Highly efficient deep eutectic solvent catalyzed ring opening of epoxides
Najmadin
Azizi
* and
Elham
Batebi
Chemistry and Chemical Engineering Research Center of Iran, P.O. Box 14335-186, Tehran, Iran. E-mail: azizi@ccerci.ac.ir; Fax: +98 (21)44580762; Tel: +98 (21)44580720
First published on 10th August 2012
Abstract
A choline-based deep eutectic solvent was found to be a new and effective catalyst for the construction of 1,2-difunctional ring-opening products through the reaction of epoxides with aromatic amines, thiols, alcohols, azide and cyanide in good to excellent yields with short reaction times.
Nowadays, organic reactions in green reaction media for sustainable organic synthesis, like water, supercritical media, ionic liquids, including solventless reactions, allow intensified green technologies by highly efficient synthesis of the desired products, thus avoiding tedious and costly procedures, which involve high volumes of organic solvents. In this context, designing organic reactions in deep eutectic solvent (DES) is another attractive area in green chemistry. DESs are mixtures of quaternary ammonium salts such as choline chloride with simple halide salts, which produce liquids, which have physical and solvent properties that are comparable with ionic liquids. Deep eutectic solvent is an environmentally benign, universal solvent, and it offers several benefits, including negligible vapour pressure, nonflammability, chemical and thermal stability, as well as being non-toxic and readily available as a bulk commodity chemical enabled rapid advance in numerous applications, including metal deposition, metal oxide processing, metal dissolution and a variety of synthetic processes.1
Epoxide has been recognized among the most versatile intermediates and starting materials in organic synthesis due to their ease of formation and wide reactivity with a broad range of nucleophiles with high or often complete stereo and regioselectivity.2 1,2-bifunctional ring-opened compounds represent the key step for synthesis of novel therapeutic agents, biologically active compounds and chiral auxiliaries.3 As a result of their importance, extensive studies have been done to achieve clean, selective ring opening of epoxides to convert readily available, inexpensive bulk chemicals into 1,2-difunctionalized fine chemicals.4
However, classical procedures had some limitations such as the requirement for an excess of amine and harsh reaction conditions, often working less well with poorly and sterically hindered nucleophiles, and undesirable side reactions due to the rearrangement or polymerization of sensitive epoxides. Thus, there have been incessant efforts to develop mild methodologies for the ring opening of epoxides by different nucleophiles such as thiols and amines as evidenced by recent reports.5
Throughout our investigations to develop green chemistry by using water and deep eutectic solvent (DES) as a reaction medium,6 herein we report the first example of an efficient and simple procedure for chemoselective ring opening of the epoxides with aromatic amines, thiols, alcohols and cyanide in deep eutectic solvents as a novel and selective catalyst and reaction media.
The experimental procedure is very simple and clean. DES was formed by gently stirring the tin chloride (200 mmol) and the choline chloride (100 mmol) at 130 °C in 1 h until a clear, homogenous liquid formed. Treatment of glycidyl phenylether 1 (1 mmol) with thiophenol (1 mmol) in tin chloride/choline chloride deep eutectic solvent (40 mol%) resulted in ring opening of epoxides to the corresponding 1,2-mercapto alcohols 3 as the only detectable product and isolated in 97% yield at room temperature after 10 min (Scheme 1).
 | ||
| Scheme 1 General reaction conditions. | ||
To examine the generality and scope of thiolysis in deep eutectic solvent, the reaction of sterically, electronically and functionally diverse epoxides and thiols under the same reaction conditions were screened and the results of this investigation are shown in Table 1. The reactions proceeded smoothly with almost all the commercially available epoxides such as glycidyl phenyl ether, allyl glycidyl ether, isopropyl glycidyl ether, hexane oxide and cyclohexene oxide. Both aliphatic and aromatic thiols react with a variety of epoxides, with different rates by this procedure to produce the corresponding adducts in good to excellent yields.
| Entry | Epoxides | Thiols | Time (min) | Yieldsa (%) |
|---|---|---|---|---|
| a 1H NMR yields. b Yields of other isomers. | ||||
| 1 |
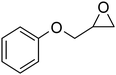
|
2a | 10 | 97 |
| 2 | 2b | 10 | 97 | |
| 3 | 2c | 10 | 97 | |
| 4 | 2f | 60 | 75 | |
| 5 | 2h | 80 | 72 | |
| 6 | 2e | 70 | 82 | |
| 7 | 2i | 80 | 68 | |
| 8 |

|
2a | 50 | 95 |
| 9 | 2c | 50 | 92 | |
| 10 |

|
2a | 60 | 95 |
| 11 | 2g | 60 | 90 | |
| 12 |

|
2a | 30 | 97 |
| 13 | 2b | 30 | 95 | |
| 14 | 2c | 70 | 95 | |
| 2d | 70 | 82 | ||
| 15 |

|
2a | 10 | 92b |
| 16 | 2b | 10 | 92b | |
| 17 |
|
2a | 80 | 94 |
| 18 | 2b | 80 | 95 | |
Aromatic thiols such as 2-naphthyl thiol and a variety of substituted thiophenols (thiophenol, 4-chloro-thiophenol, 4-bromo-thiophenol, 4-methylthiophene, 4-methoxylthiophenol, 2-methylthiophene) furnished excellent yields of the β-hydroxy sulfides under mild reaction conditions. Electron withdrawing as well as electron-donating substituents on the thiophenol did not make any difference to the reactivity and selectivity of the reaction. Similarly, the corresponding products from the reactions of aliphatic thiols were obtained albeit in moderate yields and selectivity. Furthermore, it was possible to monitor the reaction visually. A yellow solution was obtained after addition of the thiol to the epoxide in deep eutectic solvent, and the reaction mixture became yellow and viscose after complete consumption of the starting material.
Unsymmetrical alkyl oxiranes underwent cleavage by thiols with preferential attack at the less substituted carbon of epoxides, affording in most cases a single product in high to quantitative yields. Styrene oxide was reacted under these simple reaction conditions with short reaction times and high regioselectivity affording the corresponding product 3 as the minor isomer along with a major amount of other isomers by cleavage at the benzylic position with the anti-Markovnikov addition. In the case of cyclohexane oxide, trans products were obtained. The regioselectivity and stereochemistry of the 1,2-mercapto alcohols was determined by 1H NMR spectra.
Encouraged by the above successful results, we further explored the potential of this procedure for the direct ring opening reaction of various epoxides with aromatic amines and the successful results are summarized in Table 2.
| Entry | Epoxides | Amines | Time (min) | Yieldsa (%) | Ratio (5/6) |
|---|---|---|---|---|---|
| a 1H NMR yields. b Yields of 40 mol% SnCl2 alone. c Yields of 100 mol% choline chloride alone. | |||||
| 1 |

|
4a | 60 | 95(72)b(00)c | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
| 2 | 4b | 120 | 92 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
|
| 3 | 4c | 120 | 90 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
|
| 4 | 4d | 180 | 88 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
|
| 5 | 4e | 60 | 92 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
|
| 6 | 4g | 180 | 80 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
|
| 7 | 4h | 120 | 82 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
|
| 8 |

|
4a | 120 | 92 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
| 9 | 4d | 120 | 90 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
|
| 10 |

|
4a | 120 | 95 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 11 | 4f | 120 | 94 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
|
| 12 |

|
4a | 60 | 95 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
| 13 | 4b | 160 | 95 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
|
| 14 | 4e | 120 | 90 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
|
| 15 | 4f | 180 | 88 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
|
| 16 |
|
4a | 60 | 95 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
| 17 | 4b | 60 | 92 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 82 82 |
|
| 18 |

|
4a | 180 | 95 | |
| 19 | 4e | 180 | 82 | ||
| 20 | 4f | 180 | 90 | ||
It was observed that commercially available aromatic amines containing electron donating groups such as 4-methoxy aniline, 4-isopropyl aniline, 4-butyl aniline as well as withdrawing groups such as 4-chloroaniline, 4-bromoaniline, 3,4-dichloroaniline and 2-mthyl-4-bromoaniline reacted well under the present reaction conditions with aliphatic and aromatic epoxide such as glycidyl phenyl ether, glycidyl isopropyl ether, glycidyl allyl ether, 1,2-epoxy hexane, cyclohexene oxide and styrene oxide to give the corresponding amino alcohols in good to excellent yields. In general, electron-rich arylamines and mild electron-withdrawing groups such as halides afforded good to excellent yields of products in short reaction times. Sterically hindered amines like 2,4,6-trimethyl aniline gave good yields for corresponding ring opening products at longer reaction times.
The trans stereochemistry of the β-amino alcohol was determined from the coupling constants of the C–H protons α to the heteroatoms in the 1H NMR spectra. For example, the trans stereochemistry of compound 5 (Table 2, entry 18) was assigned from the coupling constants of the peaks at 3.19 ppm (ddd, J = 10.1, 9.2, 4.1 Hz, CH–NHPh) and 3.34 ppm (ddd, J = 10.3, 9.4, 4.4 Hz, CH–OH) in the 1H NMR spectrum.
Furthermore, only good yields (72%) of the corresponding product were obtained in the presence of 40 mol% of the SnCl2 alone after stirring at room temperature for 60 min4k and in the presence of choline chloride only starting material were recovered (Table 2).
In addition, TMSCN, TMSN3 and methanol were successfully used as nucleophiles in this procedure (Table 3), and the corresponding β-hydroxy azides, β-hydroxy nitrile and β-alkoxy alcohols were obtained respectively with good regio and stereoselectivity in moderate to good yields and short reaction times.
Furthermore, the convenient regeneration/recycling of deep eutectic solvent from reaction mixtures also represent significant advantages of this procedure. Recyclability of the DES was studied for the preparation of 1,2-mercapto alcohols 3 under the optimized reaction conditions.7,8 At the end of the reactions, the DES can be easily recovered (by precipitation) by addition of diethyl ether to the reaction mixture. After filtration, the remaining viscous liquid was further washed with Et2O and dried at 130 °C to retain its catalytic activity and the recovered DES was found to be recyclable for at least four successive operations (Table 4).
| a Reaction conditions: glycidyl phenylether (1 mmol), thiophenol (1 mmol) in tin chloride/choline chloride (40 mol%), rt, 10 min. | ||||
|---|---|---|---|---|
| Run | 1 | 2 | 3 | 4 |
| Yields (%) | 97 | 97 | 92 | 90 |
In summary, deep eutectic solvent9 was found to be a highly effective catalyst for the ring opening of a variety of epoxides by a range of amines, thiols, alcohols, cyanide and azide providing the anticipated products with good chemo, regio, and stereoselectivities. Furthermore, the reactions with unsymmetrical epoxides were regioselective, since the major products isolated were those arriving from the attack of the nucleophile to the less hindered carbon atom of the epoxide ring except for styrene oxide. The reaction of aromatic amines and thiols with styrene oxide regioselectively yielded the products derived from the attack on the benzylic position of the epoxide. Further studies of organic reactions in DES are in progress.
Acknowledgements
The financial support of this work provided by Iran National Science Foundation (INSF) is gratefully appreciated.Notes and references
- (a) A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 70 RSC; (b) Z. Chen, B. Zhou, H. Cai, W. Zhu and X. Zou, Green Chem., 2009, 11, 275 RSC; (c) Z. Chen, W. Zhu, Z. Zheng and X. Zou, J. Fluorine Chem., 2010, 131, 340 CrossRef CAS; (d) C. A. Angell, Y. Ansari and Z. Zhao, Faraday Discuss., 2012, 154, 9 RSC; (e) A. P. Abbott, G. Frisch, H. Garrett and J. Hartley, Chem. Commun., 2011, 47, 11876 RSC.
- (a) C. H. Behrens and K. B. Sharpless, Aldrichimica Acta, 1983, 16, 67 CAS; (b) J. G. Smith, Synthesis, 1984, 629 CrossRef; (c) I. Erdeen, in Comprehensive Heterocyclic Chemistry II, ed. A. R. Katritzky, C. W. Rees and E. F. V. Scriven, Pergamon, Oxford, 1996, vol. IA, p. 97 Search PubMed; (d) S. Bonollo, D. Lanari, A. Marrocchi and L. Vaccaro, Curr. Org. Synth., 2011, 8, 319 Search PubMed.
- (a) V. Kesavan, D. Bonnet-Delpon and J. P. Begue, Tetrahedron Lett., 2000, 41, 2895 CrossRef and references therein; (b) B. L. Chng and A. Ganesan, Bioorg. Med. Chem. Lett., 1997, 7, 1511 CrossRef.
- (a) A. K. Chakraborti, S. Rudrawar and A. Kondaskar, Org. Biomol. Chem., 2004, 2, 1277 RSC; (b) A. K. Chakraborti, A. Kondaskar and S. Rudrawar, Tetrahedron, 2004, 60, 9085 CrossRef; (c) A. K. Chakraborti, S. Rudrawar and A. Kondaskar, Eur. J. Org. Chem., 2004, 3597 CrossRef CAS; (d) J. M. Chong and K. B. Sharpless, J. Org. Chem., 1985, 50, 1560 CrossRef CAS; (e) J. Iqbal, A. Pandey, A. Shukla, R. R. Srivastava and S. Tripathi, Tetrahedron, 1990, 46, 6423 CrossRef CAS; (f) J. S. Yadav, B. V. S. Reddy and G. Baisha, Chem. Lett., 2002, 906 CrossRef CAS; (g) S. Chanrasekar, Ch. R. Reddy, B. N. Babu and G. Chandrashekar, Tetrahedron Lett., 2002, 43, 3801 CrossRef; (h) N. Devan, P. R. Sridhar, K. R. Prabhu and S. Chandrasekaran, J. Org. Chem., 2002, 67, 9417 CrossRef CAS; (i) M. Sasaki, K. Tanino, A. Hirai and M. Miyashita, Org. Lett., 2003, 5, 1789 CrossRef CAS; (j) K. Tanaka, S. Oda and M. Shiro, Chem. Commun., 2008, 820 RSC; (k) S. R. Adapa, R. Enugala and A. M. M. R. Varala, Lett. Org. Chem., 2006, 3, 187 Search PubMed.
- (a) T. Ollevier and G. Lavie-Compin, Tetrahedron Lett., 2004, 45, 49 CrossRef CAS; (b) T. Ollevier and G. Lavie-Compin, Tetrahedron Lett., 2002, 43, 7891 CrossRef CAS; (c) R.-H. Fan and X.-L. Hou, J. Org. Chem., 2003, 68, 726 CrossRef CAS; (d) Shivani, B. Pujala and A. K. Chakraborti, J. Org. Chem., 2007, 72, 3713 CrossRef; (e) B. Pujala, S. Rana and A. K. Chakraborti, J. Org. Chem., 2011, 76, 8768 CrossRef CAS; (f) Shivani and A. K. Chakraborti, J. Mol. Catal. A: Chem., 2007, 263, 137 CrossRef; (g) A. K. Chakraborti and A. Kondaskar, Tetrahedron Lett., 2003, 44, 8315 CrossRef CAS; (h) N. Azizi and M. R. Saidi, Org. Lett., 2005, 7, 3649 CrossRef CAS; (i) M. Curini, F. Epifano, M. C. Marcotullio and O. Rosati, Eur. J. Org. Chem., 2001, 4149 CrossRef CAS; (j) P.-Q. Zhao, L.-W. Xu and C.-G. Xia, Synlett, 2004, 846 CAS; (k) N. Azizi and M. R. Saidi, Tetrahedron, 2007, 63, 888 CrossRef CAS; (l) F. Fringuelli, F. Pizzo, S. Tortoioli and L. Vaccaro, Adv. Synth. Catal., 2002, 344, 379 CrossRef CAS; (m) D. Amantini, F. Fringuelli, F. Pizzo, S. Tortoioli and L. Vaccaro, Synlett, 2003, 2292 CAS; (n) F. Fringuelli, F. Pizzo, S. Tortoioli and L. Vaccaro, J. Org. Chem., 2003, 68, 8248 CrossRef CAS; (o) F. Fringuelli, F. Pizzo, S. Tortoioli and L. Vaccaro, Green Chem., 2003, 5, 436 RSC; (p) J. Wu and H.-G. Xia, Green Chem., 2005, 7, 708 RSC; (q) V. Pironti and S. Colonna, Green Chem., 2005, 7, 43 RSC; (r) S. Bonollo, D. Lanari and L. Vaccar, Eur. J. Org. Chem., 2011, 2587 CrossRef CAS; (s) S. S. Kahandal, S. R. Kale, S. T. Disale and R. V. Jayaram, Catal. Sci. Technol., 2012, 2, 1493 RSC; (t) B. Plancq and T. Ollevier, Chem. Commun., 2012, 48, 3806 RSC; (u) E. Mai and C. Schneider, Chem.–Eur. J., 2007, 13, 2729 CrossRef CAS; (v) C. Schneider, Synthesis, 2006, 3919 CrossRef CAS; (w) A. Tschöp, M. V. Nandakumar, O. Pavlyuk and C. Schneider, Tetrahedron Lett., 2008, 49, 1030 CrossRef; (x) E. Mai and C. Schneider, Synlett, 2007, 2136 CAS; (y) M. V. Nandakumar, A. Tschöp, H. Krautscheid and C. Schneider, Chem. Commun., 2007, 2756 RSC; (z) A. Tschöp, A. Marx, A. R. Sreekanth and C. Schneider, Eur. J. Org. Chem., 2007, 2318 CrossRef.
- (a) N. Azizi and M. R. Saidi, Organometallics, 2004, 23, 1457 CrossRef CAS; (b) N. Azizi, F. Aryanasab, L. Torkiyan, A. Ziyaei and M. R. Saidi, J. Org. Chem., 2006, 71, 3634 CrossRef CAS; (c) N. Azizi, L. Torkiyan and M. R. Saidi, Org. Lett., 2006, 8, 2079 CrossRef CAS; (d) N. Azizi, F. Aryanasab and M. R. Saidi, Org. Biomol. Chem., 2006, 4, 4275 RSC; (e) N. Azizi, E. Batebi, S. Bagherpour and H. Ghafuri, RSC Adv., 2012, 2, 2289 RSC; (f) N. Azizi and E. Gholibeglo, RSC Adv., 2012, 2, 7413 RSC.
- Representative procedure: Choline chloride/Tin chloride (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 40 mol%) was added to a magnetically stirred mixture of epoxide (1 mmol) and Nu (1 mmol) at rt under nitrogen. After completion of the reaction, a precipitate was formed and this served as an indicator for monitoring the reaction visually (10–180 min), the reaction mixture was diluted with Et2O (15 ml) to recover the catalyst (SnCl2/ChCl). The deep eutectic solvent was separated by decantation of the supernatant ethereal solution, was washed with Et2O (5 ml) and the combined ethereal solutions were dried (MgSO4) and concentrated under vacuum to afford 1,2-difunctional ring-opened crude products. The crude product was purified by column chromatography to provide the corresponding product. All the obtained products are known compounds to which the spectroscopic data were compared with the litreture.
2, 40 mol%) was added to a magnetically stirred mixture of epoxide (1 mmol) and Nu (1 mmol) at rt under nitrogen. After completion of the reaction, a precipitate was formed and this served as an indicator for monitoring the reaction visually (10–180 min), the reaction mixture was diluted with Et2O (15 ml) to recover the catalyst (SnCl2/ChCl). The deep eutectic solvent was separated by decantation of the supernatant ethereal solution, was washed with Et2O (5 ml) and the combined ethereal solutions were dried (MgSO4) and concentrated under vacuum to afford 1,2-difunctional ring-opened crude products. The crude product was purified by column chromatography to provide the corresponding product. All the obtained products are known compounds to which the spectroscopic data were compared with the litreture. - The general route for the synthesis of the deep eutectic solvent was as follows: Choline chloride (100 mmol) was mixed with Tin chloride (200 mmol) and heated to ca. 130 °C in air with stirring until a clear colourless liquid was obtained1.
- Deep eutectic solvent based on tin (II) chloride was known, and preparation, characterization and evaluation of the Lewis acidity of this DES were reported by using 1H NMR and ESI-MS. (a) A. P. Abbott, G. Capper, D. L. Davies, H. L. Munro, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2001, 2010 RSC; (b) F. Li, R.-h. Liu, J.-h. Wen, D.-s. Zhao, Z.-m. Sun and Y. Liu, Green Chem., 2009, 11, 883 RSC.
| This journal is © The Royal Society of Chemistry 2012 |






