Graphene oxide enwrapped Ag3PO4 composite: towards a highly efficient and stable visible-light-induced photocatalyst for water purification†
Lei
Liu
,
Jincheng
Liu
* and
Darren Delai
Sun
*
School of Civil and Environmental Engineering, Nanyang Technological University, Block N1, Nanyang Avenue, Singapore 639798. E-mail: JCLIU@ntu.edu.sg; DDSUN@ntu.edu.sg
First published on 9th August 2012
Abstract
A novel graphene oxide (GO) enwrapped Ag3PO4 (GO–Ag3PO4) composite as a visible-light-induced photocatalyst has been fabricated through an ion-exchange method of CH3COOAg and Na2HPO4 in the presence of GO sheets. Scanning electron microscope and X-ray diffraction analysis confirmed that Ag3PO4 particles have been enwrapped by GO sheets. The UV-vis spectra curve of the GO–Ag3PO4 composite showed strong absorbance in the visible light regions. The photocatalytic activity of the composite was evaluated by the degradation of organic dye (AO7) and phenol under visible-light irradiation. The results indicated that this novel GO–Ag3PO4 composite exhibited significantly higher photocatalytic activities and improved stability under visible-light irradiation, compared with bare Ag3PO4. Moreover, the GO–Ag3PO4 composite showed both excellent intrinsic antibacterial and visible-light-induced photocatalytic disinfection activities toward E. coli cells. The possible mechanism for the enhanced photocatalytic properties and antibacterial activity of the GO–Ag3PO4 composite was also discussed. Our finding paves a way to design highly efficient and stable visible-light-induced photocatalysts for the removal of organic pollutants and pathogenic bacteria for water purification.
Introduction
The growing concerns about drinking water safety have put more stress on the removal of contaminants including toxic organic pollutants and pathogenic microorganisms. Conventional technologies have some limitations in complete decontamination of those emerging anthropogenic organic pollutants or disinfection without harmful disinfection byproducts (DBPs).1–3 Semiconductor-mediated heterogeneous photocatalysis is considered as a promising alternative for the removal of organic pollutants and pathogens from wastewater, as it can be potentially used under solar irradiation without generating harmful by-products.4,5 In the past decades, a variety of semiconductor-based photocatalysts such as TiO2, ZnO, CdS, ZnS, and their derivatives have been successfully fabricated, and their photocatalytic properties have been extensively explored.6–10 However, their relatively low activities and low efficiencies in the utilization of solar irradiation concentrated in visible light regions limit their practical use in water treatment. Therefore, the exploration of new semiconductor materials as highly efficient visible-light-induced photocatalysts is of significant importance, and also a great challenge.Recently, a breakthrough was made by Ye et al., who reported the use of silver orthophosphate (Ag3PO4) as a new visible light photocatalyst for the oxidation of water and photodecomposition of organic compounds.11,12 Their results show that Ag3PO4 semiconductor has an extremely high photooxidation capability for O2 evolution from water, as well as highly efficient organic dye degradation activity under visible light, significantly higher than that of commercial TiO2−xNx. However, one major limitation of this novel photocatalyst may prevent its wide use in environmental and energy fields. The Ag3PO4 photocatalyst is unstable upon photo-illumination, and it is easily corroded by the photogenerated electrons (4Ag3PO4 + 6H2O + 12h+ + 12 e− → 12Ag + 4H3PO4 + 3O2).11 If no electron acceptor such as AgNO3 is involved in the system, the total Ag3PO4 photocatalyst could be decomposed during the photocatalysis process. Therefore, it is highly desirable to develop effective strategies to improve the stability of the Ag3PO4 photocatalyst. Very recently, AgX (X = Cl, Br, I), Ag nanoparticles and carbon quantum dots (CQDs) were introduced for the modification of Ag3PO4.13–16 The AgX, Ag nanoparticles and CQDs could promote the transfer and separation of photoexcited electron-hole pairs on the Ag3PO4 particles, so as to improve their stability by decreasing their photocorrosion. Nevertheless, a new simple method, which could improve the photocatalytic stability of Ag3PO4 while facilitating its recovery, is still required for environmental applications.
Graphene oxide (GO) is a chemically modified graphene with hydroxyl and carboxyl groups, which favour its solubility in solvents and provide opportunities for the construction of GO-based hybrid composites.17 The fabrication of GO-based hybrid catalysts, especially those for the photodegradation of pollutants, is currently an important issue of particular concern.18–20 Several well-defined hybridized composites in terms of GO and conventional semiconductors, such as GO/ZnO, GO/TiO2, and GO/Ag/AgX have been reported.18–24 The GO-based nanocrystalline composites show significantly enhanced photocatalytic performance in the degradation of pollutants, as the GO sheets could facilitate charge transfer and suppress the recombination of electron-hole pairs in the photocatalysts in these systems. Besides, biological studies indicate that graphene-based composites may have potential antibacterial properties.25,26 Our previous works have proven that GO sheets could facilitate the bacteria adhesion of GO-based composites and have a positive role in the disinfection process.21,27 All these illustrate the unique properties of GO making it an ideal support and host for enhancing the photocatalytic properties of Ag3PO4.
In this work, for the first time, a novel GO enwrapped Ag3PO4 (GO–Ag3PO4) composite is designed as a highly efficient visible-light-induced photocatalyst. A facile ion-exchange method for growing Ag3PO4 crystals on GO sheets is developed. The obtained GO–Ag3PO4 composite exhibits enhanced photocatalytic activities and stability toward organic pollutant degradation under visible-light irradiation. Moreover, the novel GO–Ag3PO4 composite displays excellent intrinsic antibacterial and enhanced photocatalytic disinfection capability of bacterial cells under visible-light irradiation, due to the synergistic effects of GO sheets and the Ag3PO4 photocatalyst. As far as we know, this is the first report concerning the photocatalytic antibacterial activity of Ag3PO4 crystals and an Ag3PO4-based composite. The advantages of involving large GO sheets into the system are: (1) GO can work as a support and electron acceptor to suppress the charge recombination of Ag3PO4 and enhance the photocatalytic activity and stability of Ag3PO4; (2) the large size of the GO sheets can facilitate the recovery of the composite by simple filtration. All these features make the GO–Ag3PO4 composite a promising candidate for water purification under visible-light irradiation.
Experimental
Materials
Natural graphite (SP1) was purchased from Bay Carbon Company (USA). Sodium nitrate (NaNO3, 99%), potassium permanganate (KMnO4, 99%), hydrogen peroxide (H2O2, 35%), concentrated sulfuric acid (36.5%), and silver acetate (CH3COOAg, 99%), silver nitrate (AgNO3, 99%), sodium hydrogen phosphate (Na2HPO4, 99%), sodium hydrate (99%), Acid Orange (AO7, 99%), and phenol (99%) were purchased from Sigma-Aldrich. All reagents were used without further purification. The deionized (DI) water was produced from a Millipore Milli-Q water purification system. Escherichia coli (E. coli) K12 ER2925 was purchased from New England Biolab.Synthesis of Ag3PO4 and GO–Ag3PO4 composite
Bare Ag3PO4 samples were prepared by the ion-exchange method reported before.12 Briefly, 0.2 g CH3COOAg was dissolved in 200 mL of DI water. Na2HPO4 aqueous solution (0.015 M) was added drop by drop to the solution with magnetically stirring, until the initial white colour changed to yellow. The mixture was then washed with water to dissolve any unreacted raw material, and dried under atmospheric conditions.GO was synthesized according to the modification of Hummers' method from natural graphite, and the process was described earlier.28,29 For the synthesis of the GO–Ag3PO4 hybrid composite, 15 mg of GO was first added to 200 mL of DI water and sonicated for 2 h to get a well dispersed GO solution. Then, 0.1 g of CH3COOAg was added to the GO solution whilst magnetically stirring for 1 h. Subsequently, Na2HPO4 aqueous solution (0.015 M) was added drop by drop to the solution, until the total mixture colour changed to olive green. The mixture was then washed with water to dissolve any unreacted raw material, and dried under atmospheric conditions.
Characterization
Atomic force microscopy (AFM) was carried out using a non-contact mode on a PSIA XE-150 scanning probe microscope. The AFM sample was prepared by spin coating the dispersion water solution of GO onto Si substrate covered with 300 nm thick SiO2. Scanning electron microscopy (SEM) images were obtained using a JEOL 2010-H microscope operating at 200 kV. Energy dispersive X-ray (EDS) measurement was conducted using the EDAX system attached to the same field emission scanning electron microscopy with carbon tape and Au sputtering used for the sample preparations. The samples for the analysis were solid after drying. X-ray powder diffraction (XRD) patterns were taken on a D8-Advance Bruker-AXS diffractometer using Cu Kα irradiation. The UV-vis absorption spectra were recorded by using an Evolution 300 spectrophotometer (UV-1700 Shimadzu). Silver ion was detected by Inductively Coupled Plasma (ICP, Perkin Elmer, optima 2000 DV).Evaluation on photocatalytic organic pollutant degradation activities of GO–Ag3PO4 composite
The photocatalytic performance of the photocatalysts was evaluated by degradation of pollutant dye AO7 and phenol. For the dye degradation, the catalysts (20 mg) were put into AO7 dye solution (50 ppm, 50 mL). For the phenol degradation, the catalysts (50 mg) were put into phenol aqueous solution (10 ppm, 50 mL). The mixtures were kept stirring in the dark for 30 min to reach the adsorption equilibrium. The light source used was 300 W Xe arc lamp equipped with a cold mirror to provide visible light (420 nm < λ ≤ 630 nm, 80 mW cm−2) (Oriel, 300 W model). At certain time intervals of visible-light irradiation, reaction solution was collected and centrifuged to remove the photocatalysts for analysis. The concentrations of AO7 and phenol were monitored by UV-vis spectroscopy, and were determined by its maximum absorption wavelength at 485 nm, and 270 nm, respectively.Evaluation on antibacterial activities of GO–Ag3PO4 composite
E. coli was chosen as the model waterborne pathogen for antibacterial activity evaluation. E. coli was cultivated in Luria-Bertani nutrient solution at 37 °C for 18 h to get the exponential growth phase. The cells were harvested by centrifugation and washed with PBS solution (0.2 M, pH 7.2) to remove residual macromolecules. All glass apparatus and solutions used in the experiments were autoclaved at 121 °C for 20 min to ensure sterility.To investigate the intrinsic antibacterial activity of the samples, E. coli cells were inoculated in PBS solution containing 20 mg L−1 Ag3PO4 and GO–Ag3PO4 composites, respectively, with a final cell concentration of ∼107 cfu mL−1. The mixture was incubated with gentle shaking for 2 h at 37 °C. The mixture was diluted with a gradient method and then applied uniformly on three LB culture medium plates per gradient solution. These plates were incubated at 37 °C for 24 h. The colony forming units were counted and compared with control plates to calculate percentage of cell viability (C/C0). Control experiments without materials were also carried out at the same time.
To investigate the photocatalytic degradation activity of GO–Ag3PO4 composite toward bacterial cells, the morphological changes of E. coli cells were monitored by scanning electron microscopy. The bacterial cells were inoculated in PBS solution containing 20 mg L−1 of Ag3PO4 and GO–Ag3PO4 composites, respectively, with a final cell concentration ∼107 cfu mL−1. The mixture solutions were kept under visible-light irradiation for 2 h. After filtering the E. coli mixture solution, the cells on the filter were quickly fixed with 2% glutaraldehyde and 1% osmium tetroxide. Then, the cells on the filter were dehydrated with sequential treatment of 50, 70, 85, 90 and 100% ethanol for 10 min. The filter was freeze dried at −50 °C before the test.
Results and discussion
Characterization of GO–Ag3PO4 composite
The strategy for the fabrication of the GO–Ag3PO4 composite is shown in Fig. 1. GO used in this work are mainly single-layer sheets, which is confirmed by their thickness from AFM images (Fig. S1, ESI†). The functional groups, such as hydroxyl and carbonyl groups, of GO could stabilize the silver ions, and the Ag3PO4 particles could form on GO sheets when eventually adding HPO42− into the solution. | ||
| Fig. 1 Schematic representation for the synthesis of GO enwrapped Ag3PO4 composite. | ||
Fig. 2a and b show typical SEM images of bare Ag3PO4 particles synthesized by reacting silver acetate with Na2HPO4 in aqueous solutions with different magnifications. It can be clearly seen that the Ag3PO4 particles possess regular rhombic dodecahedral morphology with an average diameter of 500 nm, which is in agreement with the results reported by Ye, et al.12 When involving GO sheets into the system, well-defined Ag3PO4 crystals whose surfaces were distinctly enwrapped with gauze-like GO sheets could be obtained, as shown in Fig. 2c and d. The morphology and size of the Ag3PO4 in the GO–Ag3PO4 system remains the same as that of bare Ag3PO4. The color of the sample solution has been changed from the yellow of bare Ag3PO4 to the olive green of GO–Ag3PO4, due to the existence of GO sheets, as shown in the insets of Fig. 2a and c. The SEM images confirm that GO sheets could serve as a support and surfactant to enwrap Ag3PO4 particles in this particular system, which is in good agreement with the reports that GO has been demonstrated to be a surfactant in a GO-based hybrid system, and also been demonstrated to effectively retard particle aggregations.23,30 The Ag3PO4 nanoparticles enwrapped by GO sheets could be further verified by EDS analysis, and the Ag3PO4 content of the GO–Ag3PO4 composite was calculated to be around 83% according to EDS analysis (Fig. S2, ESI†).
 | ||
| Fig. 2 Typical SEM images of the synthesized Ag3PO4 particles (a) (b), and GO–Ag3PO4 composite (c) (d). Insets of (a) and (c) are photographs of the Ag3PO4 and GO–Ag3PO4 composite solutions, respectively. | ||
To further validate the Ag3PO4 nanoparticles anchored onto the GO sheets, XRD was applied to the measurements. Fig. 3a shows the XRD patterns of GO sheets, bare Ag3PO4 particles and the GO–Ag3PO4 composite. The curve of the GO sheets shows a diffraction peak at a 2θ value around 11.9°, which may be due to interlamellar water trapped between the hydrophilic graphene oxide sheets.21 Furthermore, the XRD patterns clearly show that all of the diffraction peaks of bare Ag3PO4 could be indexed to the body-centered cubic structure of Ag3PO4 (JCPDS no.06-0505), which is in good agreement with previously reported work.12 The curve of the GO–Ag3PO4 composite is without changes in comparison with the bare Ag3PO4 particles. No obvious diffraction peaks of GO are observed in the GO–Ag3PO4 composite, because the regular stack of GO is destroyed by the intercalation of Ag3PO4 particles, which is consistent with other reported works about GO-based composites.19,31–33 The XRD results confirm the Ag3PO4 crystals have been successfully located onto the GO sheets.
 | ||
| Fig. 3 (a) XRD patterns of GO sheets, Ag3PO4 particles and GO–Ag3PO4 composite, (b) UV-vis absorbance spectra of GO sheets, Ag3PO4 particles and GO–Ag3PO4 composite. | ||
UV-vis spectral measurements were employed to measure the changes in the absorption of the samples, as shown in Fig. 3b. These indicate that bare Ag3PO4 could absorb solar energy with a wavelength shorter than 530 nm. The typical UV-vis spectrum of GO exhibits two characteristic peaks, a maximum at 230 nm, which corresponds to the π–π* transitions of aromatic C–C bonds, and a shoulder at 303 nm, which is attributed to n–π* transitions of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds (Fig. 3b).34 In the GO–Ag3PO4 composite sample, the absorption toward the visible light region is remarkably enhanced compared to bare Ag3PO4 particles. This suggests that Ag3PO4 particles are enwrapped by GO sheets. This result also implies that the GO–Ag3PO4 composite is suitable to be used as visible-light-induced photocatalyst.
O bonds (Fig. 3b).34 In the GO–Ag3PO4 composite sample, the absorption toward the visible light region is remarkably enhanced compared to bare Ag3PO4 particles. This suggests that Ag3PO4 particles are enwrapped by GO sheets. This result also implies that the GO–Ag3PO4 composite is suitable to be used as visible-light-induced photocatalyst.
Visible-light-induced organic pollutant degradation performance
The photocatalytic performance of the bare Ag3PO4 and the GO–Ag3PO4 composite were investigated in terms of the photodegradation of the organic dye AO7 under visible-light irradiation. The normalized temporal concentration changes of AO7 during the photodegradation process are shown in Fig. 4, which are based on the real-time UV-vis absorption spectra of the dye, monitored continuously with increasing irradiation time (Fig. S3, ESI†). Before monitoring the photocatalytic activity of the photocatalysts, a simple adsorption of AO7 by GO sheets was investigated. It is noted that around 6% of AO7 molecules are adsorbed by the GO–Ag3PO4 composite when reaching the equilibrium adsorption state under the dark reaction conditions, while less than 2% are adsorbed on bare Ag3PO4 particles. The high adsorption capability of the composite material to dye might be attributed to the hybridization of GO sheets, which have been proven to facilitate the adsorption of pollutant molecules, owing to the non-covalent intermolecular forces.18,35 The adsorption abilities of GO to different kinds of dye molecules are quite different depending on the properties of the organic dyes, and it shows relatively low adsorption ability to acid orange.35 | ||
| Fig. 4 Photocatalytic activities of the control, Ag3PO4 and the GO–Ag3PO4 composite for photodegradation of AO7 under visible-light irradiation (20 mg of sample, initial concentration of AO7 50 ppm). Each data point and error bar represents the mean and the standard errors, respectively, of independent triplicates. | ||
The photocatalytic degradation of AO7 was carried out under visible-light irradiation (420 nm ≤ λ ≤ 630 nm, 80 mW cm−2). As shown in Fig. 4, an insignificant amount of removal of AO7 occurred in the presence of 40 min of visible-light irradiation, which was due to the weak photolysis of AO7. In the presence of bare Ag3PO4 particles, an approximate 100% degradation of AO7 is achieved within 20 min. This illustrates that bare Ag3PO4 particles exhibit excellent photocatalytic activities for the degradation of AO7 under visible-light irradiation. However, in the presence of the GO–Ag3PO4 composite, a sudden drop of the dye concentration occurs, and approximately 100% of the dye is removed within 10 min. This demonstrates that involving GO into the system could remarkably increase the photocatalytic activity of Ag3PO4 under visible-light irradiation. As the photocatalytic decontamination of the low-concentration dye solution follows a pseudo-first-order reaction,36 the corresponding degradation rate constant of bare Ag3PO4 and the GO–Ag3PO4 composite could be estimated to be 0.064 min−1 and 0.109 min−1, respectively. This result indicates that involving GO into the Ag3PO4 photocatalyst system could significantly enhance the total photocatalytic activities of the system by nearly twice as much.
To investigate the stability of Ag3PO4 and the GO–Ag3PO4 composite, we repeated the photodegradation of the AO7 dye with the photocatalysts five times, and the corresponding results are shown in Fig. 5. Fig. 5a shows that there are obvious decreases of photocatalytic degradation activity of bare Ag3PO4 toward AO7 in every repeated cycle. In the last cycle, only 50% of the dye could be removed within 40 min, which is a significant loss of photocatalytic activity compared to that of the first cycle. However, when involving GO sheets into the system, as shown in Fig. 5b, the photocatalytic efficiency of the GO–Ag3PO4 composite only displays slight decreases without a significant loss of activity after the reaction is performed consecutively five times. The morphology of the catalysts after repeated reaction was evaluated by SEM. An obvious particle aggregation and corrosion of bare Ag3PO4 was observed after the photocatalytic process as shown in Fig. 5c. Fig. 5d shows that the morphology of the GO–Ag3PO4 composite does not display significant changes after the repeated reactions. These results indicate that the bare Ag3PO4 photocatalyst is not stable during the photocatalytic process, and it could lose its activity under visible-light irradiation. The reason for this has been demonstrated to be that the photo-excited electrons of Ag3PO4 could simultaneously reduce the photocatalyst if no sacrificial reagent was involved in the system.11 The process could be concluded as:
| 4Ag3PO4 + 6H2O + 12h+ + 12e− → 12Ag + 4 H3PO4 + 3O2. |
 | ||
| Fig. 5 Five consecutive cycling photodegradation curves of the AO7 dye over Ag3PO4 (a) and the GO–Ag3PO4 composite (b), SEM images of Ag3PO4 (c) and the GO–Ag3PO4 composite (d) after decontamination experiments. Each data point and error bar represents the mean and the standard errors, respectively, of independent triplicates. | ||
The photocatalytic performance of bare Ag3PO4 and the GO–Ag3PO4 composite was also evaluated by the photodegration of phenol aqueous solution under visible-light irradiation, as shown in Fig. 6. Prior to irradiation, the adsorption rate of phenol to bare Ag3PO4 and the GO–Ag3PO4 composite was around 6% and 10%, respectively. The high adsorption capability of the composite material to phenol could be attributed to the hybridization of the GO sheets, which is in agreement with the results of the dye adsorption. Meanwhile, there was no phenol removal under visible-light irradiation without the photocatalysts, implying that the phenol has no photolysis. In the presence of bare Ag3PO4 particles, an approximate 100% degradation of phenol is achieved within 30 min, with a degradation rate constant of 0.027 min−1. In the presence of the GO–Ag3PO4 composite, approximately 100% of the phenol is removed within 20 min, with a degradation rate constant of 0.038 min−1. The GO–Ag3PO4 composite shows higher photocatalytic activity for the decomposition of phenol compared with bare Ag3PO4 particles, which further proves that involving GO into the system could remarkably increase the photocatalytic activity of Ag3PO4 under visible-light irradiation. Moreover, the stability tests shown in Fig. 7a and b further confirm that the GO–Ag3PO4 composite is also demonstrated to be a stable and efficient visible light photocatalyst compared with bare Ag3PO4 particles.
 | ||
| Fig. 6 Photocatalytic activities of the control, Ag3PO4 and the GO–Ag3PO4 composite for photodegradation of phenol under visible-light irradiation (50 mg of sample, initial concentration of phenol 10 ppm). Each data point and error bar represents the mean and the standard errors, respectively, of independent triplicates. | ||
 | ||
| Fig. 7 Five consecutive cycling photodegradation curves of phenol over Ag3PO4 (a) and the GO–Ag3PO4 composite (b). Each data point and error bar represents the mean and the standard errors, respectively, of independent triplicates. | ||
Antibacterial and visible-light-induced disinfection performance
Ag and Ag-based composites are known to be effective biocides against numerous kinds of bacteria, fungi and virus.38 Besides, the semiconductor-mediated photocatalytic disinfection has been considered as a promising and an effective alternative for microorganism removal.39 Thus, it is reasonable to assume that the novel GO–Ag3PO4 composite could be suitable for the disinfection application. Nevertheless, little is known about the antibacterial performance of bare Ag3PO4 or Ag3PO4-based composites. As a result, for the first time, this work investigates the intrinsic antibacterial and photocatalytic disinfection of both Ag3PO4 and the GO–Ag3PO4 composite by using the model waterborne pathogen E. coli.Fig. 8a shows that 100% bacterial cell removal occurred both in the presence of bare Ag3PO4 and the GO–Ag3PO4 composite system at a sample concentration of 20 mg L−1. The bactericidal activities of the samples are further confirmed by the images of colonies incubated on an agar plate with and without samples. According to Fig. 8b, E. coli can be completely killed in the presence of bare Ag3PO4 and the GO–Ag3PO4 composite. Our previous work proved that the large GO sheets synthesized have no obvious antibacterial activity toward E. coli cells within 100 mg L−1.21,27 Therefore, the excellent antibacterial activities of bare Ag3PO4 and the GO–Ag3PO4 composite should be due to the intrinsic property of Ag3PO4.
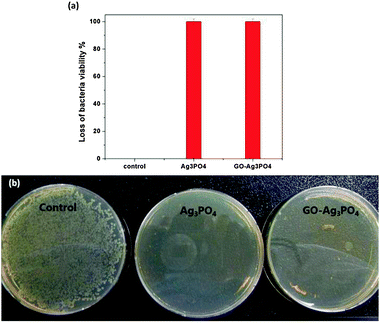 | ||
| Fig. 8 The results of the antibacterial tests applying different samples: control, Ag3PO4, and GO–Ag3PO4 composite (a). Images of E. coli colonies on an agar plate incubated with different samples: control, Ag3PO4, and GO–Ag3PO4 composite (b) (sample concentration: 20 mg L−1, initial bacterial concentration: ∼107 cfu mL−1). Each data point and error bar represents the mean and the standard errors, respectively, of independent triplicates. | ||
It is generally accepted that Ag+ at high concentrations exhibits bactericidal activity.40,41 The Ag+ of the sample solution was monitored by inductively coupled plasma (ICP) measurements. The bare Ag3PO4 solution contains 4.9 ppm (mg L−1) Ag+, while the GO–Ag3PO4 composite solution contains 4.6 ppm Ag+. The high level of Ag+ in the sample solution is due to the slight solubility of Ag3PO4.42 The control experiment shows that total bacterial removal was observed in the presence of 4.5 ppm Ag+ (in the form of AgNO3), as shown in Fig. S4 (ESI†). These facts suggest that the antibacterial activity of Ag3PO4 and the GO–Ag3PO4 composite should result from dissolved Ag+. It is worth mentioning that the relatively high level of Ag+ depends on the solubility of the Ag3PO4, and nearly 6 mg L−1 of Ag3PO4 (in terms of 4.6 ppm Ag+) was dissolved under this experiment condition, which led to the complete removal of E. coli cells. As a result, a study on the relationship between sample concentration and disinfection efficiency is out of discussion in this particular system. A low concentration of 20 mg L−1 of sample was used in all the disinfection experiments.
The SEM was used to investigate the morphology changes of E. coli cells after the disinfection reaction. The E. coli cells remained in a good state in the absence of samples as shown in Fig. 9a and b. However, in the presence of Ag3PO4 and the GO–Ag3PO4 composite, obvious cell damages were observed as shown in Fig. 9d, e, g and h. These results imply that Ag+ could react with some membrane proteins to damage the cell membrane and its relative function, which is in good agreement with previous research.38 Moreover, in the presence of the GO–Ag3PO4 composite, most of the cells were gathered on GO sheets in Fig. 9g and h. This implies that the water soluble GO sheets may adsorb and gather the bacteria onto the surface, which has also been observed in our previous works.21,27 Therefore, this could enhance the interaction between bacteria and the materials on the GO sheets. Unfortunately, the Ag3PO4 crystals were destroyed during the disinfection process in both Ag3PO4 and the GO–Ag3PO4 system, as shown in Fig. 9e and h, although it is much better in the GO–Ag3PO4 system than for Ag3PO4. This may be due to the interaction between Ag3PO4 and the bacteria or bacterial secretion containing other ions like chloride ions and thiol groups, as the solubility of Ag3PO4 is higher than some other silver compounds such as AgCl.42 This could affect the reusability of Ag3PO4-based composites in the disinfection process. Future work should be focused on finding methods to control the solubility of Ag3PO4-based materials.
 | ||
| Fig. 9 SEM images of E. coli cells after treatment with different samples for 2 h: control without (a) (b) and with (c) visible-light irradiation, Ag3PO4 without (d) (e) and with (f) visible-light irradiation, GO–Ag3PO4 composite without (g) (h) and with (i) visible-light irradiation (sample concentration: 20 mg L−1, initial bacterial concentration: ∼107 cfu mL−1). | ||
The photocatalytic disinfection of the novel GO–Ag3PO4 composite was further investigated by SEM measurements after visible-light irradiation. No obvious change of the bacterial cells was observed after 2 h of visible-light irradiation in the absence of catalysts, as shown in Fig. 9c. This implies that short time visible-light irradiation has no obvious effects on bacteria damage. However, in the presence of Ag3PO4 photocatalysts, most of the bacterial cells were damaged, as shown in Fig. 9f. Some cell degradation was observed in Fig. 9f, compared with Fig. 9e. In the presence of the GO–Ag3PO4 composite, bacterial cells were enwrapped by GO sheets, and obvious cell degradation was observed under visible-light irradiation as shown in Fig. 9i. These results show that the photocatalytic degradation capability of bacterial cells in the GO–Ag3PO4 composite system was enhanced in comparison with that in the bare Ag3PO4 system under visible-light irradiation. The enhanced photocatalytic disinfection properties of the GO–Ag3PO4 composite may be due to the good adsorption ability of GO for bacteria, facilitating the interaction between bacterial cells and catalysts. It could also be due to the enhanced photocatalytic activity of the GO–Ag3PO4 composite compared with bare Ag3PO4, which has been proven in the photocatalytic organic pollutant degradation experiments.
Photocatalytic mechanism
As is well known, an efficient charge separation/transfer is crucial for the enhancement of photocatalytic activities.5,18,43 GO has been shown to be an effective electron transporter and acceptor in the systems of GO/TiO2, GO/ZnO and Ag/AgX/GO (X = Br, Cl).18,19,22,23,44,45 The GO sheets could facilitate charge transfer and suppress the recombination of electron-hole pairs of the GO-based photocatalysts. Thus, it is reasonable to assume that a reinforced charge separation/transfer might be achieved in our GO–Ag3PO4 composite system, where GO could act as the electron acceptor and Ag3PO4 as the electron donor. Moreover, recent research showed that AgX, Ag nanoparticles and CQDs could act as electron acceptors to make the photoexcited electrons transfer from Ag3PO4, and effectively protect Ag3PO4 from photocorrosion.13–16 On the basis of the experimental facts and analysis, we could propose an explanation for the enhanced photocatalytic activity and stability observed from our GO–Ag3PO4 composite system, as shown in Fig. 10. When the GO–Ag3PO4 composite is irradiated with visible light, the photogenerated electrons could be transferred to the GO sheets, thus inhibiting the charge recombination and promoting the photocatalytic activity. The efficient electron transfer from Ag3PO4 to GO sheets also sustains the stability of the GO–Ag3PO4 composite by keeping electrons away from the Ag3PO4. Meanwhile, the photogenerated holes on Ag3PO4 could oxidize polluted dyes, while the electrons on GO could adsorb surface O2 to form various reactive oxygen species (ROSs), thus could assist the degradation of organic pollutants. In addition to the enhanced charge separation, the photocatalytic reaction also involves the transportation of contaminant molecules over the catalyst surface. Previous reports have shown that GO sheets have a relatively high adsorption ability towards organic pollutants, such as methylene blue, rhodamine B and acid orange.35 Depending on different properties of organics, the adsorption abilities of GO to organics are different. The high adsorption abilities of the organic pollutant on the catalyst surface would enhance the electron transfer efficiency and contact opportunity with photogenerated active species. As a result, the high photocatalytic activity towards our GO–Ag3PO4 composite system might also due to the adsorption ability of GO to organics. | ||
| Fig. 10 Schematic drawing showing the process of the photocatalytic dye degradation and disinfection over the GO–Ag3PO4 composite. | ||
As for the mechanism of antibacterial and photocatalytic disinfection activity of GO–Ag3PO4 composite system, an explanation is shown in Fig. 10. Firstly, the excellent intrinsic antibacterial activity of the GO–Ag3PO4 composite is mainly caused by the high level of soluble Ag+ due to the slight solubility of Ag3PO4. Meanwhile, the water soluble GO sheets adsorb and gather the bacteria onto the surface, which may enhance the interaction between bacterial cells and Ag3PO4 photocatalysts on the GO sheets. Eventually, the bacterial cell degradation could be achieved by Ag3PO4 photocatalysts under visible-light irradiation, which is similar to the photocatalytic degradation of the organic dye. To the synergistic effects of a high level of Ag+ release, good adsorbtion capability of GO to bacterial cells, and enhanced photocatalytic activity of the GO–Ag3PO4 composite are attributed to the excellent antibacterial and photocatalytic disinfection activity of this novel composite.
Conclusion
In summary, a novel visible-light-induced photocatalyst in terms of Ag3PO4 and GO has been fabricated. The GO–Ag3PO4 composite shows high-performance photocatalytic degradation activity toward organic pollutants with enhanced efficiency and stability compared with bare Ag3PO4 particles. This novel composite also shows excellent intrinsic antibacterial activity towards E. coli. Moreover, the GO–Ag3PO4/visible-light system illustrates enhanced photocatalytic cell degradation compared with bare Ag3PO4, due to the synergistic effects of excellent antibacterial Ag+, good adsorption ability of GO toward bacterial cells and high-efficient photocatalytic activity of Ag3PO4. The recovery of the GO–Ag3PO4 composite can be implemented by a simple filtration process due to the large GO sheets. As a result, this new GO–Ag3PO4 composite may find promising applications in environmental and water treatment.Acknowledgements
Authors would like to acknowledge the Clean Energy Research Programme under National Research Foundation of Singapore and the Singapore Environment & Water Industry (EWI) Development Council for their research grant (grant no. NRF2007EWT-CERP01-0420 and MEWR 651/06/166) in support for this work. The scholarship provided by EWI is also gratefully appreciated.Notes and references
- T. Wintgens, F. Salehi, R. Hochstrat and T. Melin, Water Sci. Technol., 2008, 57, 99–107 CrossRef CAS.
- H. Yang and H. Cheng, Sep. Purif. Technol., 2007, 56, 392–396 CrossRef CAS.
- J. F. Lu, T. Zhang, J. Ma and Z. L. Chen, J. Hazard. Mater., 2009, 162, 140–145 CrossRef CAS.
- A. Mills, R. H. Davies and D. Worsley, Chem. Soc. Rev., 1993, 22, 417–425 RSC.
- M. R. Hoffmann, S. T. Martin, W. Y. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69–96 CrossRef CAS.
- X. W. Zhang, T. Zhang, J. Ng and D. D. Sun, Adv. Funct. Mater., 2009, 19, 3731–3736 CrossRef CAS.
- S. Anandan, N. Ohashi and M. Miyauchi, Appl. Catal., B, 2010, 100, 502–509 CrossRef CAS.
- C. J. Lin, Y. H. Yu and Y. H. Liou, Appl. Catal., B, 2009, 93, 119–125 CrossRef CAS.
- M. Muruganandham, R. Amutha, E. Repo, M. Sillanpää, Y. Kusumoto and M. Abdulla-Al-Mamun, J. Photochem. Photobiol., A, 2010, 216, 133–141 CrossRef CAS.
- M. Artemyev, E. Ustinovich and I. Nabiev, J. Am. Chem. Soc., 2009, 131, 8061–8065 CrossRef CAS.
- Z. G. Yi, J. H. Ye, N. Kikugawa, T. Kako, S. X. Ouyang, H. Stuart-Williams, H. Yang, J. Y. Cao, W. J. Luo, Z. S. Li, Y. Liu and R. L. Withers, Nat. Mater., 2010, 9, 559–564 CrossRef CAS.
- Y. Bi, S. Ouyang, N. Umezawa, J. Cao and J. Ye, J. Am. Chem. Soc., 2011, 133, 6490–6492 CrossRef CAS.
- Y. Bi, S. Ouyang, J. Cao and J. Ye, Phys. Chem. Chem. Phys., 2011, 13, 10071–10075 RSC.
- Y. Liu, L. Fang, H. Lu, L. Liu, H. Wang and C. Hu, Catal. Commun., 2012, 17, 200–204 CrossRef CAS.
- Y. Liu, L. Fang, H. Lu, Y. Li, C. Hu and H. Yu, Appl. Catal., B, 2012, 115–116, 245–252 CrossRef CAS.
- H. Zhang, H. Huang, H. Ming, H. Li, L. Zhang, Y. Liu and Z. Kang, J. Mater. Chem., 2012, 22, 10501–10506 RSC.
- D. A. Dikin, S. Stankovich, E. J. Zimney, R. D. Piner, G. H. B. Dommett, G. Evmenenko, S. T. Nguyen and R. S. Ruoff, Nature, 2007, 448, 457–460 CrossRef CAS.
- H. Zhang, X. J. Lv, Y. M. Li, Y. Wang and J. H. Li, ACS Nano, 2010, 4, 380–386 CrossRef CAS.
- J. Liu, H. Bai, Y. Wang, Z. Liu, X. Zhang and D. D. Sun, Adv. Funct. Mater., 2010, 20, 4175–4181 CrossRef CAS.
- C. Chen, W. M. Cai, M. C. Long, B. X. Zhou, Y. H. Wu, D. Y. Wu and Y. J. Feng, ACS Nano, 2010, 4, 6425–6432 CrossRef CAS.
- J. Liu, L. Liu, H. Bai, Y. Wang and D. D. Sun, Appl. Catal., B, 2011, 106, 76–82 CrossRef CAS.
- T. G. Xu, L. W. Zhang, H. Y. Cheng and Y. F. Zhu, Appl. Catal., B, 2011, 101, 382–387 CrossRef CAS.
- M. Zhu, P. Chen and M. Liu, ACS Nano, 2011, 5, 4529–4536 CrossRef CAS.
- H. Zhang, X. Fan, X. Quan, S. Chen and H. Yu, Environ. Sci. Technol., 2011, 45, 5731–5736 CrossRef CAS.
- W. B. Hu, C. Peng, W. J. Luo, M. Lv, X. M. Li, D. Li, Q. Huang and C. H. Fan, ACS Nano, 2010, 4, 4317–4323 CrossRef CAS.
- S. Liu, T. H. Zeng, M. Hofmann, E. Burcombe, J. Wei, R. Jiang, J. Kong and Y. Chen, ACS Nano, 2011, 5, 6971–6980 CrossRef CAS.
- L. Liu, J. Liu, Y. Wang, X. Yan and D. D. Sun, New J. Chem., 2011, 35, 1418–1423 RSC.
- J. Liu, H. Jeong, K. Lee, J. Y. Park, Y. H. Ahn and S. Lee, Carbon, 2010, 48, 2282–2289 CrossRef CAS.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339–1339 CrossRef CAS.
- R. Pasricha, S. Gupta and A. K. Srivastava, Small, 2009, 5, 2253–2259 CrossRef CAS.
- J. F. Shen, M. Shi, N. Li, B. Yan, H. W. Ma, Y. Z. Hu and M. X. Ye, Nano Res., 2010, 3, 339–349 CrossRef CAS.
- M. R. Das, R. K. Sarma, R. Saikia, V. S. Kale, M. V. Shelke and P. Sengupta, Colloids Surf., B, 2011, 83, 16–22 CrossRef CAS.
- X. Q. Fu, F. L. Bei, X. Wang, S. O'Brien and J. R. Lombardi, Nanoscale, 2010, 2, 1461–1466 RSC.
- J. I. Paredes, S. Villar-Rodil, A. Martinez-Alonso and J. M. D. Tascon, Langmuir, 2008, 24, 10560–10564 CrossRef CAS.
- Z. G. Xiong, L. L. Zhang, J. Z. Ma and X. S. Zhao, Chem. Commun., 2010, 46, 6099–6101 RSC.
- J. G. Yu, H. G. Yu, B. Cheng, X. J. Zhao, J. C. Yu and W. K. Ho, J. Phys. Chem. B, 2003, 107, 13871–13879 CrossRef CAS.
- D. R. Dreyer, S. Park, C. W. Bielawski and R. S. Ruoff, Chem. Soc. Rev., 2010, 39, 228–240 RSC.
- C. Marambio-Jones and E. M. V. Hoek, J. Nanopart. Res., 2010, 12, 1531–1551 CrossRef CAS.
- S. Malato, P. Fernandez-Ibanez, M. I. Maldonado, J. Blanco and W. Gernjak, Catal. Today, 2009, 147, 1–59 CrossRef CAS.
- S. Pal, Y. K. Tak and J. M. Song, Appl. Environ. Microbiol., 2007, 73, 1712–1720 CrossRef CAS.
- S. W. Kim, Y. W. Baek and Y. J. An, Appl. Microbiol. Biotechnol., 2011, 92, 1045–1052 CrossRef CAS.
- W. Stumm and J. J. Morgan, A Wiley-Interscience publication, 3rd edn, 1996 Search PubMed.
- C. C. Chen, W. H. Ma and J. C. Zhao, Chem. Soc. Rev., 2010, 39, 4206–4219 RSC.
- J. Du, X. Y. Lai, N. L. Yang, J. Zhai, D. Kisailus, F. B. Su, D. Wang and L. Jiang, ACS Nano, 2011, 5, 590–596 CrossRef CAS.
- D. H. Yoo, V. C. Tran, V. H. Pham, J. S. Chung, N. T. Khoa, E. J. Kim and S. H. Hahn, Curr. Appl. Phys., 2011, 11, 805–808 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Additional figures include AFM of GO sheets, EDS spectra and element contents of GO–Ag3PO4 composite, absorption spectra of AO7 dye during the photodegradation process, and image of E. coli colonies on an agar plate incubated with Ag+. See DOI: 10.1039/c2cy20483e |
| This journal is © The Royal Society of Chemistry 2012 |
