (±2 standard errors) of 4.4 (±1.7) to 8.6 (±3.7) ng g−1 was found in ringed seals 5–25 years old from Amundsen Gulf in Canada.24 Aubail et al.25 reported dental Hg concentrations (±sd) of 2.94 (±1.99) ng g−1 in ringed seals from West Greenland and of 5.75 (±6.20) ng g−1 in ringed seals from East Greenland. However, dental Hg concentrations measured in polar bears are lower than those found in beluga whales by Outridge et al.,23 who reported a mean Hg concentration (±sd) of 98.4 (±109) ng g−1 dw for animals 6–26 years old and Hg concentrations ranging from 6.4 (±13.3) to 292.3 (±36) ng g−1 in individuals 10–60 years old from the Beaufort Sea.24 A geographical difference in general environmental Hg exposure could likely contribute to this difference, since polar bears from Svalbard and their primary food the ringed seal have been shown to generally exhibit lower Hg concentrations than Canadian or Greenlandic individuals.6,9,48–50 Moreover, it has been shown that during periods of reliable food access, polar bears mainly consume seal blubber,51 which is not exhibiting high Hg contents52 and could therefore result in lower dental Hg levels in polar bears compared to beluga whales. However, again the excretion also plays a major role as toothed whales do not have the hair excretion route like the polar bears which results in relatively higher concentrations in e.g. meat and brain.47Biological factors
Although diet may vary substantially between genders, no difference in Hg concentrations was found between males and females, which fits the lack of difference between males and females previously reported in soft tissues of polar bears.7–9 In contrast, dental Hg concentrations were correlated with age (Table 4). Polar bears generally exhibit a cumulative pattern of this metal especially in their liver.7–9,11 However, age effects on dental Hg concentrations in Arctic marine mammals are not consistent in the literature. Outridge et al.22 showed that Hg concentrations in teeth of beluga whales increased with age, while Kinghorn et al.53 did not find a significant age effect in the same species. Outridge et al.24 observed a positive correlation between age and Hg concentrations in teeth of ringed seals, while Aubail et al.25 did find a significant decrease of the dental Hg concentrations during the first years of life of Greenland ringed seals; this trend was explained by early maternal transfer of Hg to pre- and postnatal individuals. Indeed, since ringed seals acquire their permanent dentition at the foetal stage,54 the prenatal transfer of Hg may represent an important source of Hg for the dental tissues mineralised during the foetal stage, compared to the postnatal Hg incorporated from diet. In this study, a general increase of Hg concentrations in the teeth of polar bears was observed for the first 10 years of life and was followed by a more constant plateau phase (Fig. 2a). Thus, this trend could be explained by the fact that polar bears, in contrast to ringed seals, would acquire their permanent dentition at the postnatal stage. This results in the progressive accumulation of Hg throughout years with a low intake of Hg from the maternal milk, to greater Hg concentrations with an increasing efficiency of the hunting strategy and thus, the amount of prey and Hg ingested. The stabilized levels of Hg observed afterwards may likely be related to the late dentinal occlusion of the tooth. Indeed, the deposition of dentine reduces the pulp area and in some species, the pulp cavity has been shown to occlude at some point of their life with the subsequent suspension of the deposition of dentine55 and likely also elements such as Hg from blood vessels.The highest dental Hg content in our study (72.3 ng g−1 dw) was displayed by one of the five cubs of the year, whereas the four others exhibited low dental Hg concentrations (from 0.9 to 3.4 ng g−1 dw). Kenny and Bickel56 reported that a 6 month old polar bear cub still had some deciduous incisors at its age, thus, the hypothesis that the deciduous incisor may actually have been sampled and analysed instead of the permanent one for that yearling cannot be eliminated. The high Hg value displayed by that particular individual could likely correspond to the Hg maternal input during the foetal stage, an input which has been shown to be of important contribution for the dental tissues of ringed seals.25
Since integration time and process of both isotopic and metallic elements are considered to be relatively similar, δ15N or δ13C values have been used to relate Hg concentrations to the trophic position or foraging location, respectively. However, Hg concentrations were not correlated with δ15N or δ13C values in this study and no age effect was found for δ15N values, which is different from other previous investigations in which high δ15N values were measured in muscle of different marine mammals in the first years of life, relative to dietary inputs from mother's milk during the period of suckling.57–59 In those studies, the decrease in nitrogen isotope values reflects ontogenetic changes and is likely to be associated with the period from primarily feeding on milk towards primarily feeding on live prey. Likewise, no age effects were found for δ13C values in our study, whereas cubs of the year were expected to present low δ13C values, in response to depleted 13C directly derived from maternal milk lipids.
Time trend
In contrast to soft tissues, which are generally degraded over time, dental tissues are well preserved from degradation. In addition, mammalian teeth are stored and preserved within jaws in museum collections for decades, and their easy access allows thus to provide long-term time series of data. As biological archives, teeth are thought to reflect the Hg exposure through diet at the time of formation since dental Hg matches blood Hg at the time of formation and mineralisation of the tooth. Therefore, Hg concentrations in teeth reflect an average exposure of the period of life during which the dental tissues have been mineralised.29 Thus, it is worth noticing that the sampling year (in this case equalling death of the animal) does not represent the year of Hg exposure or accumulation for this animal, but terminates a lifetime period of exposure.A decrease in Hg levels over time was found in the teeth of polar bears from Svalbard. The absence of temporal trends in δ15N and δ13C values over the four studied decades did not support the hypothesis of a temporal variation in feeding or foraging habits, but did mostly point towards a reduction in environmental Hg exposure. A temporal decrease in Hg concentrations has been observed previously in polar bear hair from East Greenland between 1973 and 2001. This was explained by reduced general environmental Hg levels attributed to a reduction in Hg emissions from European and North American sources.12 In addition, our results are also in agreement with previous investigations on the Hg content in human deciduous teeth from Norway, which described a decreasing time trend of the dental Hg levels from the 1970s to the 1990s likely reflecting a decrease in environmental Hg burden in Norway.60
The atmospheric transport is the main pathway for Hg to reach the Arctic region,5 and the Svalbard area seems to be under the influence of wind flows from Eastern North America, Europe and Russia.61Mercury emissions have been reported as decreasing substantially from the North American, European and Russian sources in the 1990s, due to a general diminution of industrial activities and consumption of raw materials.62–64 Although the processes between Hg emissions from a source and its accumulation by organisms are long and complex, changes in dental Hg concentrations over time in Svalbard polar bears are likely to be explained by a decrease in emissions of this metal from remote sources and subsequent transport and delivery by the winds to the Svalbard ecosystem.
Conclusion
Teeth can provide long term elemental and isotopic composition and important knowledge on lifetime dietary and contaminant exposure of the studied animals. However, biological factors like age are important to investigate, when using dental tissues to assess temporal trends of Hg. Indeed, age seems to have a great influence on Hg concentrations and in addition, it seems necessary to determine if the studied species develops its permanent dentition at the foetal or postnatal stage for a better understanding of the relation age–Hg concentrations. In our study, the combined use of the elemental and isotopic measurements allowed elimination of feeding behavior as a factor of influence on Hg temporal patterns. Thus, the temporal trend observed in the teeth of polar bears from Svalbard over the recent four decades seemed to reflect the decrease in the deposition and subsequent bioaccumulation of Hg in the Svalbard ecosystem, following the decrease in Hg emissions to the atmosphere from Europe and North America during the late 20th century.Acknowledgements
We greatly acknowledge Carine Churlaud from the Centre Commun d'Analyses (La Rochelle, France), Gert Asmund, Sigga Joensen and Lene Bruun from the NERI for their valuable input on the Hg analysis, and Pierre Richard, Benoît Lebreton and Gaël Guillou from LIENSs (UMR 6250 CNRS-La Rochelle University, France) for their advice on and contribution to the stable isotope analysis. We also would like to thank Thea Bechshøft from NERI for her help regarding the samples. We are grateful to Peter Outridge, Canadian Geological Survey and Michael Goodsite, National Environmental Research Institute (NERI, Roskilde, Denmark) for advice and discussion underway. This study was partly financed by The Dancea Programme, KVUG (Kommissionen for Videnskabelige Undersøgelser i Grønland) and the Prince Albert II Foundation. Finally, the Poitou-Charentes region and Fund M. L. Furnestin-Faure did support financially the PhD research.References
- S. E. Lindberg, S. Brooks, C. J. Lin, K. Scott, T. Meyers, L. Chambers, M. R. Landis and R. Stevens, Water, Air, Soil Pollut., 2001, 295–302 Search PubMed.
- P. A. Ariya, A. P. Dastoor, M. Amyot, W. H. Schroeder, L. Barrie, K. Anlauf, F. Raofie, A. Ryzhkov, D. Davignon, J. Lalonde and A. Steffen, Tellus, Ser. B, 2004, 56, 397–403 CrossRef.
- G. Stern, L. Loseto, R. W. Macdonald, F. Wang, C. Zdanowicz, P. M. Outridge, A. Cole, J. Chételat, H. Hintelmann and A. Steffen, in AMAP Assessment 2011: Mercury in the Arctic, Arctic Monitoring and Assessment Programme (AMAP), Oslo, Norway, 2011, ch. 4, pp. 67–83. Search PubMed.
- L. Atwell, K. A. Hobson and H. E. Welch, Can. J. Fish. Aquat. Sci., 1998, 55, 1114–1121 CrossRef CAS.
- AMAP, in AMAP Assessment 2002: Heavy Metals in the Arctic, Arctic Monitoring and Assessment Programme (AMAP), Oslo, Norway, 2005 Search PubMed.
- E. W. Born, A. Renzoni and R. Dietz, Polar Res., 1991, 9, 113–130 Search PubMed.
- B. M. Braune, R. J. Norstrom, M. P. Wong, B. T. Collins and J. Lee, Sci. Total Environ., 1991, 100, 283–299 CrossRef CAS.
- R. Dietz, E. W. Born, C. T. Agger and C. O. Nielsen, Polar Biol., 1995, 15, 175–185 Search PubMed.
- R. Dietz, F. Riget and E. W. Born, Sci. Total Environ., 2000, 245, 25–47 CrossRef CAS.
- G. Norheim, J. U. Skaare and O. Wiig, Environ. Pollut., 1992, 77, 51–57 Search PubMed.
- S. A. Rush, K. Borga, R. Dietz, E. W. Born, C. Sonne, T. Evans, D. C. G. Muir, R. J. Letcher, R. J. Norstrom and A. T. Fisk, Environ. Pollut., 2008, 153, 618–626 CrossRef CAS.
- R. Dietz, F. Riget, E. W. Born, C. Sonne, P. Grandjean, M. Kirkegaard, M. T. Olsen, G. Asmund, A. Renzoni, H. Baagoe and C. Andreasen, Environ. Sci. Technol., 2006, 40, 1120–1125 CrossRef CAS.
- R. Dietz, E. W. Born, F. Rigét, A. Aubail, C. Sonne, R. Drimmie and N. Basu, Environ. Sci. Technol., 2011, 45, 1458–1465 CrossRef CAS.
- R. D. P. Eaton and J. P. Farant, Arctic, 1982, 35, 422–425 Search PubMed.
- T. W. Horton, J. D. Blum, Z. Xie, M. Hren and C. P. Chamberlain, Polar Res., 2009, 28, 443–454 CrossRef.
- T. Cardona-Marek, K. K. Knott, B. E. Meyer and T. M. O'Hara, Environ. Toxicol. Chem., 2009, 28, 1416–1424 CrossRef CAS.
- WHO, IPCS/International Programme on Chemical Safety, WHO (World Health Organization), Environmental Health Criteria 101, Geneva, 1990 Search PubMed.
- N. Miyazaki, in Encyclopedia of Marine Mammals, ed. W. F. Perrin, B. Würsig and J. G. M. Thewissen, Academic Press, 2002, pp. 1227–1232 Search PubMed.
- R. Eide, G. B. Wesenberg and G. Fosse, Scand. J. Dent. Res., 1993, 101, 1–4 Search PubMed.
- H. M. Tvinnereim, R. Eide and T. Riise, Sci. Total Environ., 2000, 255, 21–27 CrossRef CAS.
- R. Eide and G. R. Wesenberg, Environ. Res., 1993, 61, 212–222 CrossRef CAS.
- P. M. Outridge, R. Wagemann and R. McNeely, Environ. Toxicol. Chem., 2000, 19, 1517–1522 CrossRef CAS.
- P. M. Outridge, K. A. Hobson, R. McNeely and A. Dyke, Arctic, 2002, 55, 123–132 Search PubMed.
- P. M. Outridge, K. A. Hobson and J. Savelle, Sci. Total Environ., 2009, 407, 6044–6051 CrossRef CAS.
- A. Aubail, R. Dietz, F. Rigét, B. Simon-Bouhet and F. Caurant, Sci. Total Environ., 2010, 408, 5137–5146 CrossRef CAS.
- B. J. Peterson and B. Fry, Annu. Rev. Ecol. Syst., 1987, 18, 293–320 Search PubMed.
- B. S. Chisholm, D. E. Nelson and H. P. Schwarcz, Science, 1982, 216, 1131–1132 CAS.
- M. Minagawa and E. Wada, Geochim. Cosmochim. Acta, 1984, 48, 1135–1140 Search PubMed.
- P. M. Outridge, in Mercury: Sources, Measurements, Cycles and Effects, Mineralogical Association of Canada, Ottawa, 2005, ch. 11, pp. 217–234 Search PubMed.
- I. Stirling, in Encyclopedia of Marine Mammals, ed. W. F. Perrin, B. Würsig and J. G. M. Thewissen, Academic Press, 2002, pp. 945–948 Search PubMed.
- S. J. Iverson, S. Stirling and S. L. Lang, in Top Predators in Marine Ecosystems, ed. I. L. Boyd, S. Wanless and C. J. Camphuysen, Cambridge University Press, 2006, pp. 98–117 Search PubMed.
- M. A. McKinney, R. J. Letcher, J. Aars, E. W. Born, M. Branigan, R. Dietz, T. J. Evans, G. W. Gabrielsen, D. C. G. Muir, E. Peacock and C. Sonne, Environ. Sci. Technol., 2011, 45(3), 896–902 CrossRef CAS.
- A. E. Derocher, O. Wiig and M. Andersen, Polar Biol., 2002, 25, 448–452 Search PubMed.
- W. Calvert and I. Stirling, Bears: Biol. Manage., 1990, 8, 351–356 Search PubMed.
- L. F. Lowry, J. J. Burns and R. R. Nelson, Can. Field Nat., 1987, 101, 141–146 Search PubMed.
- A. E. Derocher, O. Wiig and G. Bangjord, Polar Biol., 2000, 23, 675–678 Search PubMed.
- K. L. Laidre, I. Stirling, L. F. Lowry, O. Wiig, M. P. Heide-Jorgensen and S. H. Ferguson, Ecol. Appl., 2008, 18, 97–125 Search PubMed.
- K. M. Kovacs, C. Lydersen, J. E. Overland and S. E. Moore, Mar. Biodiversity, 2011, 41(1), 181–194 Search PubMed.
- R. Dietz, M. P. Heide-Jørgensen, T. Harkonen, J. Teilmann and N. Valentin, Sarsia, 1991, 76, 17–21 Search PubMed.
- G. E. M. Hall and P. Pelchat, Analyst, 1997, 122(9), 921–924 RSC.
- J. H. Zar, in Biostatistical Analysis: International Edition, Pearson Education, 2009 Search PubMed.
- R Development Core Team, in R: a Language and Environment for Statistical Computing, Foundation for Statistical Computing, Vienna, Austria, 2008, ISBN 3-900051-07-0, http://www.r-project.org/ Search PubMed.
- M. A. Ramsay and I. Stirling, J. Zool., 1988, 214, 601–634 Search PubMed.
- A. Aguilar, A. Borrell and T. Pastor, J. Cetacean Res. Manage., 1999, 1, 83–116 Search PubMed , special issue.
- T. J. Brookens, J. T. Harvey and T. M. O'Hara, Sci. Total Environ., 2007, 372, 676–692 Search PubMed.
- T. J. Brookens, T. M. O'Hara, R. J. Taylor, G. R. Bratton and J. T. Harvey, Mar. Pollut. Bull., 2008, 56, 27–41 Search PubMed.
- R. Dietz, N. Basu, B. Braune, T. O'Hara, T. Scheuhammer and C. Sonne, in AMAP Assessment 2011: Mercury in the Arctic, Arctic Monitoring and Assessment Programme (AMAP), Oslo, Norway, 2011, ch. 6, pp. 113–137 Search PubMed.
- R. Dietz, P. Paludan-Müller, C. T. Agger and C. O. Nielsen, NAMMCO Scientific contributions, 1998, 1, 242–273 Search PubMed.
- R. J. Norstrom, R. E. Sweinsberg and B. T. Collins, Sci. Total Environ., 1986, 48, 195–212 CrossRef CAS.
- F. Riget, D. Muir, M. Kwan, T. Savinova, M. Nyman, V. Woshner and T. O'Hara, Sci. Total Environ., 2005, 351, 312–322 Search PubMed.
- I. Stirling and E. H. McEwan, Can. J. Zool., 1975, 53, 1021–1027 CAS.
- P. Johansen, D. Muir, G. Asmund and F. Riget, Sci. Total Environ., 2004, 331, 189–206 CrossRef CAS.
- A. Kinghorn, M. M. Humphries, P. Outridge and H. M. Chan, Sci. Total Environ., 2008, 402, 43–50 Search PubMed.
- B. E. Stewart, S. Innes and R. E. A. Stewart, Mar. Mamm. Sci., 1998, 14, 221–231 Search PubMed.
- A. A. Hohn, in Encyclopedia of Marine Mammals, ed. W. F. Perrin, B. Würsig and J. G. M. Thewissen, Academic Press, 2002, pp. 6–13 Search PubMed.
- D. E. Kenny and C. Bickel, Int. Zoo Yearb., 2005, 39, 205–214 Search PubMed.
- R. Dietz, F. Riget, K. A. Hobson, M. P. Heide-Jorgensen, P. Moller, M. Cleeman, J. de Boer and M. Glasius, Sci. Total Environ., 2004, 331, 83–105 CrossRef CAS.
- K. A. Hobson and J. L. Sease, Mar. Mamm. Sci., 1998, 14, 116–129 Search PubMed.
- S. C. Polischuk, K. A. Hobson and M. A. Ramsay, Can. J. Zool., 2001, 79, 499–511 Search PubMed.
- H. M. Tvinnereim, R. Eide and G. R. Fosse, International Conference on Human Health Effects of Mercury Exposure, Faroe Islands, 1997 Search PubMed.
- R. W. Macdonald, T. Harner, J. Fyfe, H. Loeng and T. Weingartner, in AMAP, Assessment 2002: the Influence of Global Change on Contaminant Pathways to, Within, and from the Arctic, Arctic Monitoring and Assessment Programme (AMAP), Oslo, Norway, 2003 Search PubMed.
- AMAP/UNEP, in Technical Background Report to the Global Atmospheric Mercury Assessment, Arctic Monitoring and Assessment Programme/UNEP Chemicals Branch, 2008, http://www.chem.unep.ch/mercury/Atmospheric_Emissions/Technical_background_report.pdf Search PubMed.
- J. Munthe, M. Goodsite, T. Berg, J. Chetelat, A. Cole, A. Dastoor, T. Douglas, R. W. Macdonald, D. Muir, P. Outridge, J. Pacyna, H. Skov, A. Steffen, K. Sundseth, O. Travnikov, I. Wangberg and S. Wilson, in AMAP Assessment 2011: Mercury in the Arctic, Arctic Monitoring and Assessment Programme (AMAP), Oslo, Norway, 2011, ch. 2, pp. 9–44 Search PubMed.
- E. G. Pacyna and J. M. Pacyna, Water, Air, Soil Pollut., 2002, 137, 149–165 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
