POCIS sampling in combination with ELISA: Screening of sulfonamide residues in surface and waste waters
Ivo
Černoch
a,
Milan
Fránek
a,
Iva
Diblíková
a,
Klára
Hilscherová
b,
Tomáš
Randák
c,
Tomáš
Ocelka
d and
Luděk
Bláha
*b
aVeterinary Research Institute, Hudcova 70, 62100 Brno, Czech Republic
bResearch Centre for Toxic Compounds in the Environment (RECETOX), Faculty of Science, Masaryk University, Kamenice 3, 62500 Brno, Czech Republic. E-mail: blaha@recetox.muni.cz; Fax: +420-549492840; Tel: +420-549493194
cUniversity of South Bohemia in Ceske Budejovice, Faculty of Fisheries and Protection of Waters, South Bohemian Research Center of Aquaculture and Biodiversity of Hydrocenoses, Vodnany, Czech Republic
dInstitute of Public Health in Ostrava, Ostrava, Czech Republic
First published on 17th November 2011
Abstract
Sulfonamide antibiotics coming from both human and veterinary medicine are among the most common emerging pollutants in freshwater. The present paper shows the successful application of passive sampling using POCIS in combination with an immunochemical ELISA technique and HPLC/MS/MS analysis to study the distribution of sulfonamides in streams around small towns in the Czech Republic, as well as around a major agglomeration of the city of Brno, including its waste water treatment plant (WWTP). Results indicated the presence of sulfonamides at most studied sites with concentrations ranging from <20 up to 736 ng of sulfamethoxazole equivalents per POCIS. Very high levels were detected in both the influent and effluent of the Brno WWTP with maxima > 8000 ng SMX per POCIS. All samplers collected down-stream of the studied towns and WWTPs clearly showed an increase in sulfonamide drug residues. Higher concentrations were determined in rivers at the city of Brno agglomeration. In agreement with other available studies, these findings indicate low efficiency of conventional WWTPs to eliminate polar pharmaceuticals such as sulfonamides. Good performance and correlation with the LC/MS results, as well as ease of use, indicate good potential for the immunochemical ELISA technique to become the screening tool for sulfonamide determination in surface waters including passive samplers.
Environmental impactThe present research contributes to the development and application of sampling and analytical methods for the monitoring of water contamination. We have successfully analysed levels of sulfonamide drug residues in environmental samples by combination of POCIS (polar organic compound integrated sampler) and ELISA (enzyme-linked immunosorbent assay), and the results are correlated with the standard LC/MS/MS method. Elevated concentrations of sulfonamide residues were determined in Czech river waters, especially below the outlets of the waste water treatment plants. The results show good potential of ELISA in combination with POCIS as a cost-effective screening tool for the environmental monitoring of emerging polar contaminants in water. |
Introduction
The conventional methods for the monitoring of surface water rely on discontinuous grab or composite sampling. These sampling approaches are shown to be effective for documenting the occurrence of pollutants but they only provide information about concentration levels at the time of sampling, and may miss episodic events such as a spill or storm related run-off, chemical inputs or the influence of precipitation. This can be addressed by collecting many representative samples over a time period, but it increases the financial requirements of the analyses.1–4On the other hand, novel passive sampling devices offer a cheaper and more effective procedure for measuring time-weighted average (TWA) analyte concentrations.5 Over the last two decades, passive sampling devices using various receiving phases have been employed successfully for continuous monitoring of pollutants in surface water. These include semipermeable membrane device (SPMD) samplers described by Huckins et al.,6 the Chemcatcher passive sampler with polar receiving phase4,7,8 and also the polar organic chemical integrative sampler—POCIS.9–12 So far, two general configurations of POCIS have been used, a generic design suitable for pesticides and virtually all hydrophilic organic contaminants (POCISpest), and the specific sampler design for sampling of pharmaceuticals (POCISpharm).
Chromatographic methods combined with various types of detectors have been commonly employed for environmental analyses using POCIS sampling.10,12–16 Although these systems allowed a specific and sensitive assay, they are relatively expensive and thus less suitable for screening large sample loads. Immunochemical methods such as enzyme-linked immunosorbent assays (ELISA) can provide a cost effective and fast screening alternative to instrumental techniques. Moreover, sufficient dilution of the concentrated POCIS extracts with assay buffer satisfies the conditions of the highly sensitive ELISA analysis with respect to low matrix effect interferences. ELISA has been used for direct screening of environmental samples,17 however its use in combination with passive sampling (especially POCIS) has been rarely reported.18,19
Sulfonamides are antimicrobial agents still widely used in human and veterinary medicine. The unchanged parent compounds and their metabolites are excreted from human and animal organisms and discharged to sewers. Then, sulfonamide residues mainly enter the aquatic environment after incomplete elimination during municipal waste water treatment. Although these compounds are normally present in the environment at low levels, they have a long life-time, can accumulate in organisms and may cause bacterial drug resistance.20,21 From the wide spectrum of sulfonamide drugs, sulfamethoxazole (SMX) followed by sulfapyridine and their acetyl metabolites are the most frequently detected compounds in the aquatic environment. SMX has been detected widely in European and American rivers and WWTP effluents in concentrations higher than 0.5 μg L−1.22 These findings also support results obtained by Tamtam et al.21 in the Seine river. They detected SMX in every sample with individual concentrations reaching 0.544 μg L−1. Similarly to SMX, concentrations of sulfapyridine also reached μg L−1 values in surface waters.23 The main source of sulfapyridine entering the municipal waste water is the administered pharmaceutical sulfasalazine.
In this work, an application of the ELISA kit for the determination of sulfonamide residues especially SMX in POCIS sample extracts is presented. Within the study, two environmental projects focused on the monitoring of sulfonamide drug residues in surface water sampled by POCIS technology in various localities of the Czech Republic. Besides the evaluation of the ELISA technique for sulfonamide screening in POCIS samples, an impact of the waste water treatment plants (WWTPs) at small towns as well as a major city on the quality of the aquatic environment is discussed too.
Materials and methods
Instrumentation
Passive sampler devices POCISpest and POCISpharm were purchased from ExposMeter AB (Sweden), and the sample concentrator DB-3D (Techne, United Kingdom) was applied to prepare extracts. Vortex MS3 basic (IKA Germany) was utilized as the sample mixing tool. Sulfamethoxazole ELISA kits (96T) for sulfonamide determination in samples were supplied by Abraxis LLC (USA). ELISA microtitre plates were washed with the auto strip washer ELX50 (Bio-Tec Instruments, USA). Ultra microplate reader EL 808 with KC4 v.3.1 software (Bio-Tec Instruments, USA) was used for the ELISA absorbance measurements and processing the results. A HTS PAL (CTC) autosampler, Rheos2000 (Flux) quaternary pump and TSQ Quantum Access™ (ThermoScientific, USA) triple quadrupole tandem mass spectrometer were used for reference analysis by LC-MS/MS.Site locations
Two environmental studies were conducted. The first project addressed the impact of small towns in the Czech Republic (4000–13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 inhabitants) on the water quality at the upper parts of several rivers (Fig. 1). For each site, samples with POCISpest and POCISpharm (about three weeks deployment) were collected up-stream and down-stream of the towns during 2008. The down-stream localities were located 50 to 300 m below the discharges of municipal WWTP. The second study focused on two rivers (Svratka and Svitava) in the major city of Brno, in the Czech Republic (400
000 inhabitants) on the water quality at the upper parts of several rivers (Fig. 1). For each site, samples with POCISpest and POCISpharm (about three weeks deployment) were collected up-stream and down-stream of the towns during 2008. The down-stream localities were located 50 to 300 m below the discharges of municipal WWTP. The second study focused on two rivers (Svratka and Svitava) in the major city of Brno, in the Czech Republic (400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 inhabitants). POCISpest samples were collected (21–28 days) at upstream and downstream sites from the WWTP at seven small towns. In addition, POCISpest samples were also deployed in the inlet and outlet of the WWTP (Fig. 1 insert).
000 inhabitants). POCISpest samples were collected (21–28 days) at upstream and downstream sites from the WWTP at seven small towns. In addition, POCISpest samples were also deployed in the inlet and outlet of the WWTP (Fig. 1 insert).
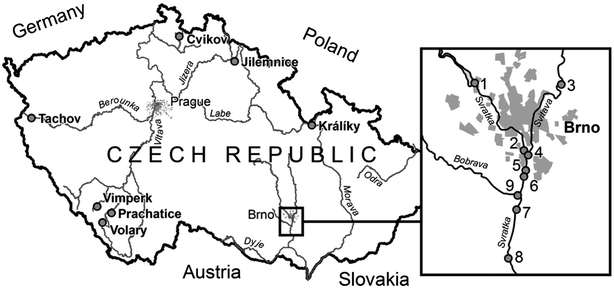 | ||
| Fig. 1 Map of the Czech Republic with sampling localities. Large map—position of seven small towns situated on the upper parts of the rivers (clock-wise: Cvikov, Jilemnice, Králíky, Volary, Prachatice, Vimperk, Tachov); Insert—city of Brno with nine sampling sites at rivers and the waste water treatment plant (WWTP): 1: Svratka river—Kninicky, 2: Svratka river before confluence, 3: Svitava river—Bilovice, 4: Svitava river before confluence, 5: WWTP influent, 6: WWTP effluent, 7: Svratka river—Rajhradice, 8: Svratka river—Zidlochovice, 9: Bobrava river. | ||
POCIS sampling
The POCIS consists of a hydrophilic microporous polyethersulfone membrane, which encloses the sequestration medium. For POCISpest, triphasic sorbent is used consisting of Isolute ENV+ polystyrene divinylbenzene (Argonaut Technologies, Redwood City, CA, USA) and Ambersorb 1500 carbon (Rohm and Haas, Philadelphia, PA, USA) dispersed on S-X3 Biobeads (200–400 mesh, Bio-Rad, Hercules, CA, USA). The POCISpharm variant contains Oasis HLB sorbent (Waters, Milford, MA, USA) designed to sample most of the pharmaceutical classes (24; ExposMeter, Sweden). Both POCISpest and POCISpharm devices (placed in the deployment apparatus, which was a stainless steel canister to protect samplers from bio-fouling and direct impact of the water flow) were installed submersed approximately 0.5 m below the water-level. After the exposure period, samplers were recovered, washed with clean water, sealed in airtight metal cans and transported to laboratories on ice. The samplers were stored at −18 °C until analysis.POCIS extraction
For analyses, sorbents were extracted and eluted using the appropriate solvent as described previously.9,25 Briefly, the residues sampled by POCISpharm were recovered by methanol and water (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 v/v) acidified with 0.1% trifluoroacetic acid, TFA. POCISpest sorbents were extracted by methanol
10 v/v) acidified with 0.1% trifluoroacetic acid, TFA. POCISpest sorbents were extracted by methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene
toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dichloromethane (1
dichloromethane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, v/v/v). The volumes of extracts were reduced by evaporation under a gentle stream of nitrogen to 1 mL, and for POCISpest the sample solvent was changed to pure methanol. The sample extracts were shaken for 20 s on vortex and diluted 100-fold with miliQ water before ELISA analyses.
8, v/v/v). The volumes of extracts were reduced by evaporation under a gentle stream of nitrogen to 1 mL, and for POCISpest the sample solvent was changed to pure methanol. The sample extracts were shaken for 20 s on vortex and diluted 100-fold with miliQ water before ELISA analyses.
ELISA analyses
The ELISA performance was carried out according to instructions for users attached in the SMX ELISA kit (Abraxis LLC, USA). 75 μL of calibration standards (0, 0.025, 0.05, 0.10, 0.25 and 1.00 μg L−1), control samples or analyzed samples were added in duplicates into wells on the microtitre plate followed by the addition of 50 μL of SMX antibody solution. Reagents in wells were shaken (30 s) and incubated for 20 min at room temperature (20–26 °C). After the incubation, 50 μL of enzyme conjugate was added into each well, the plate was shaken again and incubated for 40 min at room temperature. The content of the wells was vigorously shaken into a waste and the rest of the unbound compounds were removed by washing three times (using wash buffer, 350 μL per well). 150 μL of the substrate solution was added into each well. The enzymatic reaction was stopped after 30 min incubation at room temperature by the addition of 100 μL of stopping solution into the wells. The absorbance was measured at 450 nm and the concentration of the analyte in a POCIS extract was calculated from a calibration curve; the results were expressed as ng/POCIS.LC-MS analyses
100 μL of sample extract was mixed with 100 μL of water with a spike of a cocktail of internal standards, and the samples were analyzed with LC-MS/MS for sulfapyridin (SP) and sulfamethoxazol (SMX). The process blank samples were analyzed in parallel. The analytical column was a Phenomenex C18 Aqua (2 mm × 50 mm, 5 μm particles) coupled to a HTS PAL (CTC) autosampler, Rheos2000 (Flux) quaternary pump and TSQ Quantum Access™ triple quadrupole tandem mass spectrometer (ThermoScientific, USA). Two MS/MS transitions were monitored (where possible) for all native analytes to confirm identity. Isotope dilution and internal standard methods were used for the quantification of target compounds. An agreement of the calculated results better than 30% was accepted as a confirmed result. If one of the values was below the limit of quantification (<LOQ), such a limit was reported.Results and discussion
Traditional chromatographic methods linked with MS technologies are most frequently used for water analysis, they are well characterized and they can be employed for both screening and quantitative analysis.26 Immunochemical methods, namely ELISA, are cost-effective and can serve as the tools for the semi-quantitative screening of environmental samples when validity of the used immunochemical method is verified by comparison with instrumental methods. To our knowledge, the application of ELISA together with passive sampling has been rarely investigated,18,19 and sulfonamide screening by POCIS/ELISA has not been published until now.Analytical characterization of sulfonamide ELISA
In the present study, a commercial SMX ELISA Kit 96T (Abraxis L.L.C, USA) was used for the assessment of sulfonamides in the POCIS samples. A standard calibration curve was set up using SMX standards from 0.025 to 1.00 μg L−1, with corresponding ELISA absorbance values ranging normally from 1.6 to 0.2. The detection limit (LOD) determined in assay buffer was in compliance with the LOD stated in the kit (0.015 μg L−1). A control sample for SMX (0.2 μg L−1) was included in every assay run and treated in the same manner as unknown samples. The intra-assay variation coefficients ranged from 5 to 15%.An analytical recovery was estimated for 5, 10 and 50 ng of SMX added into eight POCIS methanol extracts diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 with assay buffer. The samples were then analysed by ELISA. Except sample No. 8, the recovery determined in the POCIS samples varied from 58 to 122% for 5 and 10 ng SMX fortifications (Table 1). A good recovery, around 100%, was achieved when 50 ng of SMX was added into POCIS extracts. The results demonstrate that the ELISA enables reliable detection of elevated SMX concentrations and other sulfonamides in POCIS sample extracts.
100 with assay buffer. The samples were then analysed by ELISA. Except sample No. 8, the recovery determined in the POCIS samples varied from 58 to 122% for 5 and 10 ng SMX fortifications (Table 1). A good recovery, around 100%, was achieved when 50 ng of SMX was added into POCIS extracts. The results demonstrate that the ELISA enables reliable detection of elevated SMX concentrations and other sulfonamides in POCIS sample extracts.
| POCIS sample | Endogenous sulfonamidesa | Fortified SMX | Recovery | Fortified SMX | Recovery | Fortified SMX | Recovery |
|---|---|---|---|---|---|---|---|
| (ng/POCIS) | (5 ng/POCIS) | (%) | (10 ng/POCIS) | (%) | (50 ng/POCIS) | (%) | |
| a SMX concentration equivalents | |||||||
| 1 | 2.11 | 6.14 | 81 | 8.95 | 68 | 49.9 | 96 |
| 2 | 6.77 | 12.9 | 122 | 14.5 | 78 | 54.3 | 95 |
| 3 | 11.1 | 15.3 | 84 | 16.9 | 58 | 57.7 | 93 |
| 4 | 14.6 | 18.2 | 73 | 20.6 | 61 | 60.8 | 93 |
| 5 | 25.0 | 33.6 | 172 | 33.4 | 85 | 72.3 | 95 |
| 6 | 9.48 | 14.2 | 94 | 16.1 | 66 | 55.1 | 91 |
| 7 | 7.75 | 13.0 | 105 | 16.2 | 85 | 58.3 | 101 |
| 8 | 38.0 | 47.6 | 191 | 52.4 | 143 | 90.1 | 104 |
Although the applied ELISA is intended primarily for SMX, a broad specific (generic) antibody is used in the kit, and it allows the determination of other sulfonamides as we have described previously.27 Thus, the assay is also highly sensitive to sulfamethoxypyridazine, sulfachloropyridazine and sulfadimethoxine with cross-reactivities based on SMX (=100%) 174.7%, 141.7% and 60.7%, respectively. Lower cross-reactivity was found for sulfamethizole (10.2%), sulfapyridine (3.4%) and sulfasalazine (3.2%), whereas other tested sulfonamides showed cross-reactivity below 1%. As ELISA can only provide screening results, the SMX concentration equivalents are introduced in this study to express measured sulfonamide levels.
Monitoring of sulfonamides in surface water sampled by POCIS technology
The POCIS samplers have been used previously for both qualitative and quantitative evaluation of pharmaceuticals, pesticides and hormones in surface waters.2 From the two available configurations, POCISpharm seems to be most suitable for the sampling of sulfonamide drug residues in surface water. On the other hand, most of the sulfonamides are polar substances and they can be bound also on the triphasic sorbent incorporated in the POCISpest sampler, which is designed for analyses of general hydrophilic organic contaminants. The final recovery from both sorbents may be different, although there is significant overlap in the sequestration of chemicals by both POCIS devices.24In this study, two environmental projects focused on the monitoring of sulfonamide drug occurrence in surface water sampled by POCIS technology in various localities of the Czech Republic. Although the ELISA used in this work is intended mainly for SMX, due to the generic properties of the antibody higher concentrations of other sulfonamides could also be detected.
 | ||
| Fig. 2 Sulfamethoxazole (SMX) concentration equivalents determined by ELISA in POCISpest and POCISpharm sample extracts collected up-stream and down-stream of seven small towns. | ||
With the exception of Vimperk town, SMX concentrations were always elevated in rivers down-stream of the town (Fig. 2). The highest values in both POCISpharm and POCISpest extracts (335 and 210 ng of SMX equivalents per POCIS) were found below the town of Prachatice, where more than 30-times higher levels were detected down-stream of the town in comparison to the up-stream site. At other localities, SMX equivalents did not exceed 100 ng per POCIS. These results clearly indicate significant contributions of small towns (including the WWTPs) to contamination of surface and ground waters by sulfonamides as confirmed also in our further project around the city of Brno (see below) as well as in other studies.28–30
| Sampling localities | Spring 2007 | Autumn 2007 | |||||
|---|---|---|---|---|---|---|---|
| ELISA | LC-MS/MS | ELISA | LC-MS/MS | ||||
| SMX | SP | SMX | SP | ||||
| SMX equivalents | (ng/POCIS) | (ng/POCIS) | SMX equivalents | (ng/POCIS) | (ng/POCIS) | ||
| 1 | Svitava, Bílovice n. Svit. | 277 | 108 | 272 | 198 | 435 | 374 |
| 2 | Svitava, confluence | 248 | 71 | 145 | 150 | 401 | 308 |
| 3 | Svratka, Kníničky | <20 | 47 | 16 | 129 | 115 | 33 |
| 4 | Svratka, confluence | na* | na | na | 200 | 75 | 29 |
| 5 | WWTP Modřice - inlet | 1949 | 410 | 480 | 1756 | 666 | 251 |
| 6 | WWTP Modřice - outlet | 5630 | 2632 | 2060 | 8174 | 5735 | 3324 |
| 7 | Svratka, Rajhradice | na | na | na | 176 | 673 | 323 |
| 8 | Svratka, Nosislav | 421 | 293 | 203 | 216 | 581 | 290 |
| 9 | Bobrava, Popovice | 100 | 222 | 258 | 215 | 669 | 224 |
| Sampling localities | Spring 2008 | Autumn 2008 | |||||
|---|---|---|---|---|---|---|---|
| ELISA | LC-MS/MS | ELISA | LC-MS/MS | ||||
| SMX | SP | SMX | SP | ||||
| SMX equivalents | (ng/POCIS) | (ng/POCIS) | SMX equivalents | (ng/POCIS) | (ng/POCIS) | ||
| 1 | Svitava, Bílovice n. Svit. | na | na | na | 586 | 128 | 170 |
| 2 | Svitava, confluence | <20 | 87 | 71 | na | na | na |
| 3 | Svratka, Kníničky | <20 | 30 | 14 | 88 | 23 | 19 |
| 4 | Svratka, confluence | na | na | na | 384 | 17 | 14 |
| 5 | WWTP Modřice - inlet | 1929 | 1457 | 898 | 1196 | 359 | 375 |
| 6 | WWTP Modřice - outlet | 5084 | 3514 | 2223 | 4795 | 2243 | 1551 |
| 7 | Svratka, Rajhradice | 293 | 160 | 99 | 637 | 461 | 336 |
| 8 | Svratka, Nosislav | 469 | 333 | 177 | 736 | 506 | 334 |
| 9 | Bobrava, Popovice | <20 | 106 | 136 | 242 | 216 | 334 |
This observation may be partially attributed to different hydromorphology and land use at both river basins. The Svitava river flows through a rather flat and populated region (towns and villages) with intensive agriculture, while Svratka passes through diversified highlands with forests, pastures and also agricultural activities. However, the Svratka river is also substantially affected by municipal waste waters (both treated and untreated) from towns and villages. The difference between up-stream localities at both rivers may also be attributed to two large water reservoirs situated at Svratka river, which could contribute to the scavenging of sulfonamides from water.
As further shown in Table 2, markedly high concentrations of sulfonamide drug residues could be detected in WWTP samples (thousands ng of SMX equivalents per POCIS). Elevated concentrations of SMX equivalents could also be found in the Svratka river below the WWTP outlet (Rajhradice site; especially pronounced during 2008 samplings—Table 2). Lower SMX values were determined at Rajhradice and Nosislav in autumn 2007 as well as in the Bobrava river, a small tributary which is less impacted by human activities.
A number of studies have documented that sulfonamide antibiotics are among the priority emerging contaminants that have been detected in both surface and groundwaters, employing both active and passive sampling techniques.28–30 Both studies discussed in the present paper also showed a systematic increase in concentrations of sulfonamides at river samples down-stream of cities and also in WWTP effluents, which corresponds to findings all over the world. It has been reported that approximately 30% of sulfonamides (especially SMX) might not be degraded during the primary clarification and biological treatment processes at WWTPs due to their hydrophilic character,22 while hydrophobic compounds are usually effectively separated based on phase partitioning.31 In addition, the elimination efficiency of the polar substances could be influenced by rainfall events, which may increase discharges of untreated wastewaters.21
Göbel et al.28 studied elimination of selected pharmaceuticals at WWTPs, and showed that the primary treatment did not provide significant elimination of sulfonamides, and the secondary treatment of two conventional activated sludge systems resulted in highly variable removal rates. Incomplete eliminations of sulfonamides were observed in 4 out of 7 sampling periods with 41–72% removal for sulfapyridine and 9–60% for SMX. During the remaining 3 sampling periods, there were no changes or even a substantial increase in pharmaceuticals at the outflow (>2-times for SMX).28 The application of the tertiary treatment step (sand filtration) also did not significantly decrease the total sulfonamide loads.28
Regarding the samples at the city of Brno WWTP, lower SMX concentration equivalents found in the inflow (compared to the effluent; Table 2) could be attributed to lower POCIS sampling efficiencies in extremely contaminated inflow waste water.32 However, some studies showed less important effects of biofouling on the uptake of both hydrophobic and polar compounds.33,34 Nevertheless, some other studies also showed higher sulfonamide concentrations in the WWTP effluents than in the influents,35,36 and possible retransformation of the main N(4)-acetylated metabolites to the active parent sulfonamides during the wastewater treatment has been discussed. It should be noted that the ELISA employed in the present study does not enable detecting of the acetylated sulfonamides, which can also explain the elevated sulfonamide levels in the effluent. Our findings also support studies of Göbel et al.,28 where a high elimination rate or N(4)-acetylated-SMX lead to observed high variability in the elimination rate of SMX from wastewater.
 | ||
Fig. 3 Correlations between ELISA and LC-MS results. Panels A, B—all data (N = 32, including WWTP samples); C, D - river water data only (WWTP samples excluded, N = 24). Panels A + C—correlations between ELISA SMX equivalents and SMX concentrations detected by LC-MS; panels B + D—ELISA SMX equivalents vs. sum of SMX + SP concentrations. Continuous line—regression of ELISA vs.LC-MS-MS; dashed line—1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 match curve. 1 match curve. | ||
When assessing the regression coefficient between the ELISA SMX equivalent and the LC-MS data for SMX, ELISA seemed to slightly overestimate results (slope value = 0.59, Fig. 3A). However, very good correlation close to 1 (slope = 0.97) was found when ELISA SMX equivalents were compared with the sum of sulfonamides (SMX + SP) determined with LC-MS. Similarly good performance was also found in our recent study addressing atrazine concentrations in waters.37 This demonstrates that SMX ELISA may be used as a screening analytical tool, which clearly discriminates low and highly contaminated samples.
However, it should be critically pointed out that correlation values were affected by high values detected in the WWTP samples. As it is apparent from Fig. 3C, D with river water samples only (WWTP data excluded), much weaker correlations and regressions between ELISA and LC-MS results were detected. As discussed above, ELISA antibodies are able to detect several different sulfonamide antibiotics with different cross-reactivity. This could result in the variability when comparing an integrative response of ELISA to the sulfonamide mixture (SMX equivalents) to individual analytes (SMX and/or SP).
Conclusions
The determination of time-weighted average (TWA) concentrations with grab sampling methods might be time consuming and expensive, and passive samplers such as POCIS have been explored as a suitable alternative. In this work, the application of the ELISA technique in combination with POCIS sampling technology has been studied. The results of two projects focusing on the monitoring of sulfonamide drug residues in streams, rivers and WWTP samples indicated considerable variability in sulfonamide levels upstream and downstream of small towns and around the major city. The determined SMX concentration equivalents were found to be markedly higher in rivers at the Brno agglomeration compared to smaller towns. All samples collected down-stream of the studied towns and WWTPs clearly showed an increase in sulfonamide drug residues. In agreement with other available studies, these findings indicate low efficiency of conventional WWTPs to eliminate the polar pharmaceuticals such as sulfonamides from the municipal waste water. Very good correlations between ELISA and LC-MS analyses were detected when considering full data sets including WWTP samples. Good performance of the applied SMX ELISA kit, its efficiency and ease of use indicate good potential of the immunochemical technique to become the screening tool for sulfonamide determination in water, including passive samples.Acknowledgements
This work was supported by grants from the Ministry of Education, Youth and Sports of the Czech Republic (2B08036 “ENVISCREEN” and MSM0021622412 “INCHEMBIOL”) and by infrastructural projects of the European Regional Development Fund (CETOCOEN No. CZ.1.05/2.1.00/01.0001, AdmireVet No. CZ.1.05/2.1.00/01.0006-ED00060101, CENAKVA No. CZ.1.05/2.1.00/01.0024).References
- D. A. Alvarez, W. L. Cranor, S. D. Perkins, R. C. Clark and S. B. Smith, J. Environ. Qual., 2008, 37, 1024–1033 CrossRef CAS.
- S. L. Bartelt-Hunt, D. D. Snow, T. Damon, J. Shockley and K. Hoagland, Environ. Pollut., 2009, 157, 786–791 CrossRef CAS.
- L. Chimuka, T. Nemutandani, E. Cukrowska and H. Tutu, J. Environ. Monit., 2008, 10, 129–135 RSC.
- R. Gunold, R. B. Schafer, A. Paschke, G. Schuurmann and M. Liess, Environ. Pollut., 2008, 155, 52–60 Search PubMed.
- R. V. Hyne and M. Aistrope, Chemosphere, 2008, 71, 611–620 CrossRef CAS.
- J. N. Huckins, M. W. Tubergen and G. K. Manuweera, Chemosphere, 1990, 20, 533–552 CrossRef CAS.
- R. B. Schafer, A. Paschke, B. Vrana, R. Mueller and M. Liess, Water Res., 2008, 42, 2707–2717 Search PubMed.
- E. L. M. Vermeirssen, N. Bramaz, J. Hollender, H. Singer and B. I. Escher, Water Res., 2009, 43, 903–914 Search PubMed.
- D. A. Alvarez, J. D. Petty, J. N. Huckins, T. L. Jones-Lepp, D. T. Getting, J. P. Goddard and S. E. Manahan, Environ. Toxicol. Chem., 2004, 23, 1640–1648 CrossRef CAS.
- T. L. Jones-Lepp, D. A. Alvarez, J. D. Petty and J. N. Huckins, Arch. Environ. Contam. Toxicol., 2004, 47, 427–439 CrossRef CAS.
- J. Kohoutek, B. Marsalek and L. Blaha, Anal. Bioanal. Chem., 2010, 397, 823–828 CrossRef CAS.
- A. Togola and H. Budzinski, Anal. Chem., 2007, 79, 6734–6741 CrossRef CAS.
- D. A. Alvarez, W. L. Cranor, S. D. Perkins, V. L. Schroeder, L. R. Iwanowicz, R. C. Clark, C. P. Guy, A. E. Pinkney, V. S. Blazer and J. E. Mullican, Environ. Toxicol. Chem., 2009, 28, 1084–1095 CrossRef CAS.
- S. L. Macleod, E. L. Mcclure and C. S. Wong, Environ. Toxicol. Chem., 2007, 26, 2517–2529 CrossRef CAS.
- N. Mazzella, J. F. Dubernet and F. Delmas, J. Chromatogr., A, 2007, 1154, 42–51 CrossRef CAS.
- Z. L. Zhang, A. Hibberd and J. L. Zhou, Anal. Chim. Acta, 2008, 607, 37–44 CrossRef CAS.
- W. L. Shelver, N. W. Shappell, M. Franek and F. R. Rubio, J. Agric. Food Chem., 2008, 56, 6609–6615 CrossRef CAS.
- T. Wuske, I. Fittkau, J. Mahn, R. Polzius and A. Manns, Anal. Chim. Acta, 1998, 359, 321–328 CrossRef CAS.
- T. Rundberget, E. Gustad, I. A. Samdal, M. Sandvik and C. O. Miles, Toxicon, 2009, 53, 543–55 CrossRef CAS.
- W. Baran, J. Sochacka and W. Wardas, Chemosphere, 2006, 65, 1295–1299 CrossRef CAS.
- F. Tamtam, F. Mercier, B. Le Bot, J. Eurin, Q. T. Dinh, M. Clement and M. Chevreuil, Sci. Total Environ., 2008, 393, 84–95 CrossRef CAS.
- X. Peng, J. Tan, C. Tang, Y. Yu and Z. Wangt, Environ. Toxicol. Chem., 2008, 27, 73–79 CrossRef CAS.
- M. S. Diaz-Cruz, M. J. Garcia-Galan and D. Barcelo, J. Chromatogr., A, 2008, 1193, 50–59 CrossRef CAS.
- D. A. Alvarez, P. E. Stackelberg, J. D. Petty, J. N. Huckins, E. T. Furlong, S. D. Zaugg and M. T. Meyer, Chemosphere, 2005, 61, 610–622 CrossRef CAS.
- J. Kohoutek, P. Babica, L. Blaha and B. Marsalek, Anal. Bioanal. Chem., 2008, 390, 1167–1172 CrossRef CAS.
- W. W. Buchberger, J. Chromatogr., A, 2011, 1218, 603–618 Search PubMed.
- M. Franek, I. Diblikova, I. Cernoch, M. Vas and K. Hruska, Anal. Chem., 2006, 78, 1559–1567 CrossRef CAS.
- A. Gobel, C. S. McArdell, A. Joss, H. Siegrist and W. Giger, Sci. Total Environ., 2007, 372, 361–371 CrossRef.
- H. X. Li, P. A. Helm and C. D. Metcalfe, Environ. Toxicol. Chem., 2010, 29, 751–762 Search PubMed.
- R. Loos, G. Locoro, S. Comero, S. Contini, D. Schwesig, F. Werres, P. Balsaa, O. Gans, S. Weiss, L. Bláha, M. Bolchi and B. M. Gawlik, Water Res., 2010, 44, 4115–4126 CrossRef CAS.
- R. Muller, J. Y. M. Tang, R. Thierb and J. F. Mueller, J. Environ. Monit., 2007, 9, 105–109 RSC.
- P. P. Ballesta, E. G. Ferradás and A. M. Aznar, Chemosphere, 1992, 25, 1797–1809 CrossRef CAS.
- C. Harman, O. Boyum, K. V. Thomas and M. Grung, Environ. Toxicol. Chem., 2009, 28, 2324–2332 CrossRef CAS.
- K. Booij, R. van Bommel, A. Mets and R. Dekker, Chemosphere, 2006, 65, 2485–2492 CrossRef CAS.
- H. Chang, J. Y. Hu, M. Asami and S. Kunikane, J. Chromatogr., A, 2008, 1190, 390–393 CrossRef CAS.
- I. Senta, S. Terzic and M. Ahel, Chromatographia, 2008, 68, 747–758 CrossRef CAS.
- I. Černoch, M. Fránek, I. Diblíková, K. Hilscherová, T. Randák, T. Ocelka and L. Bláha, J. Environ. Monit., 2011, 13, 2582–2587 RSC.
| This journal is © The Royal Society of Chemistry 2012 |
