Composition and source apportionment of PAHs in sediments at river mouths and channel in Kaohsiung Harbor, Taiwan
Chiu-Wen
Chen
*a,
Chih-Feng
Chen
a,
Cheng-Di
Dong
a and
Yao-Ting
Tu
b
aDepartment of Marine Environmental Engineering, National Kaohsiung Marine University, Kaohsiung, 81143, Taiwan, Republic of China. E-mail: cwchen@mail.nkmu.edu.tw; Fax: +886-7-365-0548; Tel: +886-7-3617141-3762
bDepartment of Marine Environmental Engineering, National Kaohsiung Marine University, Kaohsiung, 80424, Taiwan, Republic of China
First published on 9th November 2011
Abstract
Fifty-eight sediment samples were collected in 2009 from the bottom of river mouths near Kaohsiung Harbor (Taiwan) and the harbor channel for the analyses of polycyclic aromatic hydrocarbons (PAHs) using gas chromatography-mass spectrometry (GC-MS). Concentrations of total PAHs varied from 39 to 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 521 ng g−1 (dry weight); samples collected from the mouths of Love River, Canon River, Jen-Gen River, and Salt River showed the highest PAHs concentrations. This indicates that the major sources of sediment PAHs come from those polluted urban rivers and the harbor channel. In samples collected from the Salt River mouth, approximately 43% of the PAHs are identified as PAHs with 2 or 3 rings. However, samples collected from other locations contain predominantly PAHs with 4 rings (32 to 42%) or 5 and 6 rings (36 to 44%). Emissions from traffic-related sources and waste incineration contribute to the majority of PAHs found in most channel and river mouth sediments. However, coal/oil combustion is the main cause of high concentrations of PAHs observed in the Salt River mouth sediments. Principal component analyses with multivariate linear regression (PCA/MLR) have been used to further quantify the source contributions, and the results show that the contributions of coal/oil combustion, traffic-related and waste incineration are 37%, 33% and 30%, respectively.
521 ng g−1 (dry weight); samples collected from the mouths of Love River, Canon River, Jen-Gen River, and Salt River showed the highest PAHs concentrations. This indicates that the major sources of sediment PAHs come from those polluted urban rivers and the harbor channel. In samples collected from the Salt River mouth, approximately 43% of the PAHs are identified as PAHs with 2 or 3 rings. However, samples collected from other locations contain predominantly PAHs with 4 rings (32 to 42%) or 5 and 6 rings (36 to 44%). Emissions from traffic-related sources and waste incineration contribute to the majority of PAHs found in most channel and river mouth sediments. However, coal/oil combustion is the main cause of high concentrations of PAHs observed in the Salt River mouth sediments. Principal component analyses with multivariate linear regression (PCA/MLR) have been used to further quantify the source contributions, and the results show that the contributions of coal/oil combustion, traffic-related and waste incineration are 37%, 33% and 30%, respectively.
Environmental impactPolycyclic aromatic hydrocarbons (PAHs) are one of the most important classes of organic contaminants in marine environments. Several PAHs are known carcinogens and/or mutagens or precursors to carcinogenic daughter compounds. The effect of PAHs is usually widespread and permanent in environmental media. Most PAHs have high hydrophobicity, and can be sorbed strongly by water-borne organic and inorganic particles. They may eventually sink down to the bottom sediment in the aquatic system; the PAHs found in the sediment are resistant to bacterial degradation in an anoxic environment. Even under favorable conditions, the sorbed PAHs will be released to the water as an extended source to threaten the aquatic ecosystem through bioaccumulation in food chains. Thus, understanding the contributions of the various sources is essential and important for appropriately managing PAH levels in the environment. |
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are one of the most important classes of organic contaminants in marine environments. Several PAHs are known to be carcinogens and/or mutagens or precursors to carcinogenic daughter compounds.1,2 The effect of PAHs is usually widespread and permanent in environmental media. Most PAHs have high hydrophobicity, and can be sorbed strongly by water-borne organic and inorganic particles. They may eventually sink down to the bottom sediment in the aquatic system; the PAHs found in the sediment are resistant to bacterial degradation in an anoxic environment. Even under favorable conditions, the sorbed PAHs will be released to the water as an extended source to threaten the aquatic ecosystem through bioaccumulation in food chains.3 Thus, understanding the contributions of the various sources is essential and important for appropriately managing PAHs levels in the environment.The major sources of PAHs may be either natural or anthropogenic. According to the formation mechanisms, PAHs found in marine sediments can be classified as pyrolytic or petrogenic; the pyrolytic type is usually prevalent in aquatic environments.4,5 Pyrolytic PAHs, characterized by a predominance of parent compounds with four or more aromatic rings, are produced during the combustion of all organic materials. However, petrogenic PAHs, compounds with two to three aromatic rings, are contained in petroleum and its products. The molecular indices based on the ratio of selected PAHs concentrations in sediments can be used to assess the origin of PAHs, i.e. pyrogenic or petrogenic. The ratio of some isomers (e.g., phenanthrene/anthracene, fluoranthene/pyrene, fluoranthene/(fluoranthene + pyrene)) have been widely used to distinguish PAHs from diverse origins.6–11 In addition, diagnostic ratios of benzo[a]anthracene/chrysene, benzo[b]fluoranthene/benzo[k]fluoranthene, benzo[a]pyrene/benzo[e]pyrene and indeno[1,2,3-cd]pyrene/benzo[g,h,i]perylene have been applied to identify specific types of combustion, such as vehicle exhaust, coal/coke combustion, forest fires, and smelters.9,12 However, the use of these diagnostic ratios is limited in qualitative analyses because the results are not reliable. Recently, more sophisticated statistical methods have been used to further carry out quantitative sources apportionment of PAHs found in a natural environment. Principal component analyses with multivariate linear regression (PCA/MLR) is one of such statistical methods for identifying and apportioning the sources of PAHs found in the air, soil, and sediment by many researchers.13–16
Kaohsiung Harbor, the largest international port in Taiwan located on the southwestern coast of Taiwan, faces the key trade waterway running through the Taiwan Strait and Bashi Channel. More than 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 inbound and outbound vessels used the harbor every year between 2000 and 2009, and its container traffic volume ranked the 13th highest in the world.17 The harbor has 118 docks including several industrial zone docks, fishing ports, and shipyards. Moreover, the port receives water flows from four contaminated rivers, i.e. Love River, Canon River, Jen-Gen River, and Salt River. These rivers flow through the heart of metropolitan Kaohsiung City which is the largest industrial city in Taiwan with a population of over 1.5 million. In other words, the harbor receives domestic, agricultural and industrial wastewater runoffs from upstream sections of those rivers.18 Thus, it is essential to investigate the pollution sources and their environmental impacts on Kaohsiung Harbor.
000 inbound and outbound vessels used the harbor every year between 2000 and 2009, and its container traffic volume ranked the 13th highest in the world.17 The harbor has 118 docks including several industrial zone docks, fishing ports, and shipyards. Moreover, the port receives water flows from four contaminated rivers, i.e. Love River, Canon River, Jen-Gen River, and Salt River. These rivers flow through the heart of metropolitan Kaohsiung City which is the largest industrial city in Taiwan with a population of over 1.5 million. In other words, the harbor receives domestic, agricultural and industrial wastewater runoffs from upstream sections of those rivers.18 Thus, it is essential to investigate the pollution sources and their environmental impacts on Kaohsiung Harbor.
The objectives of this study are to examine the spatial distribution, composition and source of PAHs in the sediment of Kaohsiung Harbor, and carry out quantitative sources apportionment of PAHs using source apportionment methods, and principal component analyses with multivariate linear regression (PCA/MLR). This study provides valuable information to be referenced by engineers, planners and officials for managing PAHs levels in Kaohsiung Harbor.
2. Materials and methods
2.1 Sampling strategy
Sediment samples were collected from four river mouths near Kaohsiung Harbor, and the harbor channel (Fig. 1). The fifty-eight sampling stations selected in this study included harbor channel stations (K1–K14), Love River mouth stations (L1–L10), Canon River mouth stations (C1–C12), Jen-Gen River mouth stations (J1–J11), and Salt River mouth stations (S1–S11). The channel stations cover all the main waterway of the Harbor. The four rivers contribute to increased pollution by carrying pollutants from the land sources into the Harbor. Therefore, some of the harbor stations, 10 to 12 stations, were selected for sampling each of the four river mouths. Table 1 lists the location, grain size (sand, silt, and clay) distribution, organic matter (OM), total organic carbon (TOC), and total grease (TG) content of the sediment samples collected.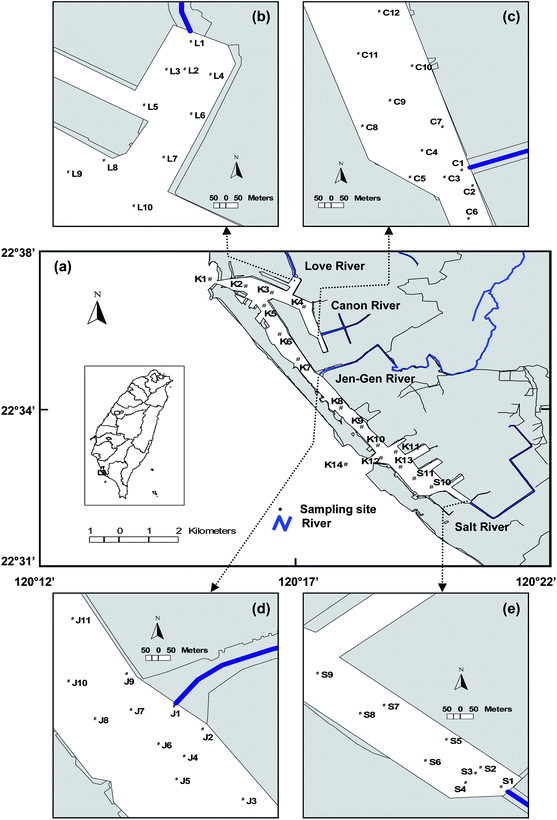 | ||
| Fig. 1 Map of the study area and sampling locations. (a) Harbor channel, (b) Love River mouth, (c) Canon River mouth, (d) Jen-Gen River mouth, and (e) Salt River mouth. | ||
| Site | Latitude (North) | Longitude (East) | Water Depth (m) | Clay (%) | Silt (%) | Sand (%) | Water content (%) | OM (%) | TG (mg kg−1) | TOC (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Harbor channel | ||||||||||
| K1 | 22°37.173′ | 120°15.294′ | 10.5 | 5.3 | 33.8 | 60.9 | 34 | 3.3 | 617 | 1.1 |
| K2 | 22°37.023′ | 120°16.005′ | 9.3 | 15.2 | 82.3 | 2.5 | 45 | 5.1 | 911 | 1.8 |
| K3 | 22°36.873′ | 120°16.516′ | 10.1 | 11.0 | 81.2 | 7.8 | 56 | 5.6 | 2025 | 2.9 |
| K4 | 22°36.582′ | 120°17.147′ | 11.2 | 12.9 | 84.3 | 2.8 | 68 | 5.1 | 1927 | 2.5 |
| K5 | 22°36.598′ | 120°16.366′ | 11.7 | 0.8 | 7.3 | 91.9 | 32 | 3.1 | 384 | 0.6 |
| K6 | 22°35.984′ | 120°16.664′ | 10.9 | 0.8 | 7.8 | 91.4 | 33 | 3.1 | 290 | 1.0 |
| K7 | 22°35.448′ | 120°17.039′ | 11.0 | 16.0 | 83.1 | 0.9 | 56 | 5.0 | 852 | 2.0 |
| K8 | 22°34.389′ | 120°17.884′ | 12.4 | 13.9 | 83.8 | 2.3 | 51 | 5.1 | 1074 | 2.0 |
| K9 | 22°33.967′ | 120°18.296′ | 14.7 | 13.2 | 86.7 | 0.1 | 48 | 4.9 | 969 | 2.1 |
| K10 | 22°33.580′ | 120°18.628′ | 14.9 | 2.0 | 14.4 | 83.6 | 37 | 3.8 | 647 | 1.4 |
| K11 | 22°33.445′ | 120°18.970′ | 14.5 | 12.5 | 87.3 | 0.2 | 48 | 4.7 | 1266 | 2.0 |
| K12 | 22°33.310′ | 120°18.675′ | 15.4 | 1.4 | 11.9 | 86.7 | 36 | 3.1 | 249 | 1.0 |
| K13 | 22°33.116′ | 120°19.056′ | 16.3 | 3.9 | 26.7 | 69.4 | 42 | 3.9 | 408 | 1.7 |
| K14 | 22°33.166′ | 120°17.983′ | 17.8 | 13.5 | 81.0 | 5.5 | 60 | 4.5 | 304 | 1.2 |
| Love River mouth | ||||||||||
| L1 | 22°37.196′ | 120°16.973′ | 4.5 | 10.6 | 81.4 | 8.0 | 64 | 5.7 | 5951 | 5.9 |
| L2 | 22°37.130′ | 120°16.958′ | 9.8 | 9.0 | 73.5 | 17.5 | 86 | 7.3 | 4518 | 5.2 |
| L3 | 22°37.128′ | 120°16.915′ | 9.4 | 9.7 | 87.8 | 2.5 | 79 | 7.5 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 739 739 |
6.6 |
| L4 | 22°37.118′ | 120°17.019′ | 6.7 | 11.3 | 79.5 | 9.2 | 101 | 7.3 | 5276 | 5.6 |
| L5 | 22°37.043′ | 120°16.862′ | 8.1 | 8.8 | 86.1 | 5.1 | 55 | 4.5 | 2141 | 2.3 |
| L6 | 22°37.023′ | 120°16.975′ | 8.5 | 11.1 | 79.0 | 9.9 | 87 | 6.2 | 4260 | 4.0 |
| L7 | 22°36.918′ | 120°16.909′ | 8.7 | 7.5 | 77.0 | 15.5 | 83 | 6.8 | 3151 | 4.9 |
| L8 | 22°36.909′ | 120°16.766′ | 10.6 | 9.5 | 87.5 | 3.0 | 67 | 4.3 | 5194 | 3.2 |
| L9 | 22°36.880′ | 120°16.681′ | 10.8 | 12.6 | 80.2 | 7.2 | 98 | 5.9 | 3163 | 4.3 |
| L10 | 22°36.800′ | 120°16.838′ | 11.4 | 12.2 | 85.9 | 1.9 | 94 | 6.8 | 7500 | 4.9 |
| Canon River mouth | ||||||||||
| C1 | 22°35.965′ | 120°17.468′ | 9.7 | 10.3 | 84.5 | 5.2 | 93 | 6.0 | 4828 | 6.8 |
| C2 | 22°35.925′ | 120°17.493′ | 12.5 | 8.9 | 81.5 | 9.6 | 61 | 5.2 | 6022 | 3.8 |
| C3 | 22°35.947′ | 120°17.429′ | 5.4 | 6.9 | 81.0 | 12.1 | 111 | 10.6 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 292 292 |
6.4 |
| C4 | 22°36.015′ | 120°17.377′ | 13.9 | 5.9 | 36.5 | 57.6 | 37 | 3.2 | 1009 | 3.0 |
| C5 | 22°35.947′ | 120°17.350′ | 12.4 | 11.1 | 82.0 | 6.9 | 60 | 4.0 | 4773 | 2.9 |
| C6 | 22°35.842′ | 120°17.484′ | 12.1 | 12.2 | 85.0 | 2.8 | 111 | 7.0 | 5486 | 4.6 |
| C7 | 22°36.076′ | 120°17.424′ | 9.6 | 11.4 | 84.2 | 4.4 | 83 | 5.8 | 2970 | 4.8 |
| C8 | 22°36.078′ | 120°17.241′ | 11.3 | 13.0 | 85.6 | 1.4 | 86 | 5.1 | 5022 | 3.7 |
| C9 | 22°36.144′ | 120°17.304′ | 12.0 | 7.5 | 39.2 | 53.3 | 55 | 4.7 | 2573 | 4.4 |
| C10 | 22°36.232′ | 120°17.353′ | 8.5 | 12.8 | 87.1 | 0.1 | 121 | 8.3 | 6391 | 4.7 |
| C11 | 22°36.262′ | 120°17.230′ | 12.0 | 13.6 | 82.9 | 3.5 | 51 | 4.2 | 1185 | 2.9 |
| C12 | 22°36.371′ | 120°17.277′ | 10.6 | 11.7 | 85.4 | 2.9 | 100 | 5.7 | 3235 | 3.8 |
| Jen-Gen River mouth | ||||||||||
| J1 | 22°35.096′ | 120°17.453′ | 10.9 | 11.5 | 84.4 | 4.1 | 102 | 6.3 | 4933 | 5.7 |
| J2 | 22°35.041′ | 120°17.517′ | 11.2 | 12.8 | 87.0 | 0.2 | 57 | 3.9 | 4526 | 2.9 |
| J3 | 22°34.872′ | 120°17.611′ | 13.1 | 13.0 | 84.3 | 2.7 | 96 | 5.9 | 3274 | 5.1 |
| J4 | 22°34.920′ | 120°17.459′ | 12.8 | 9.9 | 84.6 | 5.5 | 113 | 5.9 | 3864 | 4.7 |
| J5 | 22°34.976′ | 120°17.476′ | 10.5 | 9.9 | 87.8 | 2.3 | 56 | 5.5 | 4346 | 5.2 |
| J6 | 22°35.005′ | 120°17.417′ | 11.2 | 10.4 | 86.6 | 3.0 | 119 | 6.8 | 4926 | 5.0 |
| J7 | 22°35.087′ | 120°17.353′ | 12.6 | 12.9 | 83.9 | 3.2 | 83 | 5.3 | 3452 | 5.1 |
| J8 | 22°35.065′ | 120°17.271′ | 14.8 | 17.5 | 82.4 | 0.1 | 69 | 4.5 | 1770 | 3.1 |
| J9 | 22°35.173′ | 120°17.343′ | 11.9 | 12.1 | 86.6 | 1.3 | 98 | 4.5 | 4170 | 3.4 |
| J10 | 22°35.155′ | 120°17.211′ | 12.0 | 5.6 | 26.7 | 67.7 | 32 | 3.3 | 667 | 1.8 |
| J11 | 22°35.305′ | 120°17.219′ | 11.7 | 15.3 | 82.0 | 2.7 | 82 | 4.6 | 2819 | 2.6 |
| Salt River mouth | ||||||||||
| S1 | 22°32.354′ | 120°20.419′ | 9.8 | 8.5 | 85.0 | 6.5 | 73 | 9.5 | 3458 | 8.5 |
| S2 | 22°32.403′ | 120°20.374′ | 16.1 | 11.4 | 85.1 | 3.5 | 77 | 8.7 | 6551 | 6.3 |
| S3 | 22°32.388′ | 120°20.364′ | 14.6 | 17.3 | 81.0 | 1.8 | 62 | 11.0 | 3458 | 6.2 |
| S4 | 22°32.364′ | 120°20.341′ | 14.1 | 11.8 | 82.7 | 5.5 | 83 | 6.3 | 2528 | 5.6 |
| S5 | 22°32.472′ | 120°20.299′ | 17.3 | 12.8 | 87.1 | 0.1 | 84 | 6.4 | 4220 | 4.0 |
| S6 | 22°32.419′ | 120°20.253′ | 15.2 | 11.3 | 84.3 | 4.4 | 87 | 10.0 | 2793 | 6.4 |
| S7 | 22°32.557′ | 120°20.162′ | 17.3 | 13.9 | 86.0 | 0.1 | 44 | 6.8 | 1943 | 4.6 |
| S8 | 22°32.537′ | 120°20.110′ | 14.7 | 12.5 | 82.4 | 5.1 | 66 | 8.5 | 2190 | 5.6 |
| S9 | 22°32.636′ | 120°20.017′ | 19.5 | 12.9 | 87.0 | 0.1 | 66 | 7.1 | 2772 | 6.3 |
| S10 | 22°32.866′ | 120°19.332′ | 15.6 | 14.9 | 81.1 | 4.0 | 64 | 5.5 | 1277 | 3.1 |
| S11 | 22°32.683′ | 120°19.675′ | 9.4 | 16.4 | 83.5 | 0.1 | 45 | 5.0 | 906 | 2.5 |
2.2 Sample collection
Surface sediment samples were collected from the selected locations in Kaohsiung Harbor in May 2009 (Fig. 1) using an Ekman Dredge grab sampler (6′′ × 6′′ × 6′′) manufactured by Jae Sung International Co., Taiwan. After collection, the samples were immediately scooped into glass bottles, which were pre-washed with n-hexane and kept in an icebox, and then transported to the laboratory for analyses. In the laboratory, the samples were freeze-dried for 72 h, ground to pass through an 0.5 mm sieve and fully homogenized.19 The dried sediments were placed at −20 °C in pre-washed with n-hexane amber glass bottles covered with solvent-rinsed aluminium foil until further processing and analysis.2.3 Chemicals
All solvents and reagents used are of trace analysis (TA) grade, chromatographic (HPLC) grade or American Chemical Society (ACS) grade. Standards of sixteen United States Environmental Protection Agency (US EPA) priority PAHs contained in a mixture solution of 2000 mg L−1 were obtained from Supelco (Bellfonte, PA, USA), including naphthalene (NA), acenaphthylene (ACE), acenaphthene (AC), fluorene (FL), phenanthrene (PH), anthracene (AN), fluoranthene (FLU), pyrene (PY), benzo[a]anthracene (BaA), chrysene (CH), benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), indeno[1,2,3-cd]pyrene (IP), dibenzo[a,h]anthracene (DBA), and benzo[g,h,i]perylene (BP). Internal standard solutions (acenaphthene-d10, phenanthrene-d10, and chrysene-d12) of 4000 mg L−1 and surrogate standard solutions (2-fluorobiphenyl and 4-terphenyl-d14) of 2000 mg L−1 were obtained from AccuStandard (New Haven, CT, USA).PAH working standards, internal standard mixture solutions, and surrogate standard mixture solutions, which were properly diluted with HPLC grade n-hexane, were prepared daily before the analysis. Glassware was washed before use with n-hexane and dried in an oven at 105 °C. Other materials were previously washed with ultrapure water and acetone.
2.4 Sample preparation and analysis
Particle size was determined using a Coulter LS Particle Size Analyzer.11,18 Wet sediment samples were placed in an oven and heated at 105 °C to a constant weight. The water content of sediments was then calculated by using the weight difference before and after heating. The dry sediments were further heated to 550 °C overnight, and the weight difference with respect to dry weight was determined as the organic matter content (OM).20 Total organic carbon (TOC) was analyzed by using the Walkley–Black method, which involves titration with ferrous ammonium sulfate of the remaining dichromate after a wet combustion of the sample with potassium dichromate.21 All the sediments were analyzed twice, and the relative percent differences of the two measurements were below the 4% level for all samples. Total grease (TG) of sediment was determined following the Standard Methods 5520E.20For PAHs analyses, the sediment samples were extracted using the following procedure. One g (accuracy ±0.0001 g) of dry and homogenized sediment sample was put into a clean centrifuge tube; 5 mL of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) acetone/n-hexane and 0.1 mL of 10 mg L−1 surrogate standard mixture solutions were then added. Blanks were prepared following the same procedure but without adding sediment sample; the standard sample used for quality control was prepared by adding the standard solution to 1
1 (v/v) acetone/n-hexane and 0.1 mL of 10 mg L−1 surrogate standard mixture solutions were then added. Blanks were prepared following the same procedure but without adding sediment sample; the standard sample used for quality control was prepared by adding the standard solution to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) acetone/n-hexane. All samples were vortexed for 1 min; the mixture was subject to ultrasonic treatment for 15 min for extracting PAHs. The sample tubes were then centrifuged at 2000 rpm for 10 min. After centrifuge, the organic layer containing the extracted compounds was siphoned out with a Pasteur pipette, and the sediment was re-extracted twice with 5 mL of a 1
1 (v/v) acetone/n-hexane. All samples were vortexed for 1 min; the mixture was subject to ultrasonic treatment for 15 min for extracting PAHs. The sample tubes were then centrifuged at 2000 rpm for 10 min. After centrifuge, the organic layer containing the extracted compounds was siphoned out with a Pasteur pipette, and the sediment was re-extracted twice with 5 mL of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) acetone/n-hexane mixture. All extracts were pooled together. Activated copper was added to the combined extract for desulfurization. After subsequent drying over anhydrous sodium sulfate, and concentration to 1.0 mL using a gentle stream of nitrogen, 0.1 mL of a 5 mg L−1 internal standard mixture solution was added to the extract to be analyzed using gas chromatography (GC) with a mass selective detector (MSD).
1 (v/v) acetone/n-hexane mixture. All extracts were pooled together. Activated copper was added to the combined extract for desulfurization. After subsequent drying over anhydrous sodium sulfate, and concentration to 1.0 mL using a gentle stream of nitrogen, 0.1 mL of a 5 mg L−1 internal standard mixture solution was added to the extract to be analyzed using gas chromatography (GC) with a mass selective detector (MSD).
An Agilent 6890N GC equipped with an Agilent 7683B Injector, an HP-5MS capillary column (30 m × 0.25 mm × 1 μm) and an Agilent 5975 mass selective detector (MSD) was used to separate and quantify the extracted PAHs. The samples were injected in the splitless mode with the injection temperature maintained at 280 °C. The column temperature was initially held at 35 °C for 2 min, raised to 140 °C at the rate of 5 °C min−1, then to 300 °C at the rate of 10 °C min−1, and held at this temperature for 15 min. Detector temperature was kept at 280 °C. Helium was used as the carrier gas at a constant flow rate of 1 mL min−1. Mass spectrometry was acquired using the electron ionization (EI) and selective ion monitoring (SIM) modes. Identity of PAHs in the samples was confirmed by the retention time and abundance of quantification/confirmation ions in the authentic PAHs standards. The sixteen PAHs were quantified using the response factors related to the respective internal standards based on a five-point calibration curve for individual compounds. In this study, the concentrations of PAHs were not corrected for the surrogate standard recoveries, and are expressed on a dry-weight (dw) basis.
2.5 Quality control
Five-point calibration curves (0.08 to 4 ng μL−1), procedural blanks, checking standards, and sample duplicates were carried out for every set of samples. The response factors based on the five-point calibration curve for individual compounds show acceptable relative standard deviation (RSD) values (1.1 to 14.1%); the procedural blank values are always smaller than the detection limit; the recovery of 6 check standards range from 81% to 119% (n = 6); and the relative percent differences of 6 sample duplicates range from 3.4 to 7.5% (n = 6) for all the target analyses. The surrogate standard recoveries are 87 ± 11% for 2-fluorobiphenyl, and 96 ± 10% for 4-terphenyl-d14 for all 64 sediment samples (n = 64). The detection limits of the analytical procedure were estimated based on three times standard deviation for 7 repeated (n = 7) analyses of 16 PAHs of 8 pg, and the amount of sample extracted. The detection limits are 0.06 (FL) to 0.54 (DBA) ng g−1 (dw) for individual PAHs. The reference material SES-1 (polycyclic aromatic hydrocarbons in spiked estuarine sediment) from the National Research Council of Canada (NRCC) was used. Recoveries of all the PAHs in SES-1 are between 81 to 133% (n = 6) of the certified values.2.6 Statistical analyses
Many researchers have indicated that organic carbon plays an important role to affect the sorption of PAHs in sediments.4,19,22–24 The influence of some sediment properties (e.g., TOC content and particle size distribution) on the results was alleviated by sieving the sediment particles to less than 0.5 mm, and also by normalizing all PAHs concentrations to TOC concentrations prior to performing statistical analyses. Statistical procedures, including cluster analyses (CA), principal components analyses (PCA), and multivariate linear regression (MLR), were performed using the SPSS 12.0 software. Cluster analyses were carried out for identifying homogeneous groups of individual PAHs in the sediment samples. The normalized individual PAHs were first hierarchically clustered using weighted average linkage between the groups and the Pearson correlation for the cluster intervals.16,25PCA was used to extract a minimum number of factors (principal components) from the set of the originally measured PAHs concentrations in order to analyze the relationships among the observed variables. Factors were identified using Varimax rotation and the first three factors were extracted according to Kaiser–Meyer–Olkin (KMO) and Bartlett's test of sphericity. MLR was conducted using PCA factor scores and the standardized normal deviation of total PAHs concentrations as independent and dependent variables, respectively.13,15,16 The regression was run using a forward stepwise method; the standardized regression coefficients were used to represent the relative contributions from various sources.13,15,163. Results and discussion
3.1 Concentrations and distribution of PAHs
The PAHs concentrations in sediments collected at different sites are given in Table 2. The total amount of PAHs examined (ΣPAHs) varies drastically from 39 to 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 521 ng g−1 dw, with a mean concentration of 3326 ± 7101 ng g−1 dw. Specifically, the mean concentration of ΣPAHs in the Harbor Channel (314 ± 190 ng g−1 dw), is lower than those in the Love River mouth (1050 ± 352 ng g−1 dw), Canon River mouth (816 ± 247 ng g−1 dw), Jen-Gen River mouth (675 ± 264 ng g−1 dw), and Salt River mouth (14
521 ng g−1 dw, with a mean concentration of 3326 ± 7101 ng g−1 dw. Specifically, the mean concentration of ΣPAHs in the Harbor Channel (314 ± 190 ng g−1 dw), is lower than those in the Love River mouth (1050 ± 352 ng g−1 dw), Canon River mouth (816 ± 247 ng g−1 dw), Jen-Gen River mouth (675 ± 264 ng g−1 dw), and Salt River mouth (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 616 ± 10
616 ± 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 663 ng g−1 dw). Based on the pollutant levels suggested by Baumard et al.,26 the PAHs levels in sediments can be classified as moderate (ΣPAHs = 100–1000 ng g−1 dw) for the Harbor Channel, Jen-Gen River mouth and Canon River mouth, high (ΣPAHs = 1000–5000 ng g−1 dw) for Love River mouth, and very high (ΣPAHs > 5000 ng g−1 dw) for Salt River mouth. The Salt River mouth sediment has about 14 to 47 times higher mean concentrations of ΣPAHs than the other three river mouths and harbor channel. This result could be caused by the combined effect of industrial zone docks in the immediate vicinity and many industries, such as steel plant, machinery plant, and chemical factory, built along the Salt River.
663 ng g−1 dw). Based on the pollutant levels suggested by Baumard et al.,26 the PAHs levels in sediments can be classified as moderate (ΣPAHs = 100–1000 ng g−1 dw) for the Harbor Channel, Jen-Gen River mouth and Canon River mouth, high (ΣPAHs = 1000–5000 ng g−1 dw) for Love River mouth, and very high (ΣPAHs > 5000 ng g−1 dw) for Salt River mouth. The Salt River mouth sediment has about 14 to 47 times higher mean concentrations of ΣPAHs than the other three river mouths and harbor channel. This result could be caused by the combined effect of industrial zone docks in the immediate vicinity and many industries, such as steel plant, machinery plant, and chemical factory, built along the Salt River.
| Site | 2-Ring | 3-Ring | 4-Ring | 5-Ring | 6-Ring | LPAHsa | HPAHsa | ΣPAHsa | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NA | ACE | AC | FL | PH | AN | FLU | PY | BaA | CH | BbF | BkF | BaP | DBA | IP | BP | ||||
| a LPAHs: sum of NA, ACE, AC, FL, PH, and AN; HPAHs: sum of FLU, PY, BaA, CH. BbF, BkF, BaP, IP, DBA, and BP; ΣPAHs: sum of 16 PAHs. b Standard deviation. | |||||||||||||||||||
| Harbor channel | |||||||||||||||||||
| K1 | 6.8 | 2.0 | 0.9 | 3.0 | 28 | 7.8 | 52 | 74 | 48 | 45 | 83 | 32 | 92 | 12 | 61 | 68 | 48 | 567 | 615 |
| K2 | 18 | 6.2 | 3.5 | 7.4 | 29 | 10 | 32 | 68 | 28 | 26 | 77 | 31 | 83 | 14 | 54 | 59 | 74 | 472 | 546 |
| K3 | 20 | 6.8 | 4.3 | 11 | 41 | 14 | 44 | 53 | 29 | 23 | 60 | 19 | 46 | 12 | 47 | 59 | 97 | 392 | 489 |
| K4 | 15 | 5.0 | 2.6 | 6.1 | 27 | 12 | 31 | 45 | 29 | 21 | 60 | 19 | 45 | 12 | 44 | 55 | 68 | 360 | 428 |
| K5 | 3.8 | 1.1 | 1.0 | 1.2 | 4.4 | 1.0 | 2.3 | 4.2 | 2.2 | 1.7 | 5.0 | 1.5 | 2.9 | 1.0 | 2.5 | 3.2 | 12 | 26 | 39 |
| K6 | 5.1 | 0.9 | 1.0 | 1.5 | 6.4 | 1.6 | 5.0 | 7.8 | 4.1 | 3.5 | 8.0 | 2.2 | 5.4 | 1.1 | 5.1 | 7.0 | 16 | 49 | 65 |
| K7 | 19 | 4.3 | 3.9 | 8.9 | 33 | 13 | 36 | 48 | 30 | 25 | 56 | 18 | 45 | 11 | 41 | 46 | 82 | 355 | 438 |
| K8 | 33 | 5.1 | 5.3 | 11 | 33 | 15 | 23 | 31 | 16 | 13 | 30 | 9.0 | 22 | 5.5 | 21 | 25 | 102 | 195 | 297 |
| K9 | 22 | 5.4 | 6.3 | 12 | 38 | 18 | 30 | 43 | 21 | 20 | 37 | 12 | 27 | 6.2 | 23 | 28 | 102 | 249 | 350 |
| K10 | 12 | 2.1 | 3.4 | 4.8 | 20 | 7.1 | 14 | 23 | 11 | 10 | 16 | 5.0 | 11 | 2.6 | 8.8 | 11 | 49 | 113 | 162 |
| K11 | 30 | 6.2 | 9.2 | 17 | 49 | 14 | 37 | 43 | 24 | 22 | 42 | 13 | 31 | 6.7 | 26 | 31 | 127 | 275 | 402 |
| K12 | 10 | 1.4 | 2.3 | 4.3 | 15 | 3.5 | 8.5 | 12 | 5.4 | 4.9 | 8.8 | 2.9 | 6.4 | 1.1 | 3.2 | 4.8 | 36 | 58 | 94 |
| K13 | 28 | 6.0 | 8.3 | 15 | 42 | 13 | 33 | 41 | 22 | 21 | 39 | 14 | 31 | 5.0 | 22 | 24 | 112 | 253 | 365 |
| K14 | 9.4 | 1.4 | 1.2 | 3.4 | 13 | 3.0 | 11 | 14 | 7.2 | 7.0 | 13 | 3.9 | 8.2 | 1.0 | 5.1 | 6.3 | 31 | 77 | 108 |
| Mean | 17 | 4 | 4 | 8 | 27 | 10 | 26 | 36 | 20 | 17 | 38 | 13 | 33 | 6 | 26 | 31 | 68 | 246 | 314 |
| SDb | 10 | 2 | 3 | 5 | 14 | 6 | 15 | 22 | 13 | 12 | 26 | 10 | 28 | 5 | 20 | 23 | 37 | 169 | 190 |
| Love River mouth | |||||||||||||||||||
| L1 | 25 | 17 | 12 | 14 | 94 | 34 | 262 | 306 | 143 | 98 | 238 | 80 | 174 | 42 | 165 | 187 | 197 | 1696 | 1893 |
| L2 | 27 | 6.8 | 5.2 | 16 | 50 | 21 | 123 | 145 | 74 | 69 | 111 | 36 | 86 | 21 | 85 | 110 | 127 | 859 | 986 |
| L3 | 26 | 7.5 | 5.0 | 16 | 56 | 21 | 126 | 132 | 64 | 132 | 108 | 35 | 76 | 20 | 92 | 117 | 132 | 902 | 1034 |
| L4 | 21 | 12 | 7.9 | 19 | 95 | 34 | 205 | 224 | 110 | 84 | 173 | 56 | 122 | 32 | 116 | 140 | 188 | 1262 | 1450 |
| L5 | 16 | 6.2 | 3.7 | 8.0 | 39 | 25 | 128 | 144 | 76 | 61 | 98 | 37 | 79 | 16 | 63 | 77 | 97 | 780 | 877 |
| L6 | 25 | 5.5 | 3.4 | 10 | 35 | 12 | 106 | 121 | 64 | 64 | 108 | 34 | 82 | 19 | 74 | 93 | 90 | 766 | 856 |
| L7 | 24 | 7.6 | 4.0 | 14 | 40 | 14 | 130 | 146 | 61 | 47 | 104 | 33 | 75 | 18 | 74 | 104 | 102 | 792 | 894 |
| L8 | 25 | 10 | 4.4 | 11 | 43 | 21 | 89 | 107 | 53 | 39 | 106 | 34 | 72 | 21 | 76 | 91 | 115 | 689 | 805 |
| L9 | 18 | 9.4 | 3.9 | 9.2 | 46 | 18 | 77 | 102 | 54 | 40 | 110 | 35 | 82 | 19 | 74 | 86 | 105 | 680 | 785 |
| L10 | 22 | 10 | 5.7 | 13 | 55 | 23 | 104 | 124 | 66 | 52 | 117 | 36 | 83 | 22 | 76 | 112 | 130 | 792 | 922 |
| Mean | 23 | 9 | 6 | 13 | 55 | 22 | 135 | 155 | 77 | 69 | 127 | 42 | 93 | 23 | 90 | 112 | 128 | 922 | 1050 |
| SD | 4 | 4 | 3 | 3 | 22 | 7 | 56 | 63 | 28 | 29 | 44 | 15 | 32 | 8 | 30 | 32 | 37 | 318 | 352 |
| Canon River mouth | |||||||||||||||||||
| C1 | 46 | 7.5 | 7.7 | 20 | 71 | 22 | 108 | 136 | 59 | 62 | 101 | 27 | 61 | 16 | 57 | 104 | 173 | 732 | 905 |
| C2 | 69 | 18 | 20 | 51 | 157 | 38 | 231 | 274 | 77 | 102 | 99 | 25 | 47 | 3.5 | 25 | 16 | 352 | 900 | 1252 |
| C3 | 63 | 12 | 14 | 38 | 130 | 30 | 163 | 201 | 88 | 89 | 123 | 34 | 73 | 13 | 45 | 8.0 | 287 | 836 | 1123 |
| C4 | 16 | 2.9 | 2.9 | 7.1 | 24 | 8.6 | 34 | 48 | 24 | 21 | 42 | 13 | 29 | 8.2 | 24 | 33 | 61 | 276 | 337 |
| C5 | 35 | 7.3 | 6.0 | 19 | 55 | 20 | 80 | 101 | 51 | 44 | 84 | 23 | 51 | 11 | 36 | 40 | 142 | 521 | 663 |
| C6 | 43 | 10 | 13 | 26 | 96 | 32 | 131 | 167 | 64 | 62 | 102 | 25 | 56 | 14 | 44 | 61 | 220 | 725 | 945 |
| C7 | 37 | 10 | 8.6 | 20 | 72 | 26 | 96 | 136 | 57 | 50 | 95 | 26 | 56 | 7.8 | 46 | 62 | 174 | 632 | 806 |
| C8 | 30 | 14 | 11 | 17 | 69 | 26 | 115 | 156 | 68 | 62 | 110 | 31 | 66 | 14 | 43 | 47 | 167 | 710 | 877 |
| C9 | 30 | 7.0 | 5.9 | 13 | 47 | 18 | 58 | 79 | 44 | 36 | 77 | 21 | 48 | 13 | 41 | 47 | 121 | 464 | 585 |
| C10 | 29 | 8.3 | 7.9 | 19 | 64 | 21 | 90 | 128 | 60 | 52 | 98 | 28 | 63 | 9.1 | 46 | 59 | 149 | 632 | 781 |
| C11 | 18 | 5.4 | 5.4 | 10 | 43 | 15 | 67 | 88 | 46 | 42 | 85 | 25 | 55 | 14 | 44 | 49 | 97 | 512 | 609 |
| C12 | 26 | 24 | 5.8 | 22 | 88 | 31 | 104 | 118 | 57 | 46 | 107 | 33 | 76 | 20 | 69 | 75 | 196 | 707 | 903 |
| Mean | 37 | 11 | 9 | 22 | 76 | 24 | 107 | 136 | 58 | 56 | 94 | 26 | 57 | 12 | 43 | 50 | 178 | 637 | 816 |
| SD | 16 | 6 | 5 | 12 | 37 | 8 | 52 | 60 | 16 | 22 | 20 | 6 | 13 | 4 | 12 | 26 | 80 | 172 | 247 |
| Jen-Gen River mouth | |||||||||||||||||||
| J1 | 30 | 7.2 | 13 | 21 | 100 | 41 | 181 | 212 | 91 | 72 | 128 | 44 | 112 | 24 | 99 | 102 | 212 | 1065 | 1277 |
| J2 | 26 | 9.4 | 7.9 | 14 | 54 | 17 | 66 | 77 | 35 | 32 | 76 | 24 | 59 | 16 | 63 | 81 | 127 | 528 | 656 |
| J3 | 28 | 8.4 | 7.9 | 17 | 54 | 24 | 50 | 65 | 36 | 32 | 75 | 22 | 53 | 10 | 36 | 39 | 139 | 419 | 559 |
| J4 | 24 | 5.1 | 4.9 | 12 | 42 | 15 | 56 | 63 | 31 | 30 | 67 | 20 | 48 | 9.2 | 37 | 44 | 103 | 405 | 508 |
| J5 | 28 | 4.5 | 4.9 | 15 | 50 | 15 | 82 | 85 | 43 | 37 | 78 | 24 | 57 | 13 | 56 | 68 | 117 | 544 | 661 |
| J6 | 26 | 6.2 | 6.6 | 13 | 49 | 18 | 63 | 72 | 33 | 29 | 72 | 21 | 53 | 15 | 54 | 75 | 119 | 487 | 606 |
| J7 | 19 | 6.1 | 4.8 | 10 | 39 | 14 | 52 | 66 | 34 | 31 | 66 | 20 | 45 | 8.4 | 31 | 36 | 93 | 390 | 484 |
| J8 | 22 | 17 | 3.1 | 11 | 70 | 19 | 124 | 147 | 56 | 64 | 104 | 34 | 94 | 13 | 55 | 55 | 143 | 747 | 890 |
| J9 | 33 | 5.7 | 7.5 | 18 | 73 | 31 | 103 | 122 | 57 | 48 | 96 | 28 | 67 | 21 | 78 | 100 | 169 | 721 | 890 |
| J10 | 13 | 3.6 | 3.6 | 6.6 | 24 | 10 | 24 | 31 | 19 | 15 | 41 | 12 | 30 | 5.2 | 21 | 25 | 60 | 223 | 283 |
| J11 | 29 | 7.6 | 7.0 | 15 | 56 | 23 | 54 | 69 | 42 | 33 | 87 | 24 | 63 | 12 | 40 | 46 | 137 | 469 | 607 |
| Mean | 25 | 7 | 7 | 14 | 55 | 21 | 78 | 92 | 43 | 38 | 81 | 25 | 62 | 13 | 52 | 61 | 129 | 545 | 675 |
| SD | 6 | 4 | 3 | 4 | 20 | 9 | 44 | 51 | 19 | 17 | 23 | 8 | 23 | 5 | 23 | 26 | 40 | 227 | 264 |
| Salt River mouth | |||||||||||||||||||
| S1 | 3007 | 277 | 1244 | 2343 | 5761 | 962 | 3447 | 2586 | 1249 | 1500 | 1698 | 563 | 1564 | 276 | 1104 | 994 | 13594 | 14981 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 575 575 |
| S2 | 2361 | 358 | 1585 | 2396 | 4325 | 927 | 2825 | 2315 | 1433 | 1285 | 2421 | 744 | 2087 | 376 | 1480 | 1367 | 11952 | 16333 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 285 285 |
| S3 | 3327 | 1460 | 2057 | 3333 | 5717 | 1010 | 2660 | 2182 | 1014 | 1006 | 1667 | 523 | 1636 | 288 | 1567 | 1074 | 16903 | 13618 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 521 521 |
| S4 | 2088 | 155 | 899 | 1841 | 4020 | 887 | 1795 | 1473 | 1087 | 1393 | 1008 | 609 | 988 | 122 | 1475 | 763 | 9890 | 10713 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 603 603 |
| S5 | 1203 | 249 | 831 | 1195 | 2226 | 576 | 1627 | 1370 | 848 | 818 | 1434 | 468 | 1328 | 171 | 941 | 862 | 6280 | 9867 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 147 147 |
| S6 | 925 | 123 | 415 | 649 | 1321 | 323 | 877 | 758 | 453 | 393 | 762 | 239 | 665 | 96 | 470 | 430 | 3756 | 5143 | 8899 |
| S7 | 585 | 137 | 364 | 549 | 1029 | 267 | 881 | 830 | 454 | 372 | 739 | 243 | 677 | 94 | 467 | 426 | 2931 | 5183 | 8114 |
| S8 | 512 | 93 | 237 | 341 | 786 | 211 | 587 | 507 | 313 | 340 | 569 | 181 | 494 | 100 | 441 | 445 | 2180 | 3977 | 6157 |
| S9 | 751 | 115 | 253 | 434 | 1012 | 264 | 712 | 619 | 342 | 305 | 584 | 181 | 510 | 104 | 436 | 442 | 2829 | 4235 | 7064 |
| S10 | 209 | 47 | 93 | 132 | 307 | 102 | 291 | 293 | 153 | 125 | 294 | 101 | 247 | 22 | 198 | 183 | 889 | 1906 | 2795 |
| S11 | 222 | 57 | 109 | 144 | 357 | 111 | 415 | 384 | 207 | 180 | 400 | 139 | 334 | 54 | 270 | 235 | 1000 | 2619 | 3619 |
| Mean | 1381 | 279 | 735 | 1214 | 2442 | 513 | 1465 | 1211 | 687 | 702 | 1052 | 363 | 957 | 155 | 805 | 656 | 6564 | 8052 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 616 616 |
| SD | 1124 | 403 | 655 | 1096 | 2116 | 367 | 1088 | 829 | 453 | 516 | 667 | 223 | 609 | 111 | 524 | 380 | 5603 | 5226 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 663 663 |
3.2 PAHs composition
According to the number of aromatic rings, the 16 PAHs can be categorized into three groups, i.e. 2- & 3-ring, 4-ring, and 5- & 6-ring PAHs. The 4-ring (32 to 42%) or 5- & 6-ring (36 to 44%) PAHs are the predominant PAHs congeners in the sediments collected from all locations, except the sediments from the Salt River mouth, where the 2- & 3-ring PAHs were predominant at 43% (Fig. 2). The result suggests that the PAHs contamination in the Salt River mouth comes from a different source than those for the PAHs found in other locations. The predominance of low molecular weight PAHs in the surface sediments of the Salt River mouth reflects the presence of significant combustion products from low temperature pyrolytic processes27 and/or petrogenic sources.28 Furthermore, the type of PAHs may also indicate recent pollution and/or direct PAHs inputs into the surface water from sources such as shipping, dry and wet atmospheric deposition, and air–water exchange, among the many others.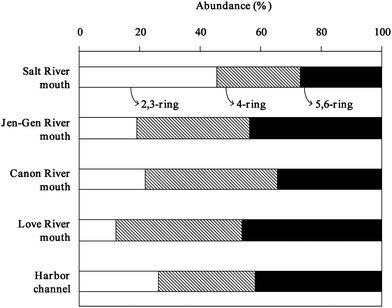 | ||
| Fig. 2 PAHs composition in sediments of Kaohsiung Harbor. (2,3-ring: NA, ACE, AC, FL, PH, AN. 4-ring: FLU, PY, BaA, CH. 5,6-ring: BbF, BkF, BaP, IP, DBA, BP). | ||
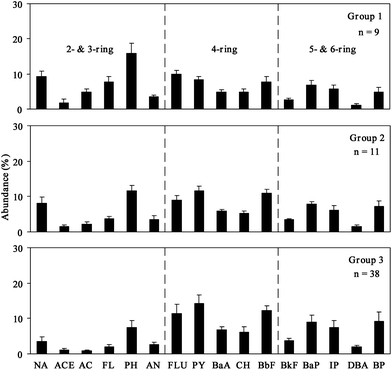
Further, a hierarchical cluster analysis was implemented to separate the homogeneous groups of the 58 sampling sites in the study areas. Results indicated that these sites can be classified into three groups as shown in Fig. 3. Group 1 includes samples from nine stations in the Salt River mouth (S1–S9); Group 2 includes samples from two stations on the Salt River mouth (S10 and S11) and harbor channel stations, except K1 to K4, and Group 3 includes samples from some stations on the harbor channel (K1 to K4) and all stations in the Love River mouth (L1–L10), Canon River mouth (C1–C12), and Jen-Gen River mouth (J1–J11). These three groups represent the respective PAHs compositions for the sediment samples collected at the Salt River mouth, harbor channel and other three river mouths.
 | ||
| Fig. 3 The compositional patterns of 16 PAHs in representative surface sediment of Kaohsiung harbor from the result of hierarchical cluster analysis. | ||
The low molecular weight (2 to 3 ring) PAHs contain on average 9.3% of NA, 4.8% of AC, 7.7% of FL, and 15.8% of the total PAHs (ΣPAHs) in Group 1. These percentages are 2 to 6 times higher than those in Group 3 (other three river mouths) (Fig. 3). For the high molecular weight (5 to 6 ring) PAHs, quantities of each PAHs in Group 1 are 1.5 times lower than those found in Group 2 (harbor channel) and Group 3. As the middle molecular weight (4 ring) PAHs are concerned, Group 1 has higher FLU than PY. However, Group 2 and Group 3 have a lower quantity of FLU than PY.
As mentioned above, PAHs found in the surface sediments of the study area have complicated compositions so that the exact source of contamination is difficult to verify but can be roughly estimated as discussed in the following paragraphs.
3.3 Relationship between sediment PAHs and characteristics
Natural characteristics of sediment, such as organic matter content and grain size influence the distribution and concentration of PAHs in sediments.22,24 Sediments with high organic matter or clay content contain abundant PAHs.19,24 In addition, Chiou et al.29 used soil-state 13C to determine the relative amounts of different structural carbon in sediment organic matter, and found that the high partitioning of PAHs to organic matter was mainly due to the presence of a significant aromatic fraction of organic matter. In this study, the influence of sediment TOC, OM, TG, water content and grain size on PAHs concentrations has been investigated. Results of linear regression analyses show that a significant correlation exists between ΣPAHs and OM (r = 0.591, p < 0.01) or TOC (r = 0.466, p < 0.01) for all sediment samples (Table 3). The observation suggests that the sediment organic phase plays an important role to affect the distribution of PAHs in sediments.| All the sediment samples (n = 58) | Group Aa (n = 11) | Group Ba (n = 47) | |
|---|---|---|---|
| a Group A: including S1–S11; Group B: including all the sediment samples, except Group A. b Correlation is significant at the 0.01 level (2-tailed). c Correlation is significant at the 0.05 level (2-tailed). | |||
| Clay | 0.191 | 0.307c | −0.282 |
| Silt | 0.187 | 0.538b | −0.025 |
| Sand | −0.192 | −0.515b | 0.312 |
| Water content | 0.051 | 0.557b | 0.382 |
| OM | 0.591b | 0.594b | 0.611c |
| TG | 0.102 | 0.713b | 0.736b |
| TOC | 0.466b | 0.740b | 0.644c |
Based on concentrations of ΣPAHs, samples collected at all stations can be categorized into two groups: (1) Group A with concentrations over 2000 ng g−1 dw of total PAHs including samples collected at the Salt River mouth (S1–S11), and (2) Group B with concentrations below 2000 ng g−1 dw of total PAHs concentration including all samples collected at the other stations. Group A shows a more significant relationship between ΣPAHs and TOC than Group B, whereas Group B has a more significant relationship between ΣPAHs and MO. Additionally, ΣPAHs and TG also appear to be significantly correlated for both Groups A and B (Table 3). These results suggest that the composition of organic matter contained in the sediment can influence the partition of PAHs in the organic matter. Salt River mouth sediments have excellent sorption capacity that suggests a possible black carbon PAHs source, such as coal.30 Salt River mouth sediments are probably bound largely to soot particles, which contribute to a variable proportion of the total organic phase in the sediments, leading to a weakly positive but nevertheless significant correlation in all samples collected at the Salt River mouth. As noted above, the FLU/(FLU + PY) ratio also suggests a coal contribution at those stations (see below).
In addition, a significant correlation (p < 0.01) is found to exist between ΣPAHs and water content or grain size in Group B but no such correlation is found in Group A. This result suggests that the physical properties of sediment with high PAHs concentration have no significant effect on the distribution of PAHs.
3.4 Estimates of PAHs sources
Diagnostic ratios, cluster analyses, and principal components analyses were used to estimate the possible sources of PAHs in sediments of the study area.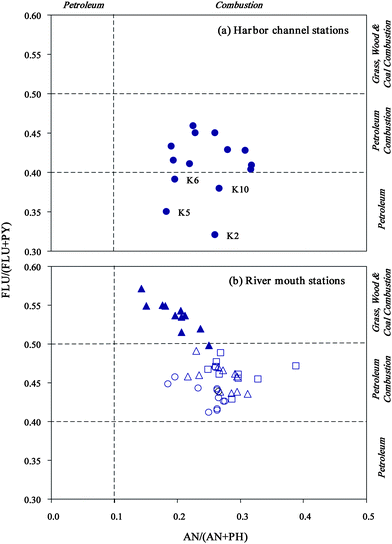 | ||
Fig. 4 PAHs cross plots for the ratios of FLU/(PY + FLU) vs.AN/(AN + PH). ( : Harbor channel, : Harbor channel,  : Love River mouth, : Love River mouth,  : Canon River mouth, : Canon River mouth,  : Jen-Gen River mouth, and : Jen-Gen River mouth, and  : Salt River mouth). : Salt River mouth). | ||
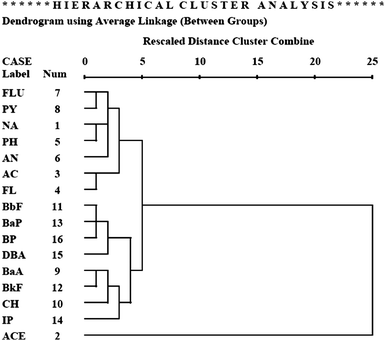 | ||
| Fig. 5 Hierarchical dendrogram for 16 PAHs in the Kaohsiung Harbor sediments using average linkage between groups and Pearson correlation as measure interval. | ||
Liu et al.16 removed data about the unknown source from the data matrix, and successful divided the pyrogenic source of PAHs into two subsets, one traffic-related and the other due to coal combustion. Based on the CA, the pyrogenic source can be subdivided into two subgroups (Fig. 5), which represent two kinds of different pyrogenic sources. Therefore, we performed the PCA again on the dataset without ACE in order to further investigate the pyrogenic sources of PAHs. The results show that three principal components, i.e.PC1, PC2, and PC3, can be identified to account for 35.8%, 35.8%, and 27.5%, respectively, of the total variance (Fig. 6).
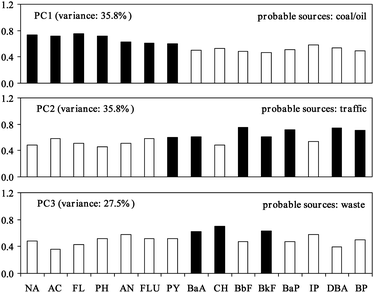 | ||
| Fig. 6 Rotated component loadings of 15 PAHs in surface sediments from Kaohsiung Harbor. (Rotation method: Varimax with Kaiser normalization, ■ loadings > 0.6). | ||
PC1 is highly weighted by NA, AC, FL, PH, AN, FLU, and PY, which are PAHs with 2 to 4 rings, and hence belong to Group 1 of the cluster analysis. Initially, this observation was believed to be indicative of petrogenic sources. However, some literature reports suggest that the combustion of oil and coal may be another potential source of this factor. Several authors reported FLU, PY, PH, and AN as predominant coal combustion profiles, and PH and PY as predominant oil burning profiles.13,15,34,36 In addition, Levendis et al.37 reported that PH, FLU, and PY are the dominant PAHs with lower concentrations of 5 to 6-ring detected in furnace effluents that come from the combustion of pulverized coal and tire crumbs at 1000 °C. Luo et al.38 reported heavily weighted PAHs species with 2 to 4 rings in the PAHs contained in suspended particulate matter. Larsen and Baker13 also reported a similar pattern in gas and particle phase PAHs. Furthermore, Yang et al.39 measured the stack emissions of twelve steel and iron plants that burned coal and heavy oil in southern Taiwan near the study area. Their results show that PAHs with low molecular weights are predominant in the particulate phase for all steel and iron plants, especially NA, AC, ACE, FL, AN, FLU, and PY. Considering the environment background, Kaohsiung Harbor is close to metropolitan Kaohsiung, the largest industrial city that hosts Linhai Industrial Park, the biggest in Taiwan with nearly 467 factories mostly in steel, chemicals, and machinery along the Salt River.40 Coal and oil are the most important energy source used widely in most factories especially the steel and power industries. Hence, a large quantity of PAHs are emitted that are either directly or indirectly discharged into the Harbor to deposit in bed sediment as sink. Therefore, assigning this factor mainly to coal and oil combustion sources of PAHs is justified
PC2 is predominately weighted by PY, BaA, BbF, BkF, BaP, DBA, and BP, with a moderate weighting of AC, FLU, and IP. The source for these species is designated as traffic-related in literature, and BP is identified as a tracer of auto emissions.13,16,34BkF, BbF, BaP, BP, FLU, DBA, and PY are indicators of emissions from vehicles;13,16,41,42 both BP and IP have been identified as tracers of diesel vehicles.13,16,42 AC, FLU, FL and PH are also suggested to indicate emission from diesel and gasoline vehicles.43 Exhaust from ships in the Kaohsiung Harbor and motor vehicles in the Kaohsiung City should be the major source contributors to this PC.
PC3 is dominated by BaA, CH, and BkF, with a moderate loading of AN and IP. Both IP and CH are suggested to indicate industrial waste incinerators.43 According to PAHs data from the stack flue gas (gas and particle phases), PH, AN, FLU and BaA are the dominant PAHs in the emissions of a medical waste incinerator44 whereas AN, FLU, BkF, IP, and BP are the dominant PAHs in the stack flue gas (gas and particle phases) of urban waste incinerators.45 Kaohsiung City has a population of 1.5 million which generates a considerable amount of waste, and all the waste is incinerated. Therefore, this factor can be considered as a waste combustion source.
3.5 Contribution of PAHs sources
Several authors13,15,16,34 have reported applying PCA/MLR to apportion the major sources of PAHs in the urban atmosphere, surface soils and sediments. The PCA/MLR method has been used in this study to calculate the percentage contributions of the major sources of PAHs in the sediments of Kaohsiung Harbor. The factor scores from the PCA without ACE and standardized normal deviation of ΣPAHs were used as independent and dependent variables, respectively, for the MLR. The mean contribution of source i is the ratio of the regression coefficient for factor i to the sum of all the regression coefficients.13 Thus, the mean contribution percents of coal and oil combustion, traffic, and waste combustion source are 37%, 33%, and 30%, respectively.The coal and oil combustion source (37%) is the first contributor to PAHs. Kaohsiung Harbor processes about 60% of Taiwan's import and export of goods in plants that are mostly located around the harbor for convenient transportation. Coal and oil are used as the major energy sources in these plants. Additionally, a coal-fired power plant is located in the south of Kaohsiung Harbor. Hence, PAHs generated by coal and oil combustion are easily discharged to Kaohsiung Harbor.
The second contributor to PAHs in the sediment is traffic-related (33%). There are two major types of traffic-related PAHs in Kaohsiung Harbor. The first type is land transport; there are 1.6 million vehicles in Kaohsiung City46 including large trucks to transport cargo on the dock. The other type is sea transport, such as vessel operations, which are very frequent in Kaohsiung Harbor. About 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cargo vessels pass in and out every year, and about 13
000 cargo vessels pass in and out every year, and about 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 fishing boats operate around the sea area near the harbor.47 Therefore, exhaust from cargo vessels, fishing boats and passenger ferries in the Kaohsiung Harbor also plays an important role as a PAHs contributor. Furthermore, compared with automobiles, more PAHs are emitted from fishing boats and ferries because in addition to not being equipped with adequate catalytic converters to clean the combustion process, most fishing boats burn fishing boat fuel oil, which can cause high emission of PAHs.48
000 fishing boats operate around the sea area near the harbor.47 Therefore, exhaust from cargo vessels, fishing boats and passenger ferries in the Kaohsiung Harbor also plays an important role as a PAHs contributor. Furthermore, compared with automobiles, more PAHs are emitted from fishing boats and ferries because in addition to not being equipped with adequate catalytic converters to clean the combustion process, most fishing boats burn fishing boat fuel oil, which can cause high emission of PAHs.48
Waste combustion is the third type of PAHs contributor to account for 30% of the total PAHs found in the harbor sediment. In Kaohsiung, there are four urban waste incinerators with a total annual handling capacity of 1.28 million tons.49 Based on the emission factor of 871 mg ton-waste−1,50 about 1118 kg year−1 of PAHs is emitted so that the waste combustion is considered as one of the main contributors.
In fact, results of PCA calculations carried out on the 16 PAHs reveal that about 25% of the source of PAHs found in Kaohsiung Harbor cannot be identified. Nevertheless, the combustion sources contribute to most PAHs in the sediment of Kaohsiung Harbor; PAHs from industry, transport and incineration of municipal waste are also significant. However, when the PAHs pollution levels are considered, industrial sources are the most important factor for PAHs pollution in Kaohsiung Harbor sediment.
4. Conclusions
The results reveal that the PAHs contained in the sediment at Kaohsiung Harbor belong to the moderate to very high pollution category. PAHs of very high concentrations exist mainly in the river mouth sediments especially Salt River that is close to a neighboring industrial park and has the highest concentration of PAHs. These results reveal that PAHs come mainly from polluted rivers particularly from rivers polluted by discharges of industrial wastewater. Compositions of the PAHs identified show that except for the sediment at the Salt River mouth, the sediment at Kaohsiung Harbor contains PAHs of high ring numbers (HPAHs, e.g. 4-ring and 5- & 6-ring PAHs) as the major PAH constituents; whereas the Salt River mouth sediment contains PAHs with low ring numbers (LPAHs, e.g. 2- & 3-ring PAHs) as the major PAH constituents with 43% of the total PAHs as 2-ring and 3-ring PAHs. Results of cluster analyses, PCA, and MLR analyses can be used as a basis to effectively define sources of the PAHs detected. The PAHs in Kaohsiung Harbor sediment originate from oil/coal combustion (37%), traffic-related emissions (33%) and waste combustion (30%). Additionally, almost all of these PAHs are contributed by pyrogenic activity. Thus, industrial activities that lead to emission of PAHs into the atmosphere is quite significant to adversely impact the environment; future pollution prevention and management should target the various industries in this region for reducing pollution.Acknowledgements
This research was supported by the Kaohsiung Harbor Bureau, Taiwan. The authors would like to thank the personnel of the Kaohsiung Harbor Bureau for their support throughout this project.References
- IARC (International Agency for Research on Cancer), 1991, Monographs on evaluation of carcinogenic risks to humans Search PubMed.
- US EPA (US Environmental Protection Agency), 1993, Provisional guidance for quantitative risk assessment of polycyclic aromatic hydrocarbons, EPA/600/R/089, Washington, DC: office of research and development, US Environmental Protection Agency Search PubMed.
- C. Zhang and G. Zheng, 2003, Characterization and desorption kinetics of PAHs from contaminated sediment in Houston Ship Channel. Environmental Institute of Houston 2003 Annual Report, 39–41 Search PubMed.
- M. P. Zakaria, H. Takada, S. Tsutsumi, K. Ohno, J. Yamada, E. Kouno and H. Kumata, Environ. Sci. Technol., 2002, 36, 1907–1918 CrossRef CAS.
- S. A. Stout, A. D. Uhler and S. D. Emsbo-Mattingly, Environ. Sci. Technol., 2004, 38, 2987–2994 CrossRef CAS.
- H. H. Soclo, P. Garrigues and M. Ewald, Mar. Pollut. Bull., 2000, 40, 387–396 CrossRef CAS.
- J. Guinan, M. Charlesworth, M. Service and T. Oliver, Mar. Pollut. Bull., 2001, 42, 1073–1081 CrossRef CAS.
- E. Magi, R. Bianco, C. Ianni and M. D. Carro, Environ. Pollut., 2002, 119, 91–98 CrossRef CAS.
- M. B. Yunker, R. W. Macdonald, R. Vingarzan, R. H. Mitchell, D. Goyette and S. Sylvestre, Org. Geochem., 2002, 33, 489–515 CrossRef CAS.
- M. D. Fang, P. C. Hsieh, F. C. Ko, J. E. Baker and C. L. Lee, Mar. Pollut. Bull., 2007, 54, 1179–1189 CrossRef CAS.
- J. J. Jiang, C. L. Lee, M. D. Fang and J. T. Liu, Mar. Pollut. Bull., 2009, 58, 752–760 CrossRef CAS.
- R. M. Dickhut, E. A. Canuel, K. E. Gustafson, K. Liu, K. M. Arzayus, S. E. Walker, G. Edgecombe, M. O. Gaylor and E. H. Macdonald, Environ. Sci. Technol., 2000, 34, 4635–4640 CrossRef CAS.
- R. K. Larsen and J. E. Baker, Environ. Sci. Technol., 2003, 37, 1873–1881 CrossRef CAS.
- G. Li, X. Xia, Z. Yang, R. Wang and N. Voulvoulis, Environ. Pollut., 2006, 144, 985–993 CrossRef CAS.
- Q. Zuo, Y. H. Duan, Y. Yang, X. J. Wang and S. Tao, Environ. Pollut., 2007, 147, 303–310 CrossRef CAS.
- Y. Liu, L. Chen, Q. H. Huang, W. Y. Li, Y. J. Tang and J. F. Zhao, Sci. Total Environ., 2009, 407, 2931–2938 CrossRef CAS.
- KHB (Kaohsiung Harbour Bureau, Taiwan, ROC), 2010, http://www.khb.gov.tw/.
- C. W. Chen, C. M. Kao, C. F. Chen and C. D. Dong, Chemosphere, 2007, 66, 1431–1440 CrossRef CAS.
- G. De Luca, A. Furesi, G. Micera, A. Panzanelli, P. C. Piu, M. I. Pilo, N. Spano and G. Sanna, Mar. Pollut. Bull., 2005, 50, 1223–1232 CrossRef CAS.
- APHA (American Public Health Association), 2001, Standard Methods for the Examination of Water and Wastewater, 21st edn, Washington, DC Search PubMed.
- S. Ünlü and B. Alpa, Chemosphere, 2006, 64, 764–777 CrossRef.
- G. B. Kim, K. A. Maruya, R. F. Lee, J. H. Lee, C. H. Koh and S. Tanabe, Mar. Pollut. Bull., 1999, 38, 7–15 CAS.
- G. P. Yang, Environ. Pollut., 2000, 108, 163–171 CrossRef CAS.
- X. C. Wang, Y. X. Zhang and R. F. Chen, Mar. Pollut. Bull., 2001, 42, 1139–1149 CrossRef CAS.
- J. Zhang, L. Z. Cai, D. X. Yuan and M. Chen, Mar. Pollut. Bull., 2004, 49, 479–486 CrossRef CAS.
- P. Baumard, H. Budzinski and P. Garrigues, Environ. Toxicol. Chem., 1998, 17, 765–776 CrossRef CAS.
- M. S. Garcia-Falcon, B. Soto-Gonzalez and J. Simal-Gandara, Environ. Sci. Technol., 2006, 40, 759–763 CrossRef CAS.
- L. F. Ping, Y. M. Luo, H. B. Zhang, Q. B. Li and L. H. Wu, Environ. Pollut., 2007, 147, 358–365 CrossRef CAS.
- C. T. Chiou, S. E. Mcgroddy and D. E. Kile, Environ. Sci. Technol., 1998, 32, 264–269 CrossRef CAS.
- C. H. Vane, I. Harrison and A. W. Kim, Sci. Total Environ., 2007, 374, 112–126 CrossRef CAS.
- M. Qiao, C. Wang, S. Huang, D. Wang and Z. Wang, Environ. Int., 2006, 32, 28–33 CrossRef CAS.
- H. Budzinski, I. Jones, J. Bellocq, C. Pierrad and P. Garrigues, Mar. Chem., 1997, 58, 85–97 CrossRef CAS.
- N. F. Y. Tam, L. Ke, X. H. Wang and Y. S. Wong, Environ. Pollut., 2001, 114, 255–263 CrossRef CAS.
- R. M. Harrison, D. J. T. Smith and L. Luhana, Environ. Sci. Technol., 1996, 30, 825–832 CrossRef CAS.
- Z. Wang, K. Li, P. Lambert and C. Yang, J. Chromatogr., A, 2007, 1139, 14–26 CrossRef CAS.
- T. Agarwal, P. S. Khillare, V. Shridhar and S. Ray, J. Hazard. Mater., 2009, 163, 1033–1039 CrossRef CAS.
- Y. A. Levendis, A. Atal and J. B. Carlson, Environ. Sci. Technol., 1998, 32, 3767–3777 CrossRef CAS.
- X. J. Luo, S. J. Chen, B. X. Mai, Q. S. Yang, G. Y. Sheng and J. M. Fu, Environ. Pollut., 2006, 139, 9–20 CrossRef CAS.
- H. H. Yang, S. O. Lai, L. T. Hsieh, H. J. Hsueh and T. W. Chi, Chemosphere, 2002, 48, 1061–1074 CrossRef CAS.
- KCG (Kaohsiung City Government, Taiwan, ROC), 2010, http://edbkcg.kcg.gov.tw/style/front001/bexfront.php?sid=bmdform& class=29.
- K. F. Ho, S. C. Lee and M. Y. Chiu, Atmos. Environ., 2002, 36, 57–65 CrossRef CAS.
- T. T. T. Dong and B. K. Lee, Chemosphere, 2009, 74, 1245–1253 CrossRef CAS.
- H. H. Yang, W. J. Lee, S. J. Chen and S. O. Lai, J. Hazard. Mater., 1998, 60, 159–174 CrossRef CAS.
- W. J. Lee, M. C. Liow, P. J. Tsai and L. T. Hsieh, Atmos. Environ., 2002, 36, 781–790 CrossRef CAS.
- C. T. Li, W. J. Lee, H. H. Mi and C. C. Su, Sci. Total Environ., 1995, 170, 171–183 CrossRef CAS.
- KCG (Kaohsiung City Government, Taiwan, ROC), 2010, http://www.tbkc.gov.tw/census-02-06.asp.
- TWCOA (Council of Agriculture, Executive Yuan, Taiwan, ROC), 2010, http://www.fa.gov. tw/pages/list.aspx?Node=242&Index=13.
- Y. C. Lin, W. J. Lee, H. W. Li, C. B. Chen, G. C. Fang and P. J. Tsai, Atmos. Environ., 2006, 40, 1601–1609 CrossRef CAS.
- TWEPA (Environmental Protection Administration, Taiwan, ROC), 2010, http://ivy4.epa.gov.tw/swims/?ctype=B&cid=swims&oid=www.
- H. H. Mi, C. F. Chiang, C. C. Lai, L. C. Wang and H. H. Yang, Aerosol Air Qual. Res., 2001, 1, 83–90 Search PubMed.
| This journal is © The Royal Society of Chemistry 2012 |
