Ce0.5Zr0.4Sn0.1O2/Al2O3 catalysts with enhanced oxygen storage capacity and high CO oxidation activity†
Qiang
Dong
*,
Shu
Yin
,
Chongshen
Guo
and
Tsugio
Sato
Institute of Multidisciplinary Research for Advanced Materials, Tohoku University, 2-1-1 Katahira, Aoba-ku Sendai 980-8577, Japan. E-mail: dong@tagen.tohoku.ac.jp; Fax: +81-22-217-5598; Tel: +81-22-217-5599
First published on 12th July 2012
Abstract
Ce0.5Zr0.4Sn0.1O2/Al2O3 catalysts were prepared by a coprecipitation route using Ce(NH4)2(NO3)6, Zr(NO3)3·2H2O, SnCl4·5H2O and Al2O3 as the starting materials, and were evaluated as a promoter in automotive catalysis by X-ray diffraction, transmission electron microscopy and the Brunauer–Emmet–Teller (BET) technique. The oxygen storage capacity (OSC) values of the samples were measured using thermogravimetric-differential thermal analysis at 600 °C with a continuous flow of CO–N2 gas and air alternately. The CO oxidation activity of the synthesized sample was evaluated via a gas FTIR spectrometer for analysis of gas compositions. The Ce0.5Zr0.4Sn0.1O2/γ-Al2O3 composite possessed excellent thermal stability, enhanced OSC values, and high CO oxidation activity at the low temperatures even after calcination at 1000 °C for 20 h.
1. Introduction
Ceria (CeO2)-based materials have attracted considerable interest for more than half a century for its far-ranging applications in catalysts, fuel cell, cosmetics, gas sensors, solid-state electrolytes, and especially its crucial application as a promoter of three-way catalysts (TWCs, this derives from its ability to simultaneously remove the three categories of pollutants, i.e. CO, NOx, and hydrocarbons (HC)), because of their excellent oxygen storage capacity (OSC).1–9 The OSC of CeO2 based on the reversible redox reaction between Ce4+ and Ce3+, is of relevant technological importance in automotive TWCs. The highest simultaneous conversions of the three pollutants CO, NOx, and HC are indeed attained over TWCs at air/fuel (A/F) ratios close to the stoichiometric value, while fuel-lean (net oxidizing) and fuel-rich (net reducing) conditions severely decrease, respectively, CO, NOx, and HC conversions. The OSC of added CeO2 acts as an oxygen partial pressure regulator, minimising these undesirable effects.10 In the last two decades it has been established that the partial incorporation of Zr4+ into the CeO2 lattice results in the formation of CexZr1−xO2 mixed oxides, which show enhanced structural/textural features and redox properties with improved thermal stability at elevated temperatures.10–12 For example, Fornasiero et al. have reported that an optimum composition, like Ce0.5Zr0.5O2 can exist as a cubic phase, which can have excellent redox properties.13 Hence, CexZr1−xO2 mixed oxides gradually replaced the pure CeO2 as the OSC promoters in TWCs.In the typical design of modern TWCs, γ-alumina (γ-Al2O3) is employed as a carrier because of its high specific surface area and relatively excellent thermal stability.14,15 At present, γ-Al2O3 supported ceria-based materials have been extensively used and proven to be the most advanced materials for TWCs.16–20 Roberta Di Monte et al.21,22 found that the homogeneous CeO2–ZrO2 structure and strong interaction between Al2O3 carriers and CeO2–ZrO2 are excellent for obtaining thermal stability. Morikawa et al.23 testified that the introduction of Al2O3 into Ce1−xZrxO2 primary particles can improve durability at high temperatures and the oxygen release rate of the materials. Besides such conventional materials, recently, Imanaka et al. described the preparation of a CeO2–ZrO2–Bi2O3 (CZB) solid solution supported on La-stabilized γ-Al2O3 (CZB/Al2O3), led to low-temperature redox activities.24 Bharali et al. synthesized two new OSC materials namely Ce0.8Tb0.2O2−x/γ-Al2O3 and Ce0.8Hf0.2O2/γ-Al2O3, which exhibit excellent OSC and superior CO oxidation activity in comparison with the most advanced CeO2–ZrO2/Al2O3 catalyst supports employed in the existing TWCs.25 However, after high-temperature treatment for a long time (for example: 10 h), retention of reduction behavior for the TWCs remains elusive because of a lowering of OSC values and phase separation.15,26 Consequently, in terms of the practical applications as an automotive exhaust catalyst, the new material with high thermal stability and enhanced OSC is a requisite for developing advanced TWCs.
On the other hand, although the reduction temperature can be decreased obviously by adding noble metal particles on the surface of the mixed oxides, the amount of such precious metals employed in the automotive catalysts has increased drastically over the last decade, reflecting the energy crisis and the growing strength of worldwide emission regulations.27,28 Therefore, it is a greatly urgent task to develop new materials which can reduce or substitute the utilization amount of noble metals in automotive exhaust catalysts.
In the present work, for the first time, we describe the preparation of Ce0.5Zr0.4Sn0.1O2/γ-Al2O3 (hereafter CZS/A) catalysts via a coprecipitation route without adding any noble metals. CeO2/γ-Al2O3 (hereafter C/A) and Ce0.5Zr0.5O2/γ-Al2O3 (hereafter CZ/A) catalysts were also prepared via the same method as the reference materials. All samples were finally calcined at 1000 °C for 20 h in air to evaluate the thermal stability.
2. Experimental
Ce(NH4)2(NO3)6, ZrO(NO3)2, SnCl4·5H2O and NH4OH were of analytical grade, and were purchased from Kanto Chemical Co. Inc., Japan (purity 99.999%). γ-Al2O3 powder (G025) was provided by Sumitomo Chemical Co., Ltd. The chemicals were used without further purification.2.1 Catalyst preparation
In a typical procedure, 1.8 g γ-Al2O3 (specific surface area of 407 m2 g−1) is firstly dispersed in 60 mL distilled water and stirred for 1 h. Separately, the stoichiometric amounts of Ce(NH4)2(NO3)6 (6 mmol), ZrO(NO3)2 (4.8 mmol) and SnCl4·5H2O (1.2 mmol) were dissolved in 60 mL distilled water and stirred for 30 min. Subsequently, the above obtained solutions were added to the alumina dispersed solution under continuous stirring for 1 h. Then, NH4OH solution was slowly dropped into the above mixed solution, and the pH value was maintained at 9, under vigorous stirring. The chemical reactions for the formation of Ce0.5Zr0.4Sn0.1O2 can be written as follows:| NH4OH → NH4+1 + OH−1 | (a) |
| Ce4+ + Zr4+ + Sn4+ + OH−1 → Ce0.5Zr0.4Sn0.1O2 + H2O | (b) |
2.2 OSC analysis
The OSC of catalysts was determined using a thermogravimetric differential thermal analysis (TG-DTA, Rigaku TAS-200). Before the measurements, the samples were held in flowing air at 600 °C for 30 min to remove residual water and other volatile gases. The mixed gas of CO–N2 (100 cm3 min−1) and air (100 cm3 min−1) was flowed alternatively at 600 °C. Finally the OSC was analyzed after getting the TGA profile.2.3 CO oxidation measurements
The CO oxidation activity of the synthesized sample was evaluated in a fixed-bed flow reactor by passing gas mixtures of 2 vol% CO in N2 and 2 vol% O2 in N2 at a rate of 500 cm3 min−1 over 0.15 g sample. The sample was pressed, crashed and sieved to obtain granules of 212–355 μm in diameter and mounted into a quartz tube reactor 6 mm in diameter. The samples were heated at a heating rate of 10 °C min−1 from room temperature to 600 °C. A gas FTIR spectrometer (MIDAC Co., IGA-4000) was employed for in situ analysis of gas composition. The conversion of CO was obtained by using the formula XCO = (1 − [CO]/[CO]0) × 100%, where [CO]0 and [CO] are initial and transient concentration of CO, respectively.2.4 Characterization
The phase composition of the catalyst was determined by X-ray diffraction analysis (XRD, Shimadzu XD-D1) using graphite-monochromized CuKα radiation. The morphology and size of the samples were determined by a transmission electron microscopy (TEM, JEOL JEM-2010). High resolution transmission electron microscopy (HRTEM) images were obtained on a ZEISS LEO 922 with an accelerating voltage of 200 kV. The specific surface area and pore size were measured using BET analysis (NOVA 4200e).3. Results and discussion
All samples of (a) C/A, (b) CZ/A and (c) CZS/A showed the characteristic XRD peaks of a fluorite structure of CeO2 (Fig. 1). No phase separation was observed even after calcination at such high temperatures as 1000 °C for 20 h. The shift of the diffraction peaks of CZ/A and CZS/A to higher angles, as compared to those of C/A, suggests that the Zr4+ and Sn4+ ions dissolve into the CeO2 lattice to form a solid solution. The calculated lattice parameters of CZ/A (a = 0.5345 nm) and CZS/A (a = 0.5317 nm) are smaller than that of C/A (a = 0.5413 nm). The observed decrease in the lattice parameter in comparison with C/A is a strong evidence of the penetration of the doped cations (Zr4+ and Sn4+) into the ceria lattice. The broadening effect of the peaks could be attributed to the nanocrystalline materials, such as 11 and 9 nm calculated by Scherer's formula for CZ/A and CZS/A, respectively.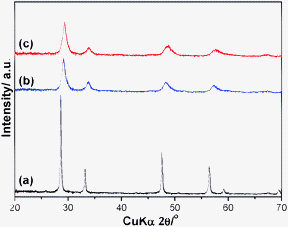 | ||
| Fig. 1 XRD patterns of the samples. (a) C/A (CeO2/γ-Al2O3), (b) CZ/A (Ce0.5Zr0.5O2/γ-Al2O3), (c) CZS/A (Ce0.5Zr0.4Sn0.1O2/γ-Al2O3). | ||
The morphology and crystalline growth of samples were confirmed by TEM (Fig. 2). The crystalline sizes of CZ and CZS on γ-Al2O3 were about 15 and 12 nm, respectively, and agreed with the calculated values using the XRD patterns and Scherer’s formula (Fig. 2(b) and (c)). However, the C/A exhibited large particle sizes of 50–60 nm (Fig. 2(a)). It has been reported that there is a synergic stabilization effect between CZ and γ-Al2O3. This effect inhibits both the transition of Al2O3 from the γ- to the α-phase and the growth of the CZ particles.13,14 The high-resolution TEM (HRTEM) images of C/A, CZ/A and CZS/A exhibited clear lattice fringes with spacings of 0.311, 0.305 and 0.302 nm (Fig. 3), respectively, which can be corresponded to the (111) planes of CeO2. The BET nitrogen adsorption–desorption analysis was undertaken to measure the specific surface area of as-prepared samples. As a result, the specific surface area of CZS/A (56 m2 g−1) was higher than C/A (28 m2 g−1) and CZ/A (41 m2 g−1), shown as in Table 1.
| Chemical composition | OSC of samples (μmol-O g−1) | Surface areas of samples (m2 g−1) |
|---|---|---|
| C/A | 111 | 28 |
| CZ/A | 596 | 41 |
| CZS/A | 774 | 56 |
 | ||
| Fig. 2 TEM images of (a) C/A, (b) CZ/A and (c) CZS/A samples. | ||
 | ||
| Fig. 3 HRTEM images of (a) C/A, (b) CZ/A and (c) CZS/A samples. | ||
The OSC values of the samples were determined at 600 °C with a continuous flow of CO–N2 gas and air alternatively. Fig. 4 shows how the typical oxygen release/storage profiles of the C/A, CZ/A, and CZS/A samples at 600 °C change with time. CZS/A exhibited the highest OSC of 774 μmol-O g−1, when compared with those of C/A (111 μmol-O g−1) and CZ/A (596 μmol-O g−1) samples (Table 1). It is accepted that the OSC is strongly depended on the specific surface area. It is obvious that CZS/A exhibited the highest specific surface area and highest OSC values. Moreover, CZS/A exhibits higher OSC than those of Ce0.5Zr0.5O2/Al2O3 (697 μmol-O g−1) and Pd/Ce0.67Zr0.33Sr0.01O2.01/Al2O3 (152 μmol-O g−1).14,29 In order to examine OSC performance stability, oxygen release/storage cycle measurements were taken, and CZS/A retained the same OSC even after 128 cycles (Fig. S1, ESI†). Comparing with the OSC of Ce0.7Zr0.3O2/Al2O3,30 the result indicates that CZS/A has good OSC performance stability.
 | ||
| Fig. 4 Oxygen release/storage properties (TG profiles) of (a) C/A, (b) CZ/A and (c) CZS/A at 600 °C. | ||
In order to test the role of Al2O3 in enhancing the OSC by increasing the surface area, supplementary experiments for the OSC analysis were carried out for the unsupported CeO2 (C), Ce0.5Zr0.5O2 (CZ) and Ce0.5Zr0.4Sn0.1O2 (CZS) samples. All the unsupported samples showed much smaller specific surface areas (C (3 m2 g−1), CZ (8 m2 g−1) and CZS (24 m2 g−1)) as shown in Fig. S2 (ESI†). As a result, the OSC was enhanced greatly by coupling with γ-Al2O3 (Fig. S3 and S4 in ESI†). The increased surface area and high OSC are thought to be related to the Al2O3 support and the involvement of Ce4+/Ce3+ and Sn4+/Sn2+ redox couples, in which the CZS lattice oxygen is thought to be utilized for CO oxidation.31 It is proposed that SnO2, being easily reducible, gives out its lattice oxygen for the oxidation reaction, which is possibly rejuvenated by subtracting oxygen from the adjacent CeO2 molecules.32 Based on these results, the improvement of the OSC can be explained by the combination of two effects: the dispersion of the nanometer-sized C, CZ and CZS particles on the Al2O3 surface, thereby increasing the surface area, and the incorporation of Sn4+ into the CZ lattice, which enhances the number of redox couples.
Moreover, the high OSC may contribute to the oxidation of CO, which is one of the important reactions of interest in the catalysis process of the automotive exhaust cleaning process. The activity curves of CO oxidation for all samples are plotted in Fig. 5. Comparison with the light-off temperatures of C/A (329 °C) and CZ/A (301 °C) CZS/A showed the lowest light-off temperature to be 275 °C, indicating that CZS/A was the most active catalyst. Furthermore, the light-off temperature of CZS/A is significantly lower than those of Ce0.5Zr0.5O2/Al2O3 (384 °C), Ce0.8Tb0.2O2−x/Al2O3 (331 °C), Ce0.8Hf0.2O2/Al2O3 (337 °C) catalysts,14,25 in particular, the light-off temperature is also lower than that of Pd/Ce0.67Zr0.33Sr0.01O2.01/Al2O3 (286 °C) catalysts,29 where Pd is used as a noble metal. It is apparently recognized that the OSC values of the catalysts are correlated to their CO oxidation activities. Based on these results, it can be suggested that the high OSC activities play an important role in the oxidation of CO at low temperatures, in other words, the order of activity is directly related to the OSC of the catalysts, which demonstrates that catalysts with higher OSC possess superior CO oxidation activity.
 | ||
| Fig. 5 CO oxidation activity curves of (a) C/A, (b) CZ/A and (c) CZS/A. | ||
4. Conclusions
A new OSC material of Ce0.5Zr0.4Sn0.1O2/Al2O3 is prepared via a coprecipitation method. Ce0.5Zr0.4Sn0.1O2/Al2O3 showed the high OSC even after calcination at 1000 °C for 20 h. Studies using CO oxidation, as a model reaction, showed that the high oxidation activity of the sample at low temperatures correlates with their enhanced OSC. The prepared Ce0.5Zr0.4Sn0.1O2/Al2O3 has the potential to be a key material in advanced catalytic converters without the help of noble metals in the design of superior three way catalysts (TWCs).Acknowledgments
This work was partly supported by the Rare Metal Substitute Materials Development Project of New Energy and the Industrial Technology Development Organization (NEDO), Japan and the Management Expenses Grants for National Universities Corporations from the Ministry of Education, Culture, Sports and Science for Technology of Japan (MEXT).Notes and references
- J. T. Kummer, Prog. Energy Combust. Sci., 1980, 6, 177 CrossRef CAS.
- H. C. Yao and Y. F. Yu, J. Catal., 1984, 86, 254 CrossRef CAS.
- R. Di Monte, J. Kasper, H. Bradshaw and C. Norman, J. Rare Earths, 2008, 26, 136 CrossRef.
- J. Kaspar, P. Fornasiero and M. Graziani, Catal. Today, 1999, 50, 285 CrossRef CAS.
- B. C. H. Steele, Nature, 1999, 400, 619 CrossRef CAS.
- B. C. H. Steele and A. Heinzel, Nature, 2001, 414, 345 CrossRef CAS.
- S. Yin, Y. Minamidate and T. Sato, Surf. Rev. Lett., 2010, 17(2), 147 CrossRef CAS.
- S. Yin, Y. Minamidate and T. Sato, Adv. Sci. Technol., 2010, 63, 30 CrossRef CAS; S. Yin, Y. Minamidate, S. Tonouchi, T. Goto, Q. Dong, H. Yamane and T. SATO, RSC Adv., 2012, 2, 5976 RSC.
- M. K. Devaraju, S. Yin and T. Sato, ACS Appl. Mater. Interfaces, 2009, 1(11), 2694 CAS.
- P. Fornasiero, G. Balducci, R. Di Monte, J. Kaspar, V. Sergo, G. Gubitosa, A. Ferrero and M. Graziani, J. Catal., 1996, 164, 173 CrossRef CAS.
- M. K. Devaraju, X. W. Liu, K. Yusuke, S. Yin and T. Sato, Nanotechnology, 2009, 20(40), 405606 CrossRef CAS.
- S. Bernal, J. J. Calvino, M. A. Gatica, C. Larese, J. A. Perez Omil and J. M. Pintado, Catal. Today, 1999, 50, 175 CrossRef CAS.
- P. Fornasiero, R. Di Monte, G. R. Rao, J. Kaspar, S. Meriani, A. Trovarelli and M. Graziani, J. Catal., 1995, 151, 168 CrossRef CAS.
- B. M. Reddy, P. Lakshmanan, P. Bharali, P. Saikia, G. Thrimurthulu, M. Muhler and W. Grulnert, J. Phys. Chem. C, 2007, 111, 10478 CAS.
- J. Wang, J. Wen and M. Q. Shen, J. Phys. Chem. C, 2008, 112, 5113 CAS.
- M. W. Zhao, M. Q. Shen and J. Wang, J. Catal., 2007, 248, 258 CrossRef CAS.
- B. M. Reddy, P. Bharali, P. Saikia, A. Khan, S. Loridant, M. Muhler and W. Gru1nert, J. Phys. Chem. C, 2007, 111, 1878 CAS.
- O. Pozdnyakova-Tellinger, D. Teschner, J. Kroehnert, F. C. Jentoft, A. Knop-Gericke, R. Schlogl and A. Wootsch, J. Phys. Chem. C, 2007, 111, 5426 CAS.
- T. Kolli, K. Rahkamaa-Tolonen, U. Lassi, A. Savimaki, R. L. Keiski, J. Z. Shyu, W. H. Weber and H. S. Gandhi, J. Phys. Chem., 1988, 92, 4964 CrossRef.
- M. H. Yao, R. J. Baird, F. W. Kunz and T. E. Hoosty, J. Catal., 1997, 166, 67 CrossRef CAS.
- R. Di Monte, P. Fornasiero, J. Kaspar, M. Graziani, J. M. Gatica, S. Bernal and H. A. Gomez, Chem. Commun., 2000, 2167 RSC.
- R. Di Monte, P. Fornasiero, S. Desinan, J. Kaspar, J. M. Gatica, J. J. Calvino and E. Fonda, Chem. Mater., 2004, 16, 4273 CrossRef CAS.
- A. Morikawa, T. Suzuki, T. Kanazawa, K. Kikuta, A. Suda and H. Shinjo, Appl. Catal., B, 2008, 78, 210 CrossRef CAS.
- N. Imanaka, T. Masui, K. Koyabu, K. Minami and T. Egawa, Adv. Mater., 2007, 19, 1608 CrossRef CAS.
- P. Bharali, P. Saikia and B. M. Reddy, Catal. Sci. Technol., 2012, 2, 931 CAS.
- C. C. Chuanga, H. I. Hsiang, J. S. Hwang and T. S. Wang, J. Alloys Compd., 2009, 470, 387 CrossRef.
- H. Tanaka, I. Tan, M. Uenishi, M. Taniguchi, M. Kimura, Y. Nishihata and J. Mizuki, J. Alloys Compd., 2006, 1071, 408 Search PubMed.
- X. Y. Zhang, E. Y. Long, Y. L. Li, J. X. Guo, L. J. Zhang, M. C. Gong, M. H. Wang and Y. Q. Chen, J. Nat. Gas Chem., 2009, 18, 139 CrossRef CAS.
- M. Q. Shen, J. Q. Wang, J. C. Shang, Y. An, J. Wang and W. L. Wang, J. Phys. Chem. C, 2009, 113, 1543 CAS.
- J. Wang, J. Wen and M. Q. Shen, J. Phys. Chem. C, 2008, 112, 5113 CAS.
- T. Baidya, A. Gupta, P. A. Deshpandey, G. Madras and M. S. Hegde, J. Phys. Chem. C, 2009, 113, 4059 CAS.
- R. Sasikala, N. M. Gupta and S. K. Kulshreshtha, Catal. Lett., 2001, 71, 69 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2cy20425h |
| This journal is © The Royal Society of Chemistry 2012 |
