Ultracentrifugation as a direct method to concentrate viruses in environmental waters: virus-like particle enumeration as a new approach to determine the efficiency of recovery
Catarina
Prata
,
Andreia
Ribeiro
,
Ângela
Cunha
,
Newton. C. M.
Gomes
and
Adelaide
Almeida
*
Department of Biology and CESAM, University of Aveiro, Campus de Santiago, 3810-193, Aveiro, Portugal. E-mail: aalmeida@ua.pt
First published on 24th November 2011
Abstract
Some health important enteric viruses are considered to be emerging waterborne pathogens and so the improvement of detection of these viruses in the aquatic environment is one of the most important steps in dealing with these pathogens. Since these viruses may be present in low numbers in water, it is necessary to concentrate water samples before viral detection. Although there are several methods to concentrate viruses in environmental waters, all present some drawbacks and consequently the method should be chosen that, despite its limitations, is adequate to achieve the aim of each study. As the effectiveness of the concentration methods is evaluated by determining the efficiency of viral recovery after concentration, it is important to use a simple and effective approach to evaluate their recovery efficiency. In this work ultracentrifugation, usually used as a secondary step for viruse concentration, was evaluated as the main method to concentrate directly viruses in environmental water samples, using the microscopic enumeration of virus-like particles (VLP) as a new approach to estimate the efficiency of recovery. As the flocculation method is currently employed to concentrate viruses in environmental waters, it was also used in this study to assess the efficiency of the ultracentrifugation as the main viral concentration method in environmental waters. The results of this study indicate that ultracentrifugation is an adequate approach to concentrate viruses directly from environmental waters (recovery percentages between 66 and 72% in wastewaters and between 66 and 76% in recreational waters) and that the determination of VLP by epifluorescence microscopy is a simple, fast and cheap alternative approach to determine the recovery efficiency of the viral concentration methods.
Environmental impactAlthough viruses are the most abundant biological component in aquatic systems, the number of health significant viruses in water is low, but even at low concentrations these pathogenic viruses can cause disease. Consequently large volumes of water are required to detect enteric viruses, which implies sample concentration to a few millilitres before analysis. Consequently it is important to have a practical viral concentration method, such as ultracentrifugation, in order to monitor pathogenic viruses. As the effectiveness of the concentration methods is evaluated by determining the efficiency of viral recovery after concentration, it is also important to use a simple, effective, fast and inexpensive approach to evaluate their recovery efficiency, such as the enumeration of virus-like particles (VLP), by epifluorescence. |
Introduction
Health relevant enteric virus groups are nowadays considered to be emerging waterborne pathogens,1,39,47 increasing the concern over the discharge of human enteric viruses not only into fresh water but also into estuarine and marine environments. In these environments they represent a health hazard in areas that are used for recreational purpose or from which shellfish are harvested for human consumption. The presence of those viruses in the aquatic environment represents a large problem for human health, economy and environmental ecology.23,33,45 A large number of human enteric viruses have been shown to be discharged into marine waters by offshore sewage outfalls and they have also been detected in coastal water polluted by sewage treatment plants and septic tanks.31Individuals suffering from diarrhea or hepatitis release a large number of viruses, values greater than 1013 and 1010 viral particles per gram of stool, respectively.12,14,40 More than 140 types of viruses that cause a variety of diseases to humans, which include hepatitis, gastroenteritis, meningitis, fever, influenza, respiratory disease, conjunctivitis, among others, can be found in wastewaters.10,23,44 However, only a small number of viruses is epidemiologically relevant9 and the most relevant viral pathogens found in water are the Norovirus, Rotavirus, Adenovirus, Astrovirus, Enterovirus and Hepatovirus.10,45
The basic steps in the virological analysis of environmental water are sampling, concentration, decontamination/removal of inhibitors and specific virus detection. Concentration is a critical step, since the viruses may be present in such low numbers that it is necessary to concentrate and reduce the volume of the sample to a few millilitres.9,52 The absence of viral concentration methods with high recuperation efficiency has been indicated as a primary reason for the low number of studies in the area of environmental virology. A variety of strategies have been used to concentrate viruses from water samples, which are based mostly on adsorption–elution techniques, flocculation, ultrafiltration and ultracentrifugation. During the last decade, a new chromatographic medium, monolithic supports, was also developed and applied successfully to the concentration of several viruses.11,19,30 However, there is not yet a single method that enables a highly efficient concentration of all viruses.2,8,10 A good concentration method should fulfil several criteria, it should be technically simple, be fast, provide high viral recovery, be adequate for a wide range of viruses, provide a small volume of concentrate, be cost effective9 and should not interfere with viral community structure.6 The last aspect is very important, namely when concentrated samples are used to get a global view of viral community composition, using for instance a high throughput DNA/cDNA sequencing approach.
Flocculation methods are most frequently used to concentrate viruses in environmental waters because they allow us to process large volumes of water13,28,44 relative to ultrafiltration methods.29,37 However, for these methods the electrostatic interaction between the virus and the surface of the filters/flakes depends on various factors, including viral isoelectric points, water pH, and salt concentration,26,43,53 which imply water sample manipulation. They are based on the ability of protein flocculation at acid pH, getting the virus trapped in protein flakes which are then released after dissolution of the flakes.41 However, not only viruses are concentrated but also PCR inhibitory substances.9,16 Ultrafiltration methods are an alternative to adsorption–elution and flocculation techniques and have been shown to be efficient to recover viruses from raw and treated sewage, surface waters and wastewaters.16,20 Ultracentrifugation is a good alternative method for viral concentration in environmental water samples since it requires minimal manipulation, samples can be processed under natural pH and an elution step is not needed. By ultracentrifugation it is possible to concentrate all viruses in a sample, by using a sufficient g-force during an adequate period of time.41 Moreover, the time needed to perform this technique is reduced, when compared with flocculation methods, and it does not introduce any PCR inhibitory substance and, consequently, concentrated samples can be successfully used for molecular detection. Although it is difficult to process large volumes of water with this method it is possible to reduce the volume of the sample to fewer millilitres than with adsorption–elution and flocculation methods.
The comparison among different concentration methods is difficult because it is necessary to take into account many variables (e.g. type of sample, volume of water and the methods used to determinate the recovery efficiency). Nevertheless, in general, for environmental waters, the ultracentrifugation method allows virus recovery efficiencies similar to those of adsorption–elution and flocculation methods.13,21,44,48
The efficiency of viral recovery after concentration is usually determined by plaque assay approaches or real time PCR.13,32,37,44 A viral suspension of known concentration is added to the sample and, after concentration, plaques of lysis are counted or real time PCR is done in order to determine recovery efficiency. However, these methods are time consuming and do not allow determination of the recovery efficiency of all viruses. It only evaluates the recovery rate of the added viruses. Moreover, most viruses cannot be cultured and, consequently, cannot be detected by lysis plaques. The enumeration of the virus-like particles (VLP), by epifluorescence, can be a simple, fast and cost-effective approach to determine viral recovery. The number of natural viruses present in the water sample can be determined before and after sample concentration, allowing evaluation of the concentration of all the viruses present in the sample. Although this technique has been frequently used to determine the viral abundance in aquatic systems5,15,48,50 it was never used to evaluate the efficiency of viral recovery of concentration methods.
The objective of this work was to evaluate ultracentrifugation as the main method to directly concentrate viruses in environmental waters, using a simple and rapid approach (determination of the VLP number) to determine the efficiency of viral recovery. As the flocculation method is normally employed to concentrate viruses in environmental waters,13,21,22,48,55 it was also used in this study to assess the efficacy of ultracentrifugation as the main concentration method in environmental waters.
Experimental
Water sampling
Wastewater and recreational water samples were tested. Wastewater samples were collected at a wastewater treatment plant of Aveiro (South ETAR) after secondary treatment and recreational water samples were collected in a brackish water zone of Ria de Aveiro. Both wastewater and recreational water were collected twice between March and August 2009. Sub-samples of 1 L for sewage treated water and of 10 L for recreational water were used for the direct flocculation method. For the ultracentrifugation method, sub-samples of 0.5 L for sewage treated water and of 1 L for recreational water were used. Three independent assays were done for each situation at each sampling date.Virus concentration by flocculation
The flocculation method used was based on the protocol described by Calgua et al.13 Water samples were acidified with HCl to pH 3.5 (±0.1) and added to 50 mL (for sewage treated water) or 100 mL (for recreational water) of skim milk solution (1% w/v) at pH 3.5 (±0.1). Samples were slowly stirred with a magnetic stirrer for 10 h at room temperature and then flocs were allowed to sediment by gravity for 8 to 10 h. Supernatants were carefully removed without disturbing the sediment and the final volume (approximately 500 mL) was centrifuged at 7000 × g for 30 min at 4 °C. Supernatant was carefully removed and the pellet was suspended in 8 mL of 1× PBS. pH was adjusted to 7.5 (±0.1) by the addition of 1 M NaOH and 1× PBS was added to a final volume of 10 mL. Concentrated samples were stored at −80 °C.Virus concentration by ultracentrifugation
Each water sample was filtered with 0.2 μm membranes (142 mm ø; Millipore Durapore) at low pressure (<200 mm Hg) using a filter system (A.E.B., S.R.L. Druck Ablassen, Italia) and then centrifuged (Beckman Optima™, LE-80 K Ultracentrifuge, rotor 50.2 Ti) at 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 1 h at 20 °C. Supernatant was removed and the pellet was suspended in 1 mL of 1× PBS. Final centrifugation was done at 100
000g for 1 h at 20 °C. Supernatant was removed and the pellet was suspended in 1 mL of 1× PBS. Final centrifugation was done at 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 1 h at 20 °C to gather the pellet. Supernatant was removed and the pellet was suspended in 200 μL of 1× PBS. Concentrated samples were stored at −80 °C.
000g for 1 h at 20 °C to gather the pellet. Supernatant was removed and the pellet was suspended in 200 μL of 1× PBS. Concentrated samples were stored at −80 °C.
Determination of the efficiency of recovery
The efficiency of recovery was determined by counting VLP before and after sample concentration by flocculation and ultracentrifugation by the epifluorescence microscopy method using a modified method of Noble and Fuhrman.39 Water samples were filtered with a 0.2 μm polycarbonate membrane and then with a 0.02 μm Al2O3 Anodisc, which were then stained in the dark for 20 min with SYBR gold dye (0.25%). Enumeration of VLP was made using a Leitz Laborlux K epifluorescence microscope. For each sub-sample, 3 replicates were observed and at least 200 viruses were counted in each replicate. Three independent assays were done for each situation of each sampling date.Detection and quantification of enteric viruses in concentrated water samples by PCR and qPCR
Both viruses, Adenoviruses and Rotaviruses, were detected in the concentrated samples (by flocculation and ultracentrifugation) by PCR in wastewater and in recreational waters, but only the Adenoviruses were quantified by qPCR for concentrated (by flocculation and ultracentrifugation) recreational waters. Adenoviruses were chosen for quantification because this viral group has been suggested as a potential indicator of the presence of viral pollution in environmental waters, namely in marine waters.2,16,37 Three independent assays were done for each situation in each sampling date.Nucleic acids were extracted from water samples using the geneMAG-RNA/DNA kit, a magnetic RNA/DNA purification kit (Chemicell®), according to the instructions of the manufacturer. For nucleic acid purification the Geneclean kit (MP Biomedicals, LLC) was used according to the instructions of the manufacturer.
Detection of Adenoviruses and Rotaviruses was performed in a Labnet TC9600-G thermocycler and amplification products were separated by electrophoresis in a 2% agarose gel stained with ethidium bromide and detected with a UV transilluminator.
Detection of Adenoviruses
Detection of Adenoviruses was made by a nested PCR technique using the primers described by Allard et al.4 The reaction mixture used consisted of 2 mM Buffer taq, 1.5 mM MgCl2, 1.28 mM of each dNTP, 0.1 U μL−1 of Taq polymerase (Fermentas) and 0.4 μM of each primer (StabVida) (hex1deg 5′-GCC SCA RTG GKC WTA CAT GCA CAT C-3′ and hex2deg 5′-CAG CAC SCC ICG RAT GTC AAA-3′), to a final volume of 25 μL. After the first PCR, 5 μL of PCR product were added to 20 μL of a new reaction mixture consisting of 1× Buffer taq, 1.5 mM MgCl2, 1.28 mM dNTPs, 0.4 μM of each primer (nehex3deg 5′ GCC CGY GCM ACI GAI ACS TAC TTC 3 and nehex4deg 5′ CCY ACR GCC AGI GTR WAI CGM RCY TTG TA 3′) and 0.1 U μL−1 of Taq polymerase.The amplification was carried out for 45 cycles at 94 °C for 10 s, 55 °C for 30 s and 72 °C for 20 s after initial denaturation at 94 °C for 4 min. A final extension step was performed at 72 °C for 5 min. The amplification products were separated by electrophoresis in a 2% agarose gel stained with ethidium bromide and detected with a UV transilluminator.
Detection of Rotaviruses
Detection of Rotaviruses was made with a PCR technique using the primers described by Villena et al.54 and using the SuperScript® II RT for the transformation of RNA to cDNA, according to the manufacturer instructions. The reaction mixture used consisted of 2 mM Buffer taq, 3 mM MgCl2, 0.08 mM of each dNTP, 0.1 U μL−1 of Taq polymerase (Fermentas) and 0.48 μM of each primer (StabVida) (VP6-3 5′-GCT TTA AAA CGA AGT CTT CAA C-3′ and VP6-4 5′-GGT AAA TTA CCA ATT CTT CCA G-3′), to a final volume of 25 μL. The amplification was carried out for 40 cycles at 94 °C for 10 s, 50 °C for 30 s and 72 °C for 20 s after initial denaturation at 95 °C for 9 minutes. A final extension step was performed at 72 °C for 7 min.The amplification products were separated by electrophoresis in a 2% agarose gel stained with ethidium bromide and detected with a UV transilluminator.
Quantification of Adenoviruses in concentrated water samples by qPCR
The quantification of Adenoviruses was performed in an iQ5 thermocycler and standards were obtained from a serial dilution of a suspension of Adenoviruses, with a known initial number of copies. The qPCR was performed using the same protocol as that described for detection of Adenovirus using the PCR technique, but with a reaction mixture of 2× iQ® SYBR® Green Super mix (2× reaction buffer with dNTPs, iTaq, DNA polymerase, 6 mM MgCl2, SYBR Green I, fluorescein and stabilizers) and 0.4 μM of each primer (nehex3deg and nehex4deg), to a final volume of 25 μL.Statistical analysis
For the enumeration of virus-like particles (VLP) and for the detection and quantification of the two enteric viruses, three sub-samples were used for both concentration methods and for both water types of each sampling date. For the enumeration of VLP, for each sub-sample, 3 replicates were analysed and for the detection and quantification of the enteric viruses only 2 replicates were done for each sub-sample. The results of the three sub samples were averaged and the standard deviation was calculated.The differences between the efficiency of recovery, determined by the enumeration of VLP, of the two concentration methods were analyzed by one-way ANOVA to check for significant differences between methods. The difference between Adenovirus quantification in recreational waters concentrated by the two methods was also evaluated using one-way ANOVA. Only the data with normal distribution (assessed by the Kolmogorov–Smirnov test) and with homogeneity of variances (assessed by Levene’s test) were used. A value of p < 0.05 was considered significant. Statistical analyses were performed by using SPSS (SPSS 15.0 for Windows, SPSS Inc., USA).
Results
Efficiency of viral recovery for the two concentration methods
The recovery efficiency for sewage samples (Fig. 1a) and recreational samples (Fig. 1b) was higher with the ultracentrifugation than with the flocculation method. Ultracentrifugation showed an average recovery efficiency of 69% for wastewater and of 76% for recreational water samples. The corresponding recovery efficiencies with the flocculation method were 38% and 22% for wastewater and recreational water, respectively.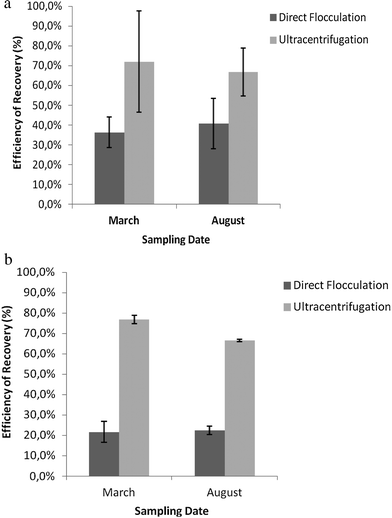 | ||
| Fig. 1 (a) Recovery rate of VLP, for both concentration methods, in sewage waters, for two sampling dates. Values represent the mean of three independent experiments; error bars indicate the standard deviation. (b): Recovery rate of VLP, for both concentration methods, in recreational waters, for two sampling dates. Values represent the mean of three independent experiments; error bars represent the standard deviation. | ||
The differences between the efficiency of recovery for the two sampling dates were not significant for wastewater (p = 0.86 and p = 0.16 respectively), but for recreational waters there were significant differences in the efficiency of recovery with the two concentration methods for both sampling dates (p = 0.01 and p = 0.00, respectively).
Detection of enteric viruses in water samples
The Adenoviruses and Rotavirus A in wastewater and recreational water samples were found in all samples after concentration by ultracentrifugation and by flocculation.Quantification of Adenoviruses in recreational water samples
The presence of Adenoviruses in recreational water samples after concentration was tested by qPCR (Fig. 2). This group of viruses was present in all samples after concentration.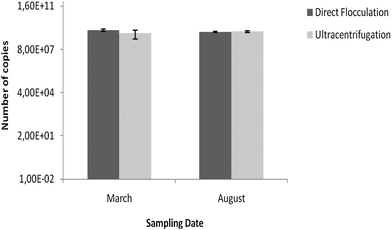 | ||
| Fig. 2 Quantification of Adenoviruses in recreational water samples after concentration by flocculation and ultracentrifugation for the two sampling dates. Values represent the mean of three independent experiments; error bars indicate the standard deviation. | ||
The differences between the quantification of Adenoviruses, for the two concentration methods, for the two sampling dates, were not significant (p = 0.057 and p = 0.868, respectively).
Discussion
Environmental samples must be sufficiently concentrated to allow efficient detection of very few viruses in a large volume of water.46 However, there is no perfect viral concentration method for water samples,2,10 the method should be chosen so that, despite its limitations, it is adequate to achieve the aim of the work. In the present work, the ultracentrifugation and the flocculation methods were compared, considering further use of the samples for molecular analysis such as PCR or pyrosequencing.The results of this study show that the ultracentrifugation method, usually used as a secondary step for virus concentration,17,29,49,51 is an adequate approach to concentrate viruses directly from environmental waters and that the determination of the VLP by microscopy is a simple, fast and cost effective method to evaluate the recovery efficiency of the concentration method.
The ultracentrifugation method recovered about 30% more viruses in residual waters and about 50% more in recreational waters than the organic flocculation method, one of the most currently used approaches to concentrate viruses from environmental waters.13,21,22,48,55 Moreover the ultracentrifugation method is simpler and faster than the flocculation approach. During flocculation, HCl is added to water samples to adjust the water pH to 3.5 and skim milk solution is added to allow virus adsorption. It is, however, well known that during the concentration step, inhibitory substances are concentrated along with the viruses and, consequently, the addition of HCl and skim milk solution may increase the inhibitory effect on subsequent PCR reactions. In ultracentrifugation, only the non-added inhibitory substances are concentrated. Although for recreational water this effect can be negligible, for wastewaters, which have large concentrations of inhibitory substances, purification of the concentrated samples is always required.18,36 For more clean environmental waters this can be an inconvenience relative to the ultracentrifugation method.
The incubation period of more than 16 hours used for flocculation may also affect the structure of the viral community. It is well known that bacteriophages represent a large fraction of the virioplankton7,35,50 and their replication cycle is frequently less than 1 hour,7,34 allowing these viruses to undergo several replications during the period of concentration by flocculation. In contrast, viruses that infect eukaryotic cells have replication times of around 40 hours56 and, consequently, it is unlikely that they replicate during the incubation period. These facts are not important if the concentration method is used to detect specific enteric viruses by PCR because specific primers are used, but if the concentrated water samples are used to study the structure of the viral community, the results will not reflect the structure of the original community. In contrast, in the ultracentrifugation method, as water samples are filtered with 0.2 μm membranes in order to remove bacteria prior to centrifugation, all cellular hosts are removed and viral replication is avoided, even for bacteriophages that have shorter life cycles. However, the water filtration before ultracentrifugation can cause viral loss due to membrane clogging. The replacement and/or the employment of large-size membranes, as used in this study, can overcome this problem. Moreover, when ultracentrifugation is used to detect specific enteric viruses, water filtration is not necessary and, consequently the loss of viruses by membrane clogging is avoided. Therefore, the concentration by ultracentrifugation provides a more realistic picture of the viral community structure than the flocculation method.
Although it is not practical to ultracentrifuge large volumes of water, it is possible to reduce the final volume of concentrated samples to a few microlitres and, consequently, efficiently concentrate viruses in environmental waters. In this study, water samples of 0.5–1.0 L were concentrated 1000–2000 times in a volume of 200 μL, while by flocculation water samples of 10 L were concentrated 100–1000 times to a final volume of 10 mL. The large amount of flocs formed during the precipitation with the skim milk prevents reduction of the concentrated water volume of the flocculated samples to less than 10 mL. Moreover, techniques of nucleic acid amplification are now the most common way for viral detection in water since they are rapid and more sensitive than traditional cell culture methods.25,42,54 Consequently, a small volume of water is sufficient to detect a specific virus. In fact, in this study enteric viruses were detected by PCR in recreational waters at sites where, in previous studies, their presence had been undetectable by cell culture and immunological methods.3
The results show that both flocculation and ultracentrifugation methods are adequate to concentrate water samples for detection and quantification of enteric viruses in environmental waters. Rotavirus A and Adenovirus were present in all samples concentrated by either of the two concentration methods and the number of Adenoviruses in recreational waters, quantified by qPCR, was similar for both concentration methods in the three independent assays of each of the two sampling dates.
The estimation of the recovery efficiency based on the enumeration of VLP by epifluorescence was similar to that achieved in other studies based on the addition of specific enteric viruses to water samples and further quantification by plaque assay22,24,27,48 or qPCR.13,23,29,37 The enumeration of VLP by epifluorescence is, however, a simpler, faster and cheaper approach relative to the traditional plaque assay and qPCR approaches. Moreover, using the VLP, it is possible to evaluate the concentration of the whole viral community and not of a specific virus, as it happens when the viruses are added to the samples and detected by plaque assay and PCR techniques. In contrast to the plaque assay enumeration, using VLP counts it is considered the infective and non-infective viruses. However, since the techniques of nucleic acid amplification are now the most common way for viral detection/quantification in environmental waters25,42,52,54 and these techniques amplify nucleic acids of both infective and non-infective viruses, the recovery efficiency based on the enumeration of VLP is not an inconvenience, reflecting even better the efficacy of the concentration methods to recover viruses in environmental waters. Consequently, the determination of VLP is a good alternative to determine the recovery efficiency of the viral concentration methods.
Acknowledgements
The authors would like to thank the University of Aveiro and Centre for Environmental and Marine Studies (CESAM) for funding the study. Financial support to C. Prata was provided by the Portuguese Foundation for Science and Technology (FCT) in the form of a PhD grant (SFRH/BD/33390/2008), São José Nascimento of University of Porto and Albert Bosh of University of Barcelona, for providing viral stocks used in this work as positive controls.Notes and references
- N. Albinana-Gimenez, P. Clemente-Casares, S. Bofill-Mas, A. Hundesa, F. Ribas and R. Girones, Distribution of human polyomaviruses, Adenoviruses and hepatitis E virus in the environmental and drinking-water treatment plant, Environ. Sci. Technol., 2006, 40, 7416–7422 CrossRef CAS.
- N. Albinana-Gimenez, M. P. Miagostovich, B. Calgua, J. M. Huguet, L. Matia and R. Girones, Analysis of Adenoviruses and polyomaviruses quantified by qPCR as indicators of water quality in source and drinking-water treatment plants, Water Res., 2009, 43, 2011–2019 CrossRef CAS.
- F. Alcântara and M. A. Almeida, Virological quality of the Ria de Aveiro: validity of potential microbial indicators, Aquat. Ecol., 1995, 29(3–4), 419–425 Search PubMed.
- A. Allard, B. Albisson and G. Wadell, Rapid typing of human Adenoviruses by a general PCR combined with restriction endonuclease analysis, J. Clin. Microbiol., 2001, 39(2), 495–505 CrossRef.
- M. A. Almeida, M. A. Cunha and F. Alcântara, Loss of estuarine bacteria by viral infection and predation in microcosm conditions, Microb. Ecol., 2001, 42(4), 562–571 CrossRef CAS.
- F. E. Angly, B. Felts, M. Breitbart, P. Salamon, R. A. Edwards, C. Carlson, A. M. Chan, M. Haynes, S. Kelley, H. Liu, J. M. Mahaffy, J. E. Mueller, J. Nulton, R. Olson, R. Parsons, S. Rayhawk, C. A. Suttle and F. Rowher, The marine viromes of four oceanic regions, PLoS Biol., 2006, 4(11), 2121–2131 CAS.
- Y. Bettarel, R. Arfi, T. B. Bouvier, M. Bouvy, E. Briand, J. Colombet, D. Corbin and T. Sime-Ngando, Virioplankton distribution and activity in a tropical eutrophicated bay, Estuarine, Coastal Shelf Sci., 2008, 80, 425–429 CrossRef.
- J. C. Block and L. Schwartzbrod, Viruses in Water Systems: Detection and Identification, VCH Publishers, Inc, New York, 1989 Search PubMed.
- A. Bosch, Human enteric viruses in the water environment: a minireview, Int. Microbiol., 1998, 1, 191–196 CAS.
- A. Bosch, S. Guix, D. Sano and R. M. Pintó, New tools for the study and direct surveillance of viral pathogens in water, Curr. Opin. Biotechnol., 2008, 19, 295–301 CrossRef CAS.
- K. Branovic, D. Forcic, J. Ivancic, A. Strancar, M. Barut, T. Kosutic-Gulija, R. Zgorelec and R. Mazuran, Application of short monolithic columns for improved detection of viruses, J. Virol. Methods, 2003, 110, 163–171 CrossRef CAS.
- S. Caballero, S. Guix, W. M. El Senousy, I. Calico, R. M. Pinto and A. Bosch, Persistent gastroenteritis in children infected with Astrovirus: association with serotype-3 strains, J. Med. Virol., 2003, 71, 245–250 CrossRef.
- B. Calgua, A. Mengewein, A. Grunert, S. Bofill-Mas, P. Clemente-Casares, A. Hundesa, A. P. Wyn-Jones, J. M. López-Pila and R. Girones, Development and application of a one-step low cost procedure to concentrate viruses from seawater samples, J. Virol. Methods, 2008, 153, 79–83 CrossRef CAS.
- M. I. Costafreda, A. Bosch and R. M. Pinto, Development, evaluation, and standardization of a real-time TaqMan reverse transcription-PCR assay for quantification of hepatitis A virus in clinical and shellfish samples, Appl. Environ. Microbiol., 2006, 72(6), 3846–3855 CrossRef CAS.
- R. Danovaro, E. Manini and A. Dell'Anno, Higher abundance of bacteria than viruses in deep Mediterranean sediments, Appl. Environ. Microbiol., 2002, 68, 1468–1472 CrossRef CAS.
- T.-T. Fong and E. Lipp, Enteric viruses of human and animals in aquatic environments: health risks, detection, and potential water quality assessment tools, Microbiol. Mol. Biol. Rev., 2005, 96(2), 357–371 CrossRef.
- G. S. Fout, B. C. Martinson, M. W. N. Moyer and D. R. Dahling, A multiplex reverse transcription-PCR method for detection of human enteric viruses in groundwater, Appl. Environ. Microbiol., 2003, 69(6), 3158–3164 CrossRef CAS.
- J. H. Guo, Y. Z. Peng, S. Y. Wang, Y. N. Zheng, H. J. Huang and S. J. Ge, Effective and robust partial nitrification to nitrite by real-time aeration duration control in an SBR treating domestic wastewater, Process Biochem., 2009, 44, 979–985 CrossRef CAS.
- I. Gutierez-Aguirre, M. Banjac, A. Steyer, M. Poljšak-Prijatelj, M. Peterka, A. Strancar and M. Ravnikar, Concentrating rotaviruses from water samples using monolithic chromatographic supports, J. Chromatogr., A, 2008, 1216, 2700–2704 CrossRef.
- T. Grassi, F. Bagordo, A. Idolo, F. Lugoli, G. Gabutti and A. De Donno, Rotavirus detection in environmental water samples by tangencial flow ultrafiltration, Environ. Monit. Assess., 2010, 164(1–4), 199–205 CrossRef CAS.
- N. Guttman-Bass and R. Armon, Concentration of Simian Rotavirus SA-11 from tap water by membrane filtration and organic flocculation, Appl. Environ. Microbiol., 1983, 45(3), 850–855 CAS.
- N. Guttman-Bass and A. Nassen, Simultaneous concentration of four enteroviruses from tap, waste and natural waters, Appl. Environ. Microbiol., 1984, 47(6), 1311–1315 CAS.
- I. A. Hamza, L. Jurzik, A. Stang, K. Sure, K. Uberla and M. Wilhelm, Detection of human viruses in rivers of a densely-populated area in Germany using a virus adsorption method optimized for PCR analyses, Water Res., 2009, 43, 2657–2668 CrossRef CAS.
- E. Haramoto, H. Katayama, K. Oguma and S. Ohgaki, Application of cation-coated filter method to detection of noroviruses, enteroviruses, Adenoviruses, and torque teno viruses in the Tamagawa river in Japan, Appl. Environ. Microbiol., 2005, 71(5), 2403–2411 CrossRef CAS.
- T. Hovi, M. Roivainen and S. Blomqvist, Enteroviruses with Special Reference to Poliovirus and Poliomyelitis Eradication, Human Viruses in Water: Perspectives in Medical, 2007, pp. 69–89 Search PubMed.
- B.-M. Hsu, C.-H. Chen, C.-M. Kung, M.-T. Wan and S.-M. Shen, Evaluation of enterovirus recovery in surface water by different adsorption and elution procedures, Chemosphere, 2007, 66, 964–969 CrossRef CAS.
- H. Katamaya, A. Shimasaki and S. Ohgaki, Development of a virus concentration method and its application to detection of enterovirus and norwalk virus from coastal seawater, Appl. Environ. Microbiol., 2002, 68(3), 1033–1039 CrossRef.
- E. Katzenelson, B. Fattal and T. Hostovesky, Organic flocculation: an efficient second-step concentration method for the detection of viruses in tap water, Appl. Environ. Microbiol., 1976, 32(4), 638–639 CAS.
- K. Kovac, I. Gutierrez-Aguirre, M. Banjac, M. Peterka, M. P. Prijatelj, M. Ravnikar, J. Z. Mijovski, A. C. Schultze and P. Raspor, A novel method for concentrating hepatitis A virus and caliciviruses from bottled water, J. Virol. Methods, 2009, 162, 272–275 CrossRef CAS.
- P. Kramberger, N. Petrovic, A. Strancar and M. Ravnikar, Concentration of plant viruses using monolithic chromatographic supports, J. Virol. Methods, 2004, 120, 51–57 CrossRef CAS.
- R. L. LaBelle, C. P. Gerba, S. M. Goyal, J. L. Melnick, I. Cech and G. F. Bogdan, Relationship between environmental factors, bacterial indicators, and the occurrence of enteric viruses in estuarine sediments, Appl. Environ. Microbiol., 1980, 39(3), 588–596 CAS.
- E. Lambertini, S. K. Spencer, P. D. Bertz, F. J. Loge, B. A. Kieke and M. A. Borchardt, Concentration of enteroviruses, Adenoviruses, and noroviruses from drinking water by use of glass wool filters, Appl. Environ. Microbiol., 2008, 74(10), 2990–2996 CrossRef CAS.
- S. H. Lee and S. J. Kim, Detection of infectious enteroviruses and Adenoviruses in tap water in urban areas in Korea, Water Res., 2002, 36(1), 248–256 CrossRef CAS.
- M. Madigan and J. Martinko, Brock Biology of Microorganisms, Pearson Prentice Hall, Inc., 2006 Search PubMed.
- T. Miki and S. Jacquet, Complex interactions in the microbial world: under-explored key links between viruses, bacteria and protozoan grazers in aquatic environments, Aquat. Microb. Ecol., 2008, 51, 195–208 CrossRef , REVIEW.
- G. Moussani, M. Mahmoudi and B. Barikbin, Biological removal of phenol from strong wastewaters using a novel MSBR, Water Res., 2009, 43, 1295–1302 CrossRef.
- M. Muscillo, M. Pourshaban, M. Iaconelli, S. Fontana, A. Di Grazia, S. Manzara, G. Fadda, R. Santangelo and G. La Rosa, Detection and quantification of human Adenoviruses in surface waters by nested PCR, TaqMan Real-Time PCR and cell culture assays, Water, Air, Soil Pollut., 2008, 191, 83–93 CrossRef CAS.
- M. Myrmel, E. M. M. Berg, B. Grinde and E. Rimstad, Enteric viruses in inlet and outlet samples from sewage treatment plants, J. Water Health, 2006, 197–209 CAS.
- R. T. Noble and J. A. Fuhrman, Use of SYBR Green I for rapid epifluorescence counts of marine viruses and bacteria, Aquat. Microb. Ecol., 1998, 14, 113–118 CrossRef.
- K. Ozawa, T. Oka, N. Takeda and G. S. Hansman, Norovirus infections in symptomatic and asymptomatic food handlers in Japan, J. Clin. Microbiol., 2007, 45(12), 3996–4005 CrossRef CAS.
- S. Percival, R. Chalmers, M. Embrey, P. Hunter, J. Sellwod and P. Wyn-Jones, Microbiology of Waterborne Diseases, Viruses, Elsevier Academic Press, London, 1st edn, 2004, pp. 337–452 Search PubMed.
- R. M. Pinto, D. Alegre, A. Dominguez, W. M. El Senousy, G. Sanchez, C. Villena, M. I. Costafreda, L. Aragones and A. Bosch, Hepatitis A virus in urban sewage from two Mediterranean countries, Epidemiol. Infect., 2007, 135, 270–273 CrossRef CAS.
- A. L. Polaczyck, J. M. Roberts and V. R. Hill, Evaluation of 1MDS electropositive microfilters to simultaneous recovery of multiple microbe classes from tap water, J. Microbiol. Methods, 2007, 68, 260–266 CrossRef.
- M. Puig, J. Jofre, F. Lucena, A. Allard, G. Wadell and R. Girones, Detection of Adenoviruses and enteroviruses in polluted water by nested PCR amplification, Appl. Environ. Microbiol., 1994, 60(8), 2963–2970 CAS.
- J. Rodríguez-Díaz, L. Querales, L. Caraballo, E. Vizzi, F. Liprandi, H. Takiff and W. Q. Betancourt, Detection and characterization of waterborne gastroenteritis viruses in urban sewage and sewage-polluted river waters in Caracas, Venezuela, Appl. Environ. Microbiol., 2009, 75(2), 387–394 CrossRef.
- K. J. Schwab, R. De Leon and M. Sobsey, Concentration and purification of beef extract mock eluates from water samples for the detection of enteroviruses, hepatitis A viruses, and norwalk viruses by reverse transcription-PCR, Appl. Environ. Microbiol., 1995, 61(2), 527–531 Search PubMed.
- G. Sedmak, D. Bina, J. Macdonald and L. Couillard, Nine-year study of the occurrence of culturable viruses in source water for two drinking water treatment plants and the influent and effluent of a wastewater-treatment plant in Milwakee, Wisconsin, Appl. Environ. Microbiol., 2005, 71, 1042–1050 CrossRef CAS.
- P. A. Shields and S. R. Farrah, Concentration of viruses in beef extract by flocculation with ammonium sulfate, Appl. Environ. Microbiol., 1986, 51(1), 211–213 CAS.
- G. F. Steward, Fingerprinting viral assemblages by pulsed Field gel electrophoresis (PFGE), Methods Microbiol., 2001, 30 Search PubMed.
- C. A. Suttle and J. A. Fuhrman, Enumeration of Virus Particles in Aquatic or Sediment Samples by Epifluorescence Microscopy, in Manual of Aquatic Viral Ecology, ed. S. W. Wilhelm, M. G. Weinbauer and C. A. Suttle, ASLO, 2010, pp. 145–153 Search PubMed.
- S. Sylvain, C. Gantzer, K. Helmi, L. Hoffmann and H.-M. Cauchie, Simultaneous concentration of enteric viruses and protozoan Parasites: a protocol based on tangential flow filtration and adapted to large volumes of surface and drinking waters, Food Environ. Virol., 2009, 1(2), 66–76 CrossRef.
- Y.-L. Tsai, M. D. Sobsey, L. R. Sangermano and C. J. Palmer, Simple method of concentrating enteroviruses and hepatitis A virus from sewage and ocean water for rapid detection by reverse transcriptase-polymerase chain reaction, Appl. Environ. Microbiol., 1993, 59(10), 3488–3491 CAS.
- J. G. Victoria, A. Kapoor, L. Li, O. Blinkova, B. Slikas, C. Wang, A. Naeem, S. Zaidi and E. Delwart, Metagenomic analysis of viruses in stool samples from children with acute flaccid paralisys, J. Virol., 2009, 83(9), 4542–4651 CrossRef.
- C. Villena, W. M. El-Senousy, F. X. Abad, R. M. Pinto and A. Bosch, Group A rotavirus in sewage samples from Barcelona and Cairo: emergence of unusual genotypes, Appl. Environ. Microbiol., 2003, 69(7), 3919–3923 CrossRef CAS.
- Virobathe, http://www.virobathe.org/, 2011.
- K. E. Wommack and R. R. Colwell, Virioplankton: viruses in aquatic ecosystems, Microbiol. Mol. Biol. Rev., 2000, 64(1), 69–114 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
