Effects of temperature and soil moisture on methyl halide and chloroform fluxes from drained peatland pasture soils
M. A. H.
Khan
*,
M. E.
Whelan
and
R. C.
Rhew
Department of Geography, University of California at Berkeley, 540 McCone Hall #4740, Berkeley, CA 94720, USA. E-mail: anwar.khan@berkeley.edu; Fax: +1 510 642 3370; Tel: +1 510 643 6984
First published on 7th November 2011
Abstract
A series of laboratory-based incubations using a stable isotope tracer technique was applied to measure the net and gross fluxes of CH3Cl and CH3Br as well as the net fluxes of CHCl3 from surface soils of the Sacramento-San Joaquin Delta of California. Annually averaged flux measurements show that these mineral/oxidized peat soils are a net source of CH3Cl (140 ± 266 nmol m−2 d−1) and CHCl3 (258 ± 288 nmol m−2 d−1), and a net sink of CH3Br (−2.3 ± 4.5 nmol m−2 d−1). Gross CH3Cl and CH3Br fluxes are strongly influenced by both soil moisture and temperature: gross production rates of CH3Cl and CH3Br are linearly correlated with temperature, whereas gross consumption rates exhibit Gaussian relationships with maximum consumption at soil moisture levels between 20 and 30% volumetric water content (VWC) and a temperature range of 25 to 35 °C. Although soil moisture and soil temperature strongly affect consumption rates, the range of gross consumption rates overall is limited (−506 ± 176 nmol m−2 d−1 for CH3Cl and −12 ± 4 nmol m−2 d−1 for CH3Br) and is similar to rates reported in previous studies. CHCl3 fluxes are not correlated with methyl halide fluxes, temperature, or soil moisture. The annual emission rates of CHCl3 from the Sacramento-San Joaquin Delta are found to be a potentially significant local source of this compound.
Environmental impactFlooded peatland is a known source or sink of atmospheric halocarbons. The peatland fluxes for halocarbons are altered following drainage and subsequent conversion to pasture. Measuring methyl halides and chloroform fluxes from drained peatland soils at the Sacramento-San Joaquin Delta of California will help to determine the potential shift in halocarbon fluxes that can occur following this major hydrological shift. The study also identified major controls on the emission or consumption rates. |
1. Introduction
Halogens (e.g., chlorine and bromine) are minor constituents in the Earth's atmosphere but are major participants in the catalytic destruction of stratospheric ozone.1,2 Among the naturally occurring halocarbons (albeit with significant anthropogenic contributions), methyl chloride (CH3Cl) and methyl bromide (CH3Br) are the most important, contributing about 16% of the total stratospheric Cl and 50% of the total stratospheric Br, respectively.3Chloroform (CHCl3) is the second largest carrier of natural Cl in the troposphere after CH3Cl, contributing to the reactive chlorine burden in the troposphere and to ozone destruction in the stratosphere.3 The global budgets of atmospheric CH3X (X = Cl and Br) and CHCl3 have many uncertainties, especially regarding the magnitude of their natural sources and sinks. Resolving this budget uncertainty is essential to more accurately determine their atmospheric lifetimes and to quantify their ozone depletion potentials (ODPs).The ODP of a halocarbon is determined by the quantity of halogens that reaches the stratosphere, depending on their atmospheric transport and chemistry.4 The loss of methyl halides in the troposphere is caused by biological degradation in the ocean, soil, and/or vegetation surfaces and by oxidation with hydroxyl radicals. Microbial and abiotic consumption in soil has the largest uncertainty with an estimated range of 100–1600 Gg yr−1 for CH3Cl5,6 and 32–154 Gg yr−1 for CH3Br.7,8 Improving the accuracy of atmospheric lifetimes necessitates better estimates of soil-driven methyl halide consumption.
Methyl halide consumption rates vary for different ecosystems and depend on soil characteristics.8 Four terrestrial biomes (temperate forests, woodlands, shrublands, and grasslands) are estimated to account for over 70% of the CH3Br soil sink.8 All of these biomes typically have non-flooded, aerated soils, and the consumption of CH3Br was identified as an aerobic prokaryotic process.9 Studies of soil microbial activity found that methyl halides may be degraded in aerobic condition by either methanotrophs10,11 or non-methanotrophic bacteria.12,13 Nitrifying bacteria also degrade methyl halidesviaammonia monooxygenase14 and additions of ammonia fertilizers stimulate consumption by agricultural soils.15
Dimmer et al.16 found that Irish peatlands were a source of CH3Cl, CH3Br, and CHCl3 under both aerobic and anaerobic conditions, with global peatland extrapolations of 0.1–151.9 Gg yr−1, 0.1–3.3 Gg yr−1, and 0.9–43.4 Gg yr−1, respectively. Northern Alaskan tundra studies17 revealed higher uptake rates of CH3Cl and CH3Br in the drained tundra and very low uptake rates in the flooded tundra. High latitude peatland measurements at Abisko, Sweden18 showed small net uptake of CH3Br and net emission of CH3Cl. That study, along with another study in New Hampshire, USA19 suggested that the uptake rates of CH3Br in peatlands (representing wetlands more broadly) are smaller than those of upland, drier ecosystems. Another study by Zaccone et al.20 suggested that most of the Br present in peat (ca. 40%) was immobilized by humic acid molecules, i.e., a recalcitrant fraction of the organic matter that acted as a geochemical sink of this element. Peatlands are a significant source of CHCl3, but their emission rates have large spatial and temporal heterogeneities.16,21 In sum, these prior studies have demonstrated that the role of peatlands as a source or sink of CH3Cl, CH3Br, and CHCl3 depends on climate and degree of waterlogging.
In the context of land use change, an important unknown is how peatland fluxes for these halomethanes are affected following drainage and subsequent conversion to pasture. Surface soils will become drier and more aerobic, and this top layer may dominate the surface-atmosphere exchange of halomethanes.8 In the San Joaquin-Sacramento River Delta, most of the peatland area (2978 km2) was drained by 1930 and converted for agricultural use,22 resulting in land surface subsidence of peat soils. Measuring methyl halides and CHCl3 fluxes from these lands will help determine the potential shift in halocarbon fluxes that can occur following this major hydrological shift. Because subsiding islands in the San Joaquin-Sacramento River Delta may be converted back into wetlands as a means of restoring lost carbon and subsiding land mass,23 it is also important to establish flux measurements in its current non-flooded state. To address this, we conducted a one year study of methyl halide (CH3Cl and CH3Br) and CHCl3 fluxes from surface soils at a drained temperate peatland pasture in California to characterize the spatial and temporal variability of the major natural fluxes of volatile halomethanes. Net fluxes (of CH3Cl, CH3Br, and CHCl3) and gross fluxes (of CH3Cl and CH3Br) were measured using laboratory-based incubations of soil cores collected monthly from the site. When conducted at field moisture and temperature, intact soil core incubations can represent in situ soil behavior for halomethane fluxes, both in terms of magnitude of fluxes as well as relationship to soil moisture.24,25
2. Materials and methods
2.1 Field site
The study site is located on the southern part of Sherman Island (38°02′13′′N, 121°45′15′′W), a peatland pasture on the west side of the Sacramento-San Joaquin River Delta and near the city of Antioch, California. The Delta surface was drained in the mid-1800s,22,26 forming an agricultural area including over 100 islands and tracts surrounded by 2250 km of man-made levees and 1130 km of waterways.27 Sherman Island (42.5 km2) is one of these islands and is currently below mean sea level (elevation −7 m),28 surrounded by levees, and sectioned with drainage channels.The regional climate is characterized as Mediterranean, with cool, wet winters and hot, dry summers.26 Mean annual precipitation and surface temperature is approximately 325 mm and 15.6 °C, respectively.29 Over 80% of the average annual precipitation occurs from November through March.22 The surface soils are dry during the warm months of the year but can be muddy during the cool winter months. At Sherman Island, the top 0.3 to 0.9 m of soil is a highly compacted, oxidized, and decomposed peat layer, which overlies a 1.5 to 2.9 m thick unoxidized peat layer.30 Sherman Island is partially comprised of some marsh deposits from San Francisco Bay, and the peats were derived from decaying marsh vegetation.31 Peat accumulation at this site started about 7000 years ago at a rate just sufficient to keep up with the average postglacial sea-level rise of about 0.2 cm per year.26 However, currently only an estimated 34 to 41% of the original peat column remains after drainage led to primary subsidence (settling and compaction of peat) and decades of secondary subsidence (microbial oxidation of peat).30 The upper layer of soil at the part of Sherman Island where this work was conducted is now largely mineral soil, classified as clay loam according to gravimetric hydrometer methods.28,32
The field site is relatively flat and predominantly covered (∼85–90%) by perennial pepperweed (Lepidiumlatifolium L.) with a small portion of C3 grass (mouse barley; Hordeum murinum L.). The remaining part (∼10–15%) was characterized by bare soil surface. New shoots of pepperweed generally appear in mid-March and persist until the end of October.33 The abundance and phenology of pepperweed is important in regulating net ecosystem exchange of carbon dioxide at this site.28 It is assumed that the sunlight is attenuated under the pepperweed canopy, causing soil moisture to be higher beneath plants relative to bare patches of soil. Recently large areas of Sherman Island have been used for irrigated pasture land for grazing cattle.
2.2 Soil core collection
At sites dominated by L. latifolium, three soil cores (17.81 cm2 surface area, 116.6 mL volume, 0 to 5 cm depth) spaced ∼100 m apart on a rough north-south transect were collected each month from June 2009 to May 2010 immediately after surface vegetation was removed. In each outing, the first core (Core 1) was taken at the north end of the transect, which was more sparsely vegetated than the middle (Core 2) and south (Core 3) end of the transect. Surface soil cores were collected using a soil corer (AMS Inc., American Falls, ID), placed in aluminum sleeves with a fitted metal base and then stored at 1 °C for 5–15 days until further experiments could be performed. The average Sherman island soil temperature (at 5 cm depth) during collection was 19.4 ± 7.3 °C.2.3 Soil core incubation
To prepare for the flux measurements, the collected surface soil cores were individually placed in glass Mason jars (1.9 L) partially submerged in a temperature controlled water/ethylene glycol bath at 20 °C (Model 1180S, VWR International, West Chester, PA, USA) at least 24 h prior to the first incubation. Three sets of incubations were conducted on each of the three soil cores collected per outing over the course of several days. To investigate temperature effects on gross production and consumption rates, additional studies were conducted on the three soil cores collected in August 2009. The volumetric water contents (VWCs) of these soils were 14%, 21%, and 23%. These cores were incubated through an increasing range of temperatures (10 °C, 15 °C, 20 °C, 25 °C, 30 °C, and 35 °C) while holding their moisture levels constant.Before the start of each incubation, the headspace was flushed with ambient air for approximately 30 s. The jar was then sealed with a Teflon wrapped Viton o-ring and a stainless steel lid. Within a few seconds of sealing, a 40–50 mL mix of 70 ppb 13CH3Cl, 7 ppb 13CH3Br, and 65 ppb CFC-113 in nitrogen was injected into the headspace through a septa in the lid using a gas tight syringe (Hamilton Company, Reno, NV, USA), resulting in respective headspace concentrations of roughly 2800–3500 ppt, 300–350 ppt, and 2800–3500 ppt. 10–15 mL air samples were withdrawn from the headspace at three different incubation times (3, 33, and 63 min) and were analyzed immediately by quadrupole GC-MS (gas chromatograph/mass spectrometer). Further descriptions of the soil core incubation technique can be found in Teh et al.34
After the incubations, the soil cores were oven dried at 105 °C overnight to determine gravimetric water content and soil bulk density (measured as weight of the soil/corresponding volume of the core size). Using these parameters, volumetric soil moisture was then calculated for each core. A portion of each dried soil core sample was homogenized, separated the coarse particles with 40 mesh sieve, and then total carbon and total nitrogen contents of 20 mg of fine particle soil were determined using a Carbon-Nitrogen analyzer (NC2100, Carlo Erba Instruments, Milan, Italy). The total carbon measured in soils from Sacramento-San Joaquin islands accurately approximates the organic carbon content, as the peat and underlying mineral soils both consist of less than 0.4% carbonate on average.30 All 36 soil samples were analyzed in duplicate to assess the precision of the method. Atropine was used as the calibration standard, with known amounts analyzed every 10 samples to monitor instrument drift.
2.4 Air sample analyses
The concentrations of the analytes were quantified using GC-MS (Agilent 6890N/5973). The custom built inlet system utilized dual cryotrapping: the first trap was a 1/8′′ stainless steel U-tube packed with HayesepD cooled with an ethanol-liquid N2 mixture (−70 °C), and the second trap was a narrow bore 1/16′′ stainless steel U-tube cooled with liquid N2 (−196 °C). Analytes were desorbed from the second trap at 100 °C under helium flow (1.4 ml min−1) and then transferred to the GC, which separated the compounds with a 60 m long, 1.4 μm thickness film DB-VRX capillary column (J&W Scientific Inc., Folsom, CA, USA). The GC oven temperature was held at 30 °C for 348 s, then was ramped up at a rate of 25 °C min−1 to 180 °C over 6 min and held at 180 °C for 8.2 min. Details of the custom inlet system and air analyses can be found in Rhew et al.17Four isotopologues of CH3Br (12CH379Br, 12CH381Br, 13CH379Br, and 13CH381Br; m/z = 94, 96, 95 and 97, respectively) and of CH3Cl (12CH335Cl, 12CH337Cl, 13CH335Cl, and 13CH337Cl; m/z = 50, 52, 51 and 53, respectively) were quantified using selective ion monitoring mode. To correct for peak enhancements due to ion fragmentation, separate runs of a ppb-level 13CH3Br and 13CH3Cl gas mixture were conducted to characterize the ion fragmentation ratios.35 A whole air working standard (SIO-2005 scale) was used for calibration curves and for monitoring instrumental drift. For this study, instrumental precision (1σ) based on daily standards after applying drift corrections were 3% for both CH3Cl and CH3Br and 5% for CHCl3.
2.5 Flux calculation
Net fluxes were calculated by applying a linear least squares fit of the measured mole fraction of analytesversus time, and then multiplying the slope by the number of moles of air in the incubation jar. The soil surface area was also included in the calculation for measuring the flux per unit area. Net flux errors were calculated by propagating the standard error on the slope with the measurement uncertainty of sample volume, temperature, and pressure. Gross fluxes were calculated using a stable isotope tracer technique in which the concentration of 12CH3X and 13CH3X were both plotted against time and subjected to an iterative procedure to determine the best fit of concentration changes to a gross production and gross consumption box model.36 Loss rates of 13CH3X represented biological and chemical consumption as well as any physical loss of the compounds from the incubation chamber. The physical loss was quantified using the inert tracer CFC-113. Gross consumption rates were calculated by multiplying the first order rate constants with seasonally averaged background concentrations in Northern Hemisphere air between 1998–2001 (10.4 ppt for CH3Br and 535.7 ppt for CH3Cl)ref.37 and the number of moles of air in the jar. These background concentrations were chosen to compare results with prior studies,25,35,36,38 although it should be noted that the uptake rates can be applied to different time periods by scaling the fluxes to recent tropospheric concentrations. Gross and net fluxes are reported as negative for uptake, positive for production. Unless otherwise specified, fluxes are reported in units of nanomoles per square meter per day (nmol m−2 d−1). For each monthly set of measurements, the fluxes are reported as the average ± 1 s.d. (1 σ) of nine separate flux values (three surface soil core experiments × three replicate flux measurements).2.6 Modeling soil consumption fluxes versus soil moisture and temperature
To model the effect of soil moisture or temperature on consumption rates, we apply a Gaussian distribution model, as used in Varner et al.39 | (1) |
 term was fitted as a constant d for all VWC:
term was fitted as a constant d for all VWC: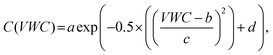 | (2) |
 | (3) |
3. Results
Sherman Island soils acted as an overall net source for CH3Cl and CHCl3 with annually averaged fluxes of 140 ± 266 nmol m−2 d−1 and 258 ± 288 nmol m−2 d−1, respectively. At the same time, soil samples were a net sink for CH3Br with averaged fluxes of −2.3 ± 4.5 nmol m−2 d−1. The monthly net flux measurements of CH3Cl, CH3Br, and CHCl3 for the soil core incubations are shown in Table 1, along with temperature, total carbon, total nitrogen, and soil moisture.| Date | Core | Soil Temp at 5 cm (°C) | Soil Total C (%) | Soil Total N (%) | Volumetric water content(%) | CH3Cl nmol m−2 d−1 | CH3Br nmol m−2 d−1 | CHCl3 nmol m−2 d−1 |
|---|---|---|---|---|---|---|---|---|
| 23 June 09 | 1 | 38.8 ± 2.1 | 6.4 ± 0.4 | 0.48 ± 0.02 | 9.5 | 572 ± 82 | 5.5 ± 2.2 | 19.9 ± 8.1 |
| 2 | 26.8 ± 1.7 | 15.5 ± 0.3 | 1.34 ± 0.02 | 23.4 | −62.4 ± 346.9 | 3.6 ± 3.4 | 18.2 ± 6.7 | |
| 3 | 33.2 ± 0.3 | 17.8 ± 0.6 | 1.34 ± 0.04 | 13.8 | −5.1 ± 73.7 | −6.2 ± 5.5 | 853 ± 493 | |
| 23 July 09 | 1 | 27.5 ± 0.4 | 9.7 ± 0.3 | 0.75 ± 0.02 | 7.0 | 408 ± 74.0 | 5.0 ± 0.6 | 329 ± 156 |
| 2 | 23.2 ± 0.7 | 16.4 ± 1.3 | 1.39 ± 0.11 | 16.6 | 71.0 ± 89.0 | 2.2 ± 4.0 | 150 ± 97.3 | |
| 3 | 29.3 ± 4.5 | 9.8 ± 0.03 | 0.69 ± 0.00 | 20.4 | −280 ± 29.8 | −7.5 ± 5.1 | 57.9 ± 36.7 | |
| 22 Aug 09 | 1 | 28.1 ± 0.3 | 7.1 ± 0.1 | 0.53 ± 0.01 | 13.8 | 1203 ± 132 | 7.1 ± 2.6 | 51.2 ± 49.5 |
| 2 | 26.8 ± 0.9 | 9.6 ± 0.04 | 0.75 ± 0.00 | 21.0 | −141 ± 67.9 | −13.4 ± 2.5 | 162 ± 147 | |
| 3 | 27.2 ± 1.1 | 9.1 ± 0.2 | 0.66 ± 0.01 | 22.7 | −62 ± 98.7 | −5.0 ± 4.0 | 224 ± 120 | |
| 25 Sep 09 | 1 | 25.5 ± 0.7 | 5.7 ± 0.01 | 0.43 ± 0.00 | 18.1 | 296 ± 72.7 | 5.2 ± 2.3 | 9.4 ± 1.6 |
| 2 | 24.6 ± 1.4 | 8.0 ± 0.3 | 0.60 ± 0.03 | 20.4 | −258 ± 51.9 | −5.2 ± 3.8 | 34.2 ± 22.9 | |
| 3 | 24.0 ± 0.8 | 12.0 ± 0.3 | 0.90 ± 0.02 | 24.4 | −201 ± 44.8 | −3.0 ± 0.2 | 67.2 ± 40.8 | |
| 23 Oct 09 | 1 | 20.6 ± 1.0 | 11.7 ± 0.06 | 0.90 ± 0.00 | 40.5 | 721 ± 145 | 1.5 ± 1.1 | 146 ± 36.8 |
| 2 | 19.6 ± 0.4 | 19.2 ± 0.6 | 1.74 ± 0.05 | 34.7 | 63.1 ± 42.3 | −4.8 ± 1.9 | 634 ± 141 | |
| 3 | 20.3 ± 0.8 | 13.8 ± 0.1 | 1.06 ± 0.02 | 42.8 | 503 ± 137.6 | −5.1 ± 1.9 | 58.6 ± 12.9 | |
| 22 Nov 09 | 1 | 12.3 ± 0.2 | 9.3 ± 0.2 | 0.71 ± 0.02 | 28.0 | 1050 ± 569 | 9.9 ± 5.9 | 103 ± 109 |
| 2 | 12.7 ± 0.1 | 8.3 ± 0.01 | 0.60 ± 0.01 | 33.9 | 185 ± 90.6 | −9.3 ± 0.6 | 833 ± 226 | |
| 3 | 12.8 ± 0.5 | 11.2 ± 0.01 | 0.85 ± 0.00 | 32.7 | 617 ± 182 | −5.1 ± 2.2 | 116 ± 83.2 | |
| 19 Dec 09 | 1 | 10.8 ± 0.1 | 6.0 ± 0.1 | 0.46 ± 0.01 | 47.1 | 5.8 ± 42.8 | −0.1 ± 0.7 | 27.9 ± 8.9 |
| 2 | 11.3 ± 0.3 | 9.6 ± 0.1 | 0.72 ± 0.00 | 46.0 | 49.9 ± 56.3 | −0.8 ± 0.9 | 145.2 ± 39.7 | |
| 3 | 11.3 ± 0.1 | 9.0 ± 0.2 | 0.67 ± 0.01 | 38.2 | 106 ± 27.1 | −0.8 ± 1.7 | 401 ± 210 | |
| 24 Jan 10 | 1 | 8.3 ± 0.2 | 6.0 ± 0.03 | 0.44 ± 0.00 | 41.5 | −3.4 ± 31.4 | −2.2 ± 1.4 | 431 ± 344 |
| 2 | 8.6 ± 0.3 | 15.8 ± 0.01 | 1.29 ± 0.00 | 53.8 | −79.4 ± 12.4 | −2.8 ± 1.8 | 3004 ± 1198 | |
| 3 | 8.5 ± 0.3 | 16.3 ± 0.2 | 1.32 ± 0.02 | 53.8 | −74.9 ± 8.4 | −0.9 ± 1.2 | 8.2 ± 5.0 | |
| 28 Feb 10 | 1 | 13.0 ± 0.8 | 12.8 ± 0.1 | 1.03 ± 0.01 | 52.1 | −16.1 ± 93.4 | −2.0 ± 4.6 | 58.8 ± 5.6 |
| 2 | 14.2 ± 0.8 | 13.4 ± 0.1 | 1.08 ± 0.01 | 49.1 | 3.8 ± 45.1 | −2.6 ± 1.4 | 32.6 ± 15.5 | |
| 3 | 14.3 ± 1.8 | 13.3 ± 0.3 | 1.06 ± 0.02 | 55.3 | −81.0 ± 24.1 | −2.5 ± 1.7 | 2.9 ± 8.6 | |
| 27 Mar 10 | 1 | 15.0 ± 0.2 | 10.6 ± 0.04 | 0.82 ± 0.00 | 25.6 | 95.8 ± 44.3 | −7.3 ± 2.4 | 193 ± 88.5 |
| 2 | 15.9 ± 0.3 | 25.1 ± 0.4 | 2.33 ± 0.03 | 29.0 | 203 ± 115 | −5.3 ± 2.9 | 288 ± 72.7 | |
| 3 | 17.8 ± 0.9 | 7.7 ± 0.2 | 0.53 ± 0.01 | 28.3 | −339 ± 72.8 | −14.7 ± 1.4 | 14.4 ± 28.2 | |
| 25 April 10 | 1 | 17.1 ± 0.2 | 7.1 ± 0.1 | 0.54 ± 0.01 | 32.9 | 1.7 ± 35.1 | 0.6 ± 1.3 | 11.2 ± 4.6 |
| 2 | 19.3 ± 0.7 | 14.3 ± 0.1 | 1.13 ± 0.01 | 31.0 | 140 ± 53.5 | −4.6 ± 3.8 | 92.5 ± 19.4 | |
| 3 | 20.4 ± 1.7 | 12.8 ± 0.2 | 1.00 ± 0.00 | 32.4 | 27.7 ± 23.1 | −7.7 ± 0.5 | 86.1 ± 61.9 | |
| 24 May 10 | 1 | 19.7 ± 0.4 | 5.8 ± 0.1 | 0.41 ± 0.01 | 16.6 | 95.9 ± 33.9 | −1.4 ± 2.0 | 91.9 ± 51.8 |
| 2 | 19.0 ± 0.7 | 12.4 ± 0.2 | 0.96 ± 0.02 | 18.0 | 269 ± 45.1 | −1.9 ± 2.0 | 593 ± 59.8 | |
| 3 | 19.5 ± 0.5 | 10.9 ± 0.2 | 0.79 ± 0.02 | 21.7 | −61.8 ± 19.7 | −2.7 ± 0.2 | 33.0 ± 39.8 |
Temporal variability in fluxes only partially accounts for the large standard deviations around the annually averaged net fluxes of CH3Cl, CH3Br, and CHCl3. Spatial variability of fluxes was also large among the three regions where the cores were taken. For example, the annual averaged net fluxes of CH3Cl for cores 1, 2, and 3 were 369 ± 430, 37.1 ± 154, and 12.4 ± 286 nmol m−2 d−1, respectively. For CH3Br, the observed average fluxes were 1.8 ± 4.8, −3.7 ± 4.6, and −5.1 ± 3.8 nmol m−2 d−1, respectively. For CHCl3, the observed average fluxes were 123 ± 134, 499 ± 834, and 159 ± 247 nmol m−2 d−1, respectively. There are significant differences of CH3Cl and CH3Br fluxes between cores 1 and 2 (t-test, t = 2.52, p = 0.0196 for CH3Cl and t = 2.87, p = 0.009 for CH3Br) and between cores 1 and 3 (t = 2.39, p = 0.0257 for CH3Cl and t = 3.88, p = 0.0008 for CH3Br). However, there is no significant difference between core 2 and core 3 flux values (t = 0.262, p = 0.7943 for CH3Cl and t = 0.794, p = 0.436 for CH3Br). The t test also shows that there are no significant differences of CHCl3 fluxes between the cores (t = 1.54, p = 0.1372 for core 1 and core 2, t = 0.443, p = 0.6612 for core 1 and core 3, and t = 1.35, p = 0.1893 for core 2 and core 3).
To explore the seasonal variability, net flux averages were determined for three seasonal periods: the dry season (May to August), the wet season (December to February), and the intermediate moist season (March to April and September to November). Soil moisture averaged 48.6 ± 4.3% VWC in the wet season, 16.8 ± 2.6% in the dry season, and 30.3 ± 6.7% in the intermediate moist season. Total carbon and nitrogen contents of the soil were very similar in magnitude for three different seasons (%C: 10.9 ± 2.1, 11.4 ± 2.8, and 11.8 ± 2.8, respectively and %N: 0.84 ± 0.19, 0.89 ± 0.24, and 0.94 ± 0.28 respectively).
Net CH3Cl fluxes were dominated by emission in the dry season (167.1 ± 118.4 nmol m−2 d−1) and intermediate moist season (207.2 ± 299.1 nmol m−2 d−1), and by consumption in the wet season (−9.9 ± 56.4 nmol m−2 d−1). In contrast, net CH3Br fluxes were dominated by consumption in all seasons: −1.7 ± 1.6, −1.6 ± 1.0, and −3.7 ± 3.2 nmol m−2 d−1 for dry, wet, and intermediate moist seasons, respectively. Unlike CH3Br, net CHCl3 fluxes were dominated by emissions in all three seasons, with average emission rates in the dry season (214.8 ± 65.9 nmol m−2 d−1) and in the intermediate moist season (179.1 ± 135.4 nmol m−2 d−1) roughly half compared to the wet season (444.2 ± 582.1 nmol m−2 d−1). Despite the seasonal variability, positive net fluxes of CHCl3 and negative net fluxes for CH3Br were observed throughout the year.
For both CH3Cl and CH3Br, the gross production and gross consumption fluxes were of similar magnitude, demonstrating how in each case the net fluxes are the sum of these two competing processes. Averaged gross production and consumption fluxes of CH3Cl were 779 ± 507 and −506 ± 176 nmol m−2 d−1, respectively. Averaged gross production and consumption fluxes of CH3Br were 6.9 ± 3.7 and −12.1 ± 4.0 nmol m−2 d−1, respectively (Fig. 1). This near balance of opposing gross fluxes produced variable net fluxes and was the reason why evaluating environmental controls on net fluxes proved to be challenging. In this case, seasonality affected both gross production and consumption fluxes in a similar way. Both production and consumption rates of CH3Cl and CH3Br were relatively high during the intermediate moist season (CH3Cl: 1084 ± 637 versus −636 ± 116 nmol m−2 d−1, CH3Br: 7.9 ± 5.2 versus −15.1 ± 2.5 nmol m−2 d−1); moderate during dry season (CH3Cl: 748 ± 158 versus −513 ± 115 nmol m−2 d−1, CH3Br: 7.3 ± 2.8 versus −11.8 ± 3.1 nmol m−2 d−1); and relatively small during the wet season (CH3Cl: 312 ± 54 versus −280 ± 69 nmol m−2 d−1, CH3Br: 4.8 ± 0.5 versus −7.3 ± 2.2 nmol m−2 d−1). These shifts in gross fluxes by season were presumably driven by soil temperature and water content, which will be discussed shortly. Gross production and consumption rates of CH3Cl and CH3Br showed no statistically significant correlations to soil total carbon and total nitrogen contents.
 | ||
Fig. 1 Annual gross and net fluxes of CH3Cl and CH3Br of soil samples collected from Sherman Island from June 2009 to May 2010. Here Month 1 is January and Month 12 is December. White circles (○) represent gross consumption rates, black circles (●) represent gross production rates, and gray triangles ( ) represent net fluxes. Each point represents the average ± 1 s.d. (1 σ) of 9 separate flux values (3 surface soil core experiments × 3 replicate flux measurements). ) represent net fluxes. Each point represents the average ± 1 s.d. (1 σ) of 9 separate flux values (3 surface soil core experiments × 3 replicate flux measurements). | ||
Flux correlations between CH3Cl and CH3Br were relatively weak for net flux (r2 = 0.4), moderate for gross production flux (r2 = 0.51), and strong for gross consumption flux (r2 = 0.97, p < 0.0001) (Fig. 2). The molar ratio of the gross consumption rates of CH3Cl and CH3Br was 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The net fluxes of CHCl3 (for which gross fluxes were not measured) did not correlate with the net fluxes of either CH3Cl or CH3Br.
1. The net fluxes of CHCl3 (for which gross fluxes were not measured) did not correlate with the net fluxes of either CH3Cl or CH3Br.
 | ||
Fig. 2 Gross and net fluxes of CH3ClversusCH3Br. Black outline circles (○) represent gross production rates, gray outline circles ( ) represent gross consumption rates and gray filled triangles ( ) represent gross consumption rates and gray filled triangles ( ) represent net fluxes. The dotted line and equations are for the linear regression between gross consumption of CH3Cl and CH3Br only. ) represent net fluxes. The dotted line and equations are for the linear regression between gross consumption of CH3Cl and CH3Br only. | ||
The gross production of CH3Cl and CH3Br did not show any correlation to soil moisture (data not shown), but the gross consumption rates of CH3Cl and CH3Br did appear to be influenced by soil moisture (Fig. 3). The gross consumption rates increased sharply at soil moistures from air dry to 20% VWC, with maximum rates observed between 20% and 30% VWC, and gradually decreased rates higher than 30% VWC. The constant temperature Gaussian models with the best goodness of fit had [a b c d] values of [1.3 27.8 13.7 0.2] for CH3Cl and [1.6 28.7 14.1 0.1] for CH3Br. The Gaussian models, however, showed a poor fit (r2 = 0.39 for both CH3Cl and CH3Br) owing to 8 instances of unusually high uptake rates at low soil moistures.
 | ||
| Fig. 3 Distribution of CH3Cl and of CH3Br consumption fluxes over soil volumetric water content (%VWC), error bars are 1 s.d. of 3 incubation experiments and typically smaller than the symbol size except where shown. White circles represent dry season, gray circles represent intermediate moist season, black circles represent wet season. The solid line is the Gaussian curve. | ||
Gross production rates of CH3Cl and CH3Br showed a positive linear correlation with temperature in the temperature manipulation experiment (Fig. 4). Among the three cores studied, the driest (14% VWC) showed the highest production rates of CH3Cl (1345 ± 837 nmol m−2 d−1) and CH3Br (13.8 ± 8.9 nmol m−2 d−1), with good correlations with temperature (r2 = 0.79, p = 0.003 for CH3Cl and r2 = 0.68, p = 0.01 for CH3Br). Lower production rates were observed in the two wetter (21–23% VWC) cores (averaged 659 ± 418 nmol m−2 d−1 CH3Cl and 4.0 ± 2.1 nmol m−2 d−1 CH3Br). Because of the poor gas diffusivity through water-filled pore spaces, it is not unusual for moist soil to have smaller emissions of soil produced gases to the atmosphere than dry soil.
 | ||
| Fig. 4 Gross production and consumption rates of CH3Cl and CH3Brversus soil temperature for 3 soil core studies from the August outing. Squares (□, ■) represent core 1 (VWC 14%), Circles (○, ●) represent core 2 (VWC 21%) and triangles (△, ▲) represent core 3 (VWC 23%). All black symbols represent production and white symbols represent consumption. The dashed lines are for linear regression between CH3Cl/CH3Br production flux and soil temperature and the solid lines are for the Gaussian curve between CH3Cl/CH3Br consumption flux and soil temperature. | ||
In contrast to the production rates, the effect of temperature on gross consumption rates was more pronounced on the wetter cores than the drier core. In fact, gross consumption rates were relatively small for the drier soil core and exhibited no obvious trend (Fig. 4).
The consumption responses for moist soil cores with VWC 21% and 23% were greatly influenced by temperature changes; the maximum consumption rates of CH3Cl and CH3Br were observed at 30 °C for both cores, declining at higher temperatures (Fig. 4). Thus the mechanism by which methyl halides are consumed in dry soil required a minimum level of soil moisture, consistent with the observed relationship for gross consumption versusVWC (Fig. 3).
The Gaussian models with the best goodness of fit for gross CH3Cl consumption as a function of temperature (eqn (3)) had [a h f g] constants of [2.3 32.5 18.4 6.4] and [2.0 34.5 16.0 5.8] for VWC 21% (r2 = 0.94, p < 0.0001) and 23% (r2 = 0.93, p = 0.0001), respectively. The best fits for gross CH3Br consumption versus temperature were [6.2 29.3 15.4 1.5] and [4.1 32.8 14.2 1.5], for VWC 21% (r2 = 0.94, p < 0.0001) and 23% (r2 = 0.83, p < 0.002), respectively.
4. Discussion
The surface (0–5 cm) soil core incubations in the current study represent the fluxes of methyl halides and chloroform expected between the top of the oxidized peat layer/mineral soils and the atmosphere, excluding the vegetation. An important uncertainty is the role of the deeper unoxidized peat layer in terms of halomethane gas production or consumption. However, a study conducted on a nearby drained peatland in the Sacramento-San Joaquin Delta showed that laboratory based incubations of surface soil cores yield similar magnitudes of CHCl3 fluxes as field flux chamber measurements conducted at the same sites where the cores were collected.38 If the underlying unoxidized peat layer played a significant role in ecosystem emissions, the field measurements of CHCl3 should yield very different results from the laboratory based incubations. For the current study, we assume that the top compacted layer of the peatland pasture dominates the surface-atmosphere exchange of methyl halides and chloroform whereas the deep underlying peat layer has minimal effect on their emission/consumption. This assumption will be further explored in a separate study of flux chamber measurements conducted on Sherman Island; however, that study, which focused on the role of vegetation, is beyond the scope of the present work.The consumption of CH3Cl and CH3Br apparently occurs through a mutual mechanism, as illustrated by the strong correlation in gross uptake rates. In addition, the molar ratio of gross consumption rates (44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for CH3Cl : CH3Br) is similar to a variety of nearby ecosystems in California, including annual grassland,35 oak-savanna woodland,25 and a rice field also located in the Sacramento-San Joaquin Delta.38 The lack of correlation between net CHCl3 fluxes with either CH3Cl or CH3Br suggests that CHCl3 was not produced or consumed through the same mechanisms as CH3Cl and CH3Br.
1 for CH3Cl : CH3Br) is similar to a variety of nearby ecosystems in California, including annual grassland,35 oak-savanna woodland,25 and a rice field also located in the Sacramento-San Joaquin Delta.38 The lack of correlation between net CHCl3 fluxes with either CH3Cl or CH3Br suggests that CHCl3 was not produced or consumed through the same mechanisms as CH3Cl and CH3Br.
Since the consumption of CH3Cl and CH3Br in soils normally occurs through oxidation by soil bacteria,8,40 the consumption response to temperature and soil moisture change should be significant when outside an optimum range. The results of the soil moisture and temperature manipulation experiments shows that both temperature and soil moisture are interdependent in their effects on the consumption rates of CH3Cl and CH3Br, with maximum consumption occurring between 20–30% VWC and 25–35 °C soil temperature. A previous study41 using agricultural soils also showed a maximum consumption of CH3Br at 25% VWC. Previous studies39,41 also showed variation of CH3Br consumption with temperature, with maximum uptake rates between 25 and 40 °C and much lower uptake rates between 5 and 15 °C. It is unclear whether temperature or soil moisture play a larger role in regulating gross uptake rates, although it should be noted that the range of uptake rates in specific soil cores between 10 and 35 °C is much smaller than the range of uptake rates observed between cores with different soil moistures. Soil moisture appears to be a much more dominant environmental factor than soil temperature in controlling biological uptake rates in an oak savannah in California,25 which also experiences a Mediterranean climate.
The production of CH3Cl and CH3Br in soils could be the result of biotic (e.g., through biosynthesis of halogenated metabolites by bacteria42 and through fungal processes43 associated with litter and/or plant roots44) or abiotic (e.g., oxidation of organic matter in the presence of iron and halide ions can produce methyl halides45). In this study, the source of emissions remains as yet undetermined. The higher emissions of both CH3Cl and CH3Br at the end of the dry season (October-November) may be a result of increased amounts of decomposing plant material providing more substrate for fungal growth. However, the linear increase of gross production in relation to temperature may be more consistent with abiotic production mechanisms.46
The emission of CHCl3 from drained pasture in the Sacramento-San Joaquin Delta of California was found higher in the wet season compared to dry season. This finding supports the Simmonds et al.21 observation of higher CHCl3 emission in saturated peatland soil and is consistent with a recent study of drained peatland soil at nearby Twitchell Island rice field38 which also showed that these soils can act as a source of CH3Cl.
The high humic substances content in peat soils47 may stimulate positive net fluxes of chloroform. The chloroform production may be mediated by chloroperoxidase enzymes that can catalyze the oxidation of inorganic chlorine in the presence of humic substances and hydrogen peroxide.48 Microorganisms producing halide-oxidizing exo-enzymes can produce HOCl which can react with soil organic matter to produce CHCl3.49 Also natural abiotic formation of CHCl3 in soils has been demonstrated in laboratory incubations of organic rich soils and organic substances believed to be constituents of humic substances.50
At the best of our knowledge, there are no previous flux measurements of halocarbons from peatlands that have been artificially drained. Nevertheless, we can compare the Sherman Island fluxes with other non-drained peatland studies (Table-2) such as the rice field on Twitchell Island,38 temperate peatlands at Mace Head, Ireland,16,51 temperate peatlands in New Hampshire, USA,19 sub-arctic wetland near Abisko, Sweden,18 and the Arctic tundra in Alaska, USA17 for a better understanding of the effect that draining peatlands has on trace gas fluxes. The flooded peatland studies at Mace Head Ireland showed higher net CH3Cl and CH3Br emission rates than the wet peatland studies in Sherman Island. The sub-arctic wetland at Abisko, Sweden also showed higher net CH3Cl emission rates with higher emissions observed at damp and dry condition.18 Because the Mace Head and Abisko measurements were performed in a peatland with vegetation, the plant species may have influenced the net emission of halocarbons from the ecosystem.
| Site | Season | CH3Cl | CH3Br | CHCl3 | Reference |
|---|---|---|---|---|---|
| na = Data not available. a Median values reported only. | |||||
| Sherman Island soil core, California, USA | Dry | 167 ± 118 | −1.7 ± 1.6 | 215 ± 66 | This study |
| Intermediate moist | 207 ± 299 | −3.7 ± 3.2 | 179 ± 135 | ||
| Wet | −9.9 ± 56.4 | −1.6 ± 1.0 | 444 ± 582 | ||
| Twitchell Island rice field soil core, California, USA | Wet | −11.8 ± 20.8 | 0.5 ± 0.7 | 10.0 ± 0.8 | 38 |
| Temperate peatlands, Mace Head, Ireland | Flooded | 287.5a | 26.0a | 105.6a | 16 |
| na | na | 88–2601 | 21 | ||
| na | na | 458–687 | 51 | ||
| Temperate peatlands, New Hampshire, USA | Flooded | na | −40 to (+50) | na | 19 |
| Sub-arctic wetland, Abisko, Sweden | Wet | 76.0 | na | na | 18 |
| Damp | 618 | na | na | ||
| Dry | 295 | na | na | ||
| Overall | 190 ± 760 | −6.3 ± 5.1 | na | ||
| Arctic wet coastal tundra, Alaska | Drained | −617 ± 43 | −9.8 ± 0.9 | 24.5 ± 4.6 | 17,24 |
| Moist | −483 ± 77 | −8.6 ± 1.3 | 74.3 ± 14.0 | ||
| Wet | −195 ± 57 | −2.1 ± 1.6 | 51.2 ± 10.4 | ||
| Flooded | −13.9 ± 3.9 | 1.1 ± 0.3 | 42 ± 7.3 |
A comparative study of the fluxes with and without vegetation in New Hampshire peatlands19 showed higher consumption of CH3Br in soil when there was no vegetation. In this study, the competing production and consumption rates of CH3Cl and CH3Br observed in temperate peatland soils are clarified through the measurement of gross fluxes. In Sherman Island, gross consumption of both compounds occurs throughout the year, but gross CH3Cl uptake is often times outweighed by gross production.
The annual CHCl3 emission rate in this study is higher than Arctic tundra and the Twitchell Island rice field, but lower than Irish peatlands. The annual average CHCl3 emission rate in the study (258 nmol m−2 d−1) is about half the rate observed by Simmonds et al.21 (585 nmol m−2 d−1), perhaps because their study measured fluxes from April to September, and fluxes were averaged only over the active season.
In addition, these results give an indication of how net fluxes of these halomethanes can be altered after temperate peatland ecosystems are drained. The aerobic environment created in a drained peatland pasture (Sherman Island) appears to promote higher consumption rates of both CH3Cl and CH3Br which would yield the observed differences in net fluxes compared to undrained peatland ecosystems studies in New Hampshire, USA19 and Mace Head, Ireland.16
5. Conclusion
The surface oxidized peat/mineral soils of the drained pasture in the Sacramento-San Joaquin Delta are found to be net sources of CH3Cl and CHCl3 and a net sink of CH3Br. Gross consumption dominates over gross production for CH3Br in these soils. The uptake rates of CH3Cl are not reflected in the net fluxes of CH3Cl because gross production predominates over gross consumption in these soils. Both gross production and consumption rates of CH3Cl and CH3Br during ‘shoulder seasons’ of intermediate moisture were much larger than those observed during either the dry or the wet months, presumably because dry conditions inhibit biological activity while flooded conditions inhibit gas diffusion in the soils. This is demonstrated by a Gaussian relationship between gross consumption rates of CH3Cl or CH3Brversus soil moisture, with maximum uptake rates between 20–30%. Gross production and gross consumption rates generally increase with temperature between 25 and 35 °C, although the effect of temperature on gross consumption is negligible under relatively dry (i.e., 14% VWC) soil moisture conditions. High net emission rates of CHCl3 were observed, averaging 258 ± 288 nmol m−2 d−1. These rates were larger than most other terrestrial ecosystems studied (e.g., temperate forests, shrublands, saltmarshes and tundra) but were less than other peatland ecosystems.Acknowledgements
We thank Y.-T. Chen, M. Song, P. Gierz, C. Dunn, D. Dualan, C. Chen, and K. Zhou for helping with sample collection and analysis. We also thank J. Verfaillie, D. Baldocchi and the support staff at Sherman Island for access to the field site.References
- S. A. Penkett, R. G. Derwent, P. Fabian, R. Borchers and U. Schmidt, Methyl chloride in the stratosphere, Nature, 1980, 283, 58–60 CrossRef CAS.
- S. M. Schauffler, E. L. Atlas, F. Flocke, R. A. Lueb, V. Stroud and W. Travnicek, Measurements of bromine containing organic compounds at the tropical tropopause, Geophys. Res. Lett., 1998, 25, 317–320 CrossRef CAS.
- S. A. Montzka, S. Reimann, Ozone-Depleting Substances (ODSs) and related chemicals, in Scientific Assessment of Ozone Depletion:2010 Chapter 1, Global Ozone Research and Monitoring Project, Report 52, pp. 1–108, World Meteorological Organization, Geneva, Switzerland, 2011 Search PubMed.
- I. Pisso, P. H. Haynes and K. S. Law, Emission location dependent ozone depletion potentials for very short-lived halogenated species, Atmos. Chem. Phys., 2010, 10, 12025–12036 CrossRef CAS.
- C. Clerbaux and D. M. Cunnold, Long-Lived Compounds. in Scientific Assessment of Ozone Depletion: 2006 (eds A-. L. N. Ajavon, D. L. Albritton, R. T. Watson) Chapter 1, World Meteorological Organization, Geneva, Switzerland, 2007 Search PubMed.
- F. Keppler, D. B. Harper, T. Rockmann, R. M. Moore and J. T. G. Hamilton, New insight into the atmospheric chloromethane budget gained using stable carbon isotope ratios, Atmos. Chem. Phys., 2005, 5, 2403–2411 CrossRef CAS.
- D. Serça, A. Guenther, L. Klinger, D. Helmig, D. Hereid and P. Zimmerman, Methyl bromide deposition to soils, Atmos. Environ., 1998, 32(9), 1581–1586 CrossRef.
- J. H. Shorter, C. E. Kolb, R. A. Kerwin, P. M. Crill, M. E. Hines, R. W. Talbot and R. C. Harriss, Rapid degradation of atmospheric methyl bromide in soils, Nature, 1995, 377, 717–719 CrossRef CAS.
- O. N. Singh and P. Fabian, Reactive bromine compounds, in The Handbook of Environmental Chemistry, Reactive Halogen Compounds in the Atmosphere, (eds P. Fabian and O. N. Singh), Vol 4E, pp. 1–43, Springer-Verlag Search PubMed.
- R. S. Oremland, L. G. Miller, C. W. Culbertson, T. L. Connell and L. Jahnke, Degradation of methyl bromide by methanotrophic bateria in cell suspensions and soils, Appl. Environ. Microbiol., 1994, 60(10), 3640–3646 CAS.
- K. D. Goodwin, J. K. Schaefer and R. S. Oremland, Bacterial oxidation of dibromomethane and methyl bromide in natural waters and enrichment cultures, Appl. Environ. Microbiol., 1998, 64, 4629–4636 CAS.
- L. G. Miller, T. L. Connell, J. R. Guidetti and R. S. Oremland, Bacterial oxidation of methyl bromide in fumigated agricultural soils, App. Environ. Microbiol., 1997, 63, 4346–4354 CAS.
- I. R. McDonald, K. L. Warner, C. McAnulla, C. A. Woodall, R. S. Oremland and J. C. Murrell, A review of bacterial methyl halide degradation: biochemistry, genetics and molecular ecology, Environ. Microbiol., 2002, 4(4), 193–203 CrossRef CAS.
- M. E. Rasche, M. R. Hyman and D. J. Arp, Biodegradation of halogenated hydrocarbon fumigants by nitrifying bacteria, Appl. Environ. Microbiol., 1990, 56(8), 2568–2571 CAS.
- L.–T. Ou, P. J. Joy, J. E. Thomas and A. G. Hornsby, Stimulation of microbial degradation of methyl bromide in soil during oxidation of an ammonia fertilizer by nitrifiers, Environ. Sci. Technol., 1997, 31(3), 717–722 CrossRef CAS.
- C. H. Dimmer, P. G. Simmonds, G. Nickless and M. R. Bassford, Biogenic fluxes of halomethanes from Irish peatland ecosystems, Atmos. Environ., 2001, 35(2), 321–330 CrossRef CAS.
- R. C. Rhew, Y. A. Teh and T. Abel, Methyl halide and methane fluxes in the northern Alaskan coastal tundra, J. Geophys. Res., 2007, B112, G02009 CrossRef.
- C. J. Hardacre, E. Blei and M. R. Heal, Growing season methyl bromide and methyl chloride fluxes at a sub-arctic wetland in Sweden, Geophys. Res. Lett., 2009, 36, L12401 CrossRef.
- M. L. White, R. K. Varner, P. M. Crill and C. H. Mosedale, Controls on the seasonal exchange of CH3Br in temperate peatlands, Global Biogeochem. Cycles, 2005, 19, GB4009 CrossRef.
- C. Zaccone, C. Cocozza, W. Shotyk and T. M. Miano, Humic acids role in Br accumulation along two ombrotrophic peat bog profiles, Geoderma, 2008, 31, 26–31 CrossRef.
- P. G. Simmonds, R. G. Derwent, A. L. Manning, S. O'Doherty and G. Spain, Natural chloroform emissions from the blanket peat bogs in the vicinity of Mace Head, Ireland over a 14-year period, Atmos. Environ., 2010, 44, 1284–1291 CrossRef CAS.
- J. Thompson, The settlement geography of the Sacramento-San Joaquin Delta, California, PhD Dissertation, Stanford University, Stanford, California, USA, 1957.
- J. Nickles, K. Taylor, and R. Fujii, California's Bay-Delta, USGS Science Supprots Decision Making: U.S. Geological Survey Fact Sheet 2010–3032, 2010, 4pp Search PubMed.
- R. C. Rhew, Y. A. Teh, T. Abel, A. Atwood and O. Mazéas, Chloroform emissions from the Alaskan Arctic tundra, Geophys. Res. Lett., 2008, 35, L21811 CrossRef.
- R. C. Rhew, C. Chen, Y. A. Teh and D. Baldocchi, Gross fluxes of methyl chloride and methyl bromide in a California oak-savanna ecosystem, Atmos. Environ., 2010, 44(16), 2054–2061 CrossRef CAS.
- B. F. Atwater, Attempts to correlate late Quaternary climatic records between San Francisco Bay, the Sacramento-San Joaquin Delta, and the Mokelumne River, California, PhD Dissertation, University of Delaware, Newark, DE, USA, 1980.
- N. P. Prokopovich, Subsidence of peat in California and Florida, Bull. Assoc. Eng. Geolog., 1985, 22, 395–420 Search PubMed.
- O. Sonnentag, M. Detto, B. R. K. Runkle, Y. A. Teh, W. L. Silver, M. Kelly and D. D. Baldocchi, Carbon dioxide exchange of a pepperweed (Lepidium latifolium L.) infestation: How do flowering and mowing affect canopy photosynthesis and autotrophic respiration?, J. Geophys. Res., 2011, 116, G01021 CrossRef.
- Y. A. Teh, W. L. Silver, O. Sonnentag, M. Detto, M. Kelly and D. D. Baldocchi, Large greenhouse gas emissions from a temperate peatland pasture, Ecosystems, 2011, 14(2), 311–325 CrossRef CAS.
- J. Z. Drexler, C. S. de Fontaine and S. J. Deverel, The legacy of wetland drainage on the remaining peat in the Sacramento-San Joaquin Delta, California, USA, Wetlands, 2009, 29, 372–386 CrossRef.
- B. F. Atwater, C. W. Hedel, and E. J. Helley, Late Quaternary depositional history, Holocene sea-level changes, and vertical crust movement, southern San Francisco Bay, California, US Geological Survey Professional Paper 1014, Washington, DC, 1977 Search PubMed.
- B. Runkle, Plant Water Use and Growth in Response to Soil Salinity in Irrigated Agriculture, Ph.D. Thesis, University of California, Berkeley, 2009.
- M. Detto, D. Baldocchi and G. G. Katul, Scaling properties of biologically active scalar concentration fluctuations in the atmosphere surface layer over a managed peatland, Boundary-Layer Meteorol., 2010, 136, 407–430 CrossRef.
- Y. A. Teh, R. C. Rhew, A. R. Atwood and T. Abel, Water, temperature and vegetation regulation of methyl chloride and methyl bromide fluxes from a shortgrass steppe ecosystem, Glob. Change Biol., 2008, 14, 77–91 Search PubMed.
- R. C. Rhew and T. Abel, Measuring simultaneous production and consumption fluxes of methyl chloride and methyl bromide in annual temperate grasslands, Environ. Sci. Technol., 2007, 41, 7837–7843 CrossRef CAS.
- R. C. Rhew, Sources and sinks of methyl bromide and methyl chloride in the tallgrass prairie: applying a stable isotope tracer technique over highly variable gross fluxes, J. Geophys. Res., 2011, 116, G03026 CrossRef.
- P. G. Simmonds, R. G. Derwent, A. J. Manning, P. J. Fraser, P. B. Krummel, S. O'Doherty, R. G. Prinn, D. M. Cunnold, R. Wang, D. B. Ryall, L. W. Porter, R. F. Weiss and P. K. Salameh, AGAGE observations of methyl bromide and methyl chloride at Mace Head, Ireland, and Cape Grim, Tasmania, 1998–2001, J. Atmos. Chem., 2004, 47, 243–269 CrossRef CAS.
- M. A. H. Khan, R. C. Rhew, M. E. Whelan, K. Zhou and S. J. Deverel, Methyl halide and chloroform emissions from a subsiding Sacramento-San Joaquin Delta island converted to rice fields, Atmos. Environ., 2011, 45(4), 977–985 CrossRef CAS.
- R. K. Varner, P. M. Crill, R. W. Talbot and J. H. Shorter, An estimate of the uptake of atmospheric methyl bromide by agricultural soils, Geophys. Res. Lett., 1999, 26(6), 727–730 CrossRef CAS.
- C. McAnulla, C. A. Woodall, I. R. McDonald, A. Studer, S. Vuilleumier, T. Leisinger and J. C. Murrell, Chloromethane utilization gene cluster from Hyphomicrobium chloromethanicum strain CM2T and development of functional gene probes to detect halomethane-degrading bacteria, Appl. Environ. Microbiol., 2001, 67, 307–316 CrossRef CAS.
- M. E. Hines, P. M. Crill, R. K. Varner, R. W. Talbot, J. H. Shorter, C. E. Kolb and R. C. Harriss, Rapid consumption of low concentrations of methyl bromide by soil bacteria, Appl. Environ. Microbiol., 1998, 64, 1864–1870 CAS.
- K.-H. van Pée and S. Unversucht, Biological dehalogenation and halogenation reactions, Chemosphere, 2003, 52, 299–312 CrossRef.
- D. B. Harper, Halomethane from halide ion-a highly efficient fungal conversion of environmental significance, Nature, 1985, 315, 55–57 CrossRef CAS.
- R. K. Varner, M. L. White, C. H. Mosedale and P. M. Crill, Production of methyl bromide in a temperate forest soil, Geophys. Res. Lett., 2003, 30(10), 1521 CrossRef.
- F. Keppler, R. Eiden, V. Niedan, J. Pracht and H. F. Schöler, Halocarbons produced by natural oxidation processes during degradation of organic matter, Nature, 2000, 403, 298–301 CrossRef CAS.
- L. Derendrop, R. Holzinger, A. Wishkerman, F. Keppler and T. Röckmann, Methyl chloride and C2–C5 hydrocarbon emissions from dry leaf litter and their dependence on temperature, Atmos. Environ., 2011, 45(18), 3112–3119 CrossRef.
- F. J. Stevenson, Humus Chemistry: Genesis, Composition, Reactions, John Wiley & Sons, New York, 1994 Search PubMed.
- E. J. Hoekstra, P. Lassen, J. G. van Leeuwen, E. W. B. de Leer, and L. Carlsen, Formulation of organic chlorine compounds of low molecular weight in the chloroperoxidase-mediated reaction between chloride and humic material, In Naturally produced organohalogens (eds A. Grimvall and E. W. B. Leer), Kluwer Academic Publishers, Dordrecht, 1995, pp 149–158 Search PubMed.
- C. N. Albers, Natural halogenated compounds in forest soils, PhD Dissertation, Roskilde University, Denmark, 2010.
- S. G. Huber, K. Kotte, H. F. Schöler and J. Williams, Natural abiotic formation of trihalomethanes in soil: Results from laboratory studies and field samples, Environ. Sci. Technol., 2009, 43, 4934–4939 CrossRef CAS.
- S. Biraud, P. Ciais, M. Ramonet, P. Simmonds, V. Kazan, P. Monfray, S. O'Doherty, G. Spain and S. G. Jennings, Quantification of carbon dioxide, methane, nitrous oxide, and chloroform emissions over Ireland from atmospheric observations at Mace Head, Tellus, 2002, 54B, 41–60 CAS.
| This journal is © The Royal Society of Chemistry 2012 |
