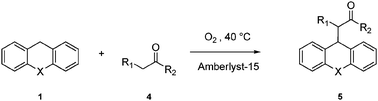
Supported sulfonic acids: reusable catalysts for more sustainable oxidative coupling of xanthene-like compounds with nucleophiles†
Calogero G.
Piscopo
,
Sofie
Bühler
,
Giovanni
Sartori
and
Raimondo
Maggi
*
“Clean Synthetic Methodology Group”, Dipartimento di Chimica Organica e Industriale dell’Università, Parco Area delle Scienze 17A, I-43124 Parma, Italy. E-mail: raimondo.maggi@unipr.it; Fax: +39 0521 905472; Tel: +39 0521 905411
First published on 10th August 2012
Abstract
The oxidative coupling of xanthene and thioxanthene with methylene active compounds can be achieved in good yields (55–92%) and selectivities (83–99%) by using the commercial catalyst Amberlyst-15, an inexpensive, safe to handle and easy to recycle sulfonic resin. The method is completely metal-free and shows a very high atom economy (99.2%).
Organic synthesis takes advantage of the high reactivity of some functional groups that allows the starting reagents to be transformed into the targeted compounds. In fact, the general strategy for forming a new bond requires the presence of a heteroatom or an unsaturation in the carbon backbone of an organic molecule, as clearly stated by Corey and Cheng.1
In recent years there has been increased interest in the catalysis using solid materials for fine chemicals preparation,2 since this approach frequently resulted in a less expensive and ecological productive process.3
In this light, it is clear that introducing a new functionality through transformation of a (sp3)C–H bond can unlock new opportunities for greener synthetic strategies.4 The high stability of this bond makes it generally unreactive, and not so useful from a synthetic point of view.5 Furthermore the direct insertion of a new group through the (sp3)C–H bond can allow organic reactions with very good atom economy, which is one of the fundamental principles of the green chemistry.6 Therefore the activation of the (sp3)C–H bond has attracted much attention at both the academic and industrial levels.
The production of a new C–C bond by coupling two sp3 carbons under oxidative conditions (Cross-Dehydrogenative Coupling – CDC) has been recently reported; in this reaction, hydrogen theoretically acts as “leaving group” and, in the best case, water is the only by-product.7 These CDC reactions can be catalytically performed simply by using metal salts or organic redox-active molecules for activating C–H bonds. For example, the coupling of two (sp3)C–H bonds, one showing acid nature such as in nitromethane8 and in a methyl ketone,9 and the other adjacent to a nitrogen atom, can be efficiently catalyzed by Cu(I) salts, in the presence of TBHP or even molecular oxygen. Furthermore, with (sp3)C–H bonds adjacent to different heteroatoms, such as oxygen, sulfur and nitrogen, a similar coupling reaction can be carried out by using an iron catalyst under similar conditions, generating the corresponding C–C bond.10 However, often aggressive oxidants, harsh conditions, and expensive reagents are required, thus diminishing the overall efficiency and sustainability of the entire process.
Very recently, Pintér and Klussmann reported interesting studies focusing on the aerobic oxidative coupling of activated benzylic CH2 groups with aldehydes,11 ketones and 1,3-dicarbonyl compounds,12 using oxygen as the oxidant. On the same topic Jiao et al. described the use of nitrocompounds,13 and an elegant CDC asymmetric version between xanthenes and different aliphatic aldehydes.14 We were particularly interested in the CDC of xanthene with ketones or 1,3-dicarbonyl compounds; the reaction proceeds smoothly under mild conditions without a metal reagent and requires the cost effective methane sulfonic acid and oxygen. This type of reaction neither involves any redox-active catalyst nor reagents commonly used for C–H bond activation; thus, there has been great interest in the design of sustainable syntheses with simple and cost effective reagents with low level of environmental impact. A possible mechanistic rationalization, proposed by Klussmann et al., involves the autoxidative formation of a xanthene hydroperoxide 2a, which would react in an acid-catalyzed SN1 type reaction with nucleophiles such as ketone 4a to form the coupling product 5aa and hydrogen peroxide (Scheme 1).
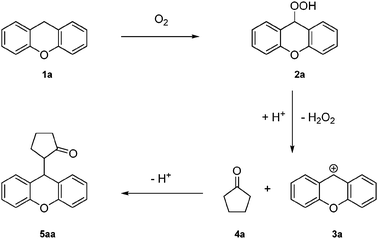 | ||
| Scheme 1 Possible mechanism for the oxidative coupling of xanthene with cyclopentanone. | ||
However, the problem of ensuring the sustainability of this process is still not completely resolved. Thus the aim of this current paper is to present a new hetero catalytic system based on supported sulfonic acids. To the best of our knowledge, this study represents the first example of CDC reactions carried out with a solid acid and reusable catalyst.
In continuation with our investigation on the preparation, characterization and use of catalysts supported on different solid materials,15 we have synthesized, according to literature data, two silica supported sulfonic acids, namely mesoporous MCM-41-supported propanesulfonic acid16 and amorphous silica supported 4-ethylphenylsulfonic acid17 (Fig. 1). In the first case a mixture of cetyltrimethylammonium chloride (C16TACl), silane bis(3-triethoxysilylpropyl)tetrasulfide (TESPT), tetraethyl orthosilicate (TEOS), NH4OH and H2O (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1140) was hydrothermally reacted at 90 °C for 48 hours, followed by washing with methanol and filtering. The filtered cake was treated with a mixture of hydrochloric acid (2 N) and methanol to remove the surfactant molecules from the mesopores. The oxidation of the sulfide groups exposed on the pore walls with Br2–HCl, followed by washing with methanol, produced MCM-41 mesoporous materials incorporating propanesulfonic acid groups.
1140) was hydrothermally reacted at 90 °C for 48 hours, followed by washing with methanol and filtering. The filtered cake was treated with a mixture of hydrochloric acid (2 N) and methanol to remove the surfactant molecules from the mesopores. The oxidation of the sulfide groups exposed on the pore walls with Br2–HCl, followed by washing with methanol, produced MCM-41 mesoporous materials incorporating propanesulfonic acid groups.
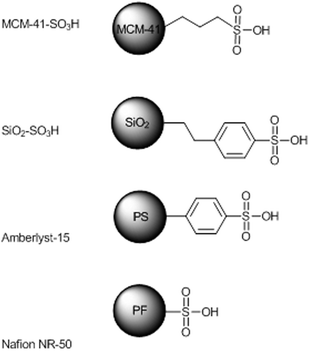 | ||
| Fig. 1 The two supported catalysts prepared, Amberlyst-15 and Nafion NR-50. | ||
Silica supported 4-ethylphenylsulfonic acid has been prepared by tethering the amorphous silica with trimethoxy(2-phenylethyl)silane followed by sulfonation with chlorosulfonic acid. The loading and surface area of all catalysts have been determined by a reported titration method18 and by the BET method,19 respectively (Table 1).
| Entry | Catalyst | Loading/mmol g−1 | Surface area/m2 g−1 | 5aa yieldb (%) | 5aa sel.c (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (1.0 mmol), 4a (5.0 mmol), and solid catalyst (5 mol% with respect to 1a) were stirred under an atmosphere of oxygen at 40 °C for 24 h. b Yields were determined by GC analysis of the crude reaction mixture using dodecane as external standard. c Calculated on xanthene. d Air atmosphere. e Addition of 1.0 mmol of 30% aq H2O2. | |||||
| 1 | MCM-41-SO3H | 2.53 | 970 | 54 | 93 |
| 2 | SiO2-SO3H | 1.14 | 293 | 74 | 95 |
| 3 | Nafion NR-50 | 0.80 | <0.1 | 40 | 98 |
| 4 | Amberlyst-15 | 4.70 | 53 | 72 | 96 |
| 5 | Amberlyst-15 | 4.70 | 53 | 43 | 98d |
| 6 | Amberlyst-15 | 4.70 | 53 | 4 | 25d,e |
The activity of the as prepared catalysts, in comparison with that of the commercially available sulfonic resins Amberlyst-15 and Nafion NR-50, was measured in the model reaction between cyclopentanone 4a and xanthene 1a carried out under an oxygen atmosphere for 24 hours by using 5 mol% of the supported sulfonic acid with respect to xanthene 1a (Scheme 1). Results reported in Table 1 show that in all cases 2-(9H-xanthen-9-yl)cyclopentanone 5aa was detected as the only product (93–95% selectivity). Commercially available superacid Nafion NR-50 afforded only a modest yield (40%; Table 1, entry 3), probably ascribable to the very low surface area. Results obtained with the silica-supported acids seem to suggest that the main factor affecting the catalytic efficiency is represented by the accessibility of the reagents to the supported acid sites rather than to the loading values. Indeed, the supported 4-ethylphenylsulfonic acid (74% yield; Table 1, entry 2) is more accessible than the supported propanesulfonic acid (54% yield; Table 1, entry 1), due to the restricted mobility of the latter that hampers its partial deactivation due to the interaction with the neighboring silanols.20
However, comparable results have been obtained with silica supported 4-ethylphenylsulfonic acid and Amberlyst-15 (74 and 72% yield, respectively; Table 1, entries 2 and 4). Hence, further studies on the parameters affecting the reaction have been developed by using Amberlyst-15 as a solid catalyst, being commercially available, more economic and safer to handle.
Preliminary studies showed that the reaction was rather slow needing 24 hours to reach the value of ∼70% yield (Fig. 2). For longer reaction times a slow, but continuous lowering of yield has been observed, due to the formation of tars and by-products (traces of bis-addition of cyclopentanone were detected).
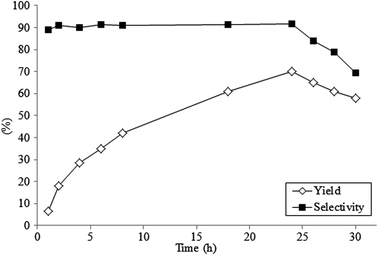 | ||
| Fig. 2 Yield and selectivity versus time in the oxidative coupling of cyclopentanone with xanthene catalyzed by Amberlyst-15. | ||
The effect of catalyst amount was then evaluated in the model reaction performed at 40 °C for 24 hours. Results of yield and selectivity shown in Fig. 3 demonstrate that the reaction gives the desired product 5aa in good yield and selectivity (58 and 85% respectively) with only 2 mol% of catalyst. A further increase in yield was observed by increasing the amount of catalyst to 5 mol%, reaching the maximum value of 72% yield and 96% selectivity. A slight lowering of yield and selectivity (to 67 and 91% respectively) has been observed with a 7 mol% catalyst amount; this negative trend was confirmed by further increasing the catalyst amount to 10 mol% (64% yield and 85% selectivity).
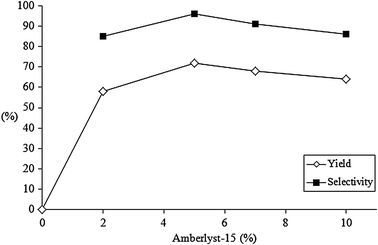 | ||
| Fig. 3 Effect of Amberlyst-15 amount on the oxidative coupling of cyclopentanone with xanthene. | ||
The model reaction can be performed with satisfactory yield (43%) under an air atmosphere instead of oxygen (Table 1, entry 5), while the addition of one equivalent of H2O2 completely blocked the reaction (Table 1, entry 6). Under the above conditions, not only the 2a–3a step is unfavorable, but also the methylene bridge of reagent 1a could undergo oxidation as previously reported.12 Although the mechanistic aspects of the reaction have not been deepened, the reaction pathway proposed for the homogeneous reaction (Scheme 1) may occur also in this case. Indeed traces of hydroperoxide 2a were detected in the reaction mixture and the presence of H2O2 was confirmed by the MnO2 test.‡
The scope and generality of the oxidative coupling process with respect to various nucleophiles was then investigated. Experimental results reported in Table 2 confirm the general applicability to different cyclic and linear ketones and to 1,3-dicarbonyl compounds. Yields of isolated products are between 55 and 90%, accompanied by excellent selectivity values (83–99%).§
| Entry | X | R1 | R2 | Product | Yieldb [Sel.] (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1 (1.0 mmol), 4 (5.0 mmol), and Amberlyst-15 (11 mg; 0.05 mmol) were stirred under an oxygen atmosphere at 40 °C for 24 h. b Yields of isolated product determined by GC analysis of the crude reaction mixture using dodecane as external standard. c Reaction temperature: 65 °C. d Reaction time: 72 h. | |||||
| 1 | O | –(CH2)3– | 5aa | 74 [95] | |
| 2 | O | –(CH2)4– | 5ab | 92 [98] | |
| 3 | O | –CH(Me)(CH2)3– | 5ac | 62 [89] | |
| 4 | O | Me | Me | 5ad | 55 [89] |
| 5 | O | Ph | Me | 5ae | 86 [99] |
| 6 | O | p-MeO-(C6H4) | Me | 5af | 65 [99] |
| 7 | O | EtO(CO) | Me | 5ag | 73 [93] |
| 8 | O | Me(CO) | Me | 5ah | 62 [83] |
| 9 | O | Ph(CO) | Me | 5ai | 66 [90]c |
| 10 | S | –(CH2)4– | 5bb | 32 [99] | |
| 11 | S | –(CH2)4– | 5bb | 55 [98]d | |
Furthermore, the reaction of thioxanthene with cyclohexanone was tested and the coupling product was obtained in good yield and excellent selectivity. It must be highlighted that, despite the oxidative reaction conditions, no traces of sulfone or sulfoxide were detected, even when the reaction was prolonged for 72 hours.
Finally the catalyst recyclability has been tested in the reaction between xanthene and cyclohexanone. After the first coupling reaction, the catalyst was filtered off, washed with methanol and water and reused for the next run. Eight runs can be efficiently performed with the recycled resin-supported sulfonic acid without a considerable decrease in its catalytic activity. The yield of each run was over 75% (Table 3).
| Run | 5ab yieldb (%) | Run | 5ab yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 1a (1.0 mmol), 4a (5.0 mmol) and Amberlyst-15 (11 mg; 0.05 mmol) were stirred under an atmosphere of oxygen at 40 °C for 24 h. b Yields were determined by GC analysis of the crude reaction mixture using dodecane as external standard. | |||
| 1 | 92 | 5 | 89 |
| 2 | 83 | 6 | 77 |
| 3 | 91 | 7 | 82 |
| 4 | 82 | 8 | 76 |
Conclusions
In summary, we have developed a clean and safe method for the oxidative coupling of xanthene and thioxanthene with methylene active compounds. The sulfonic resin catalyst has the advantage to be inexpensive, safe to handle and easy to recycle. Several important parameters of the “green chemistry” are satisfied: the atom economy is very high (99.2%), reaction conditions are mild, the reaction is completely metal-free, and the work-up is very easy.Since xanthene and its derivatives are very interesting pharmaceutically and biologically active compounds, this reaction represents a valuable chance for the synthesis of these species.
Studies are in progress in order to check the applicability of Amberlyst-15 in other CDC-type reactions.
Acknowledgements
This work was supported by the Ministero dell’Università e della Ricerca (MIUR), Italy, and the University of Parma (National Project “Attivazione ossidativa catalitica e fotocatalitica per la sintesi organica”). The Centro Interdipartimentale Misure (CIM) is acknowledged for the use of NMR instruments.Notes and references
- E. J. Corey and X.-M. Cheng, The Logic of Chemical Synthesis, Wiley, New York, 1995 Search PubMed.
- (a) G. J. Hutchings, J. Chem. Soc., Chem. Commun., 1999, 301–306 RSC; (b) A. Corma, Chem. Rev., 1995, 95, 559–614 CrossRef CAS; (c) M. Balogh and P. Laszlo, Organic Chemistry Using Clays, Springer Verlag, New York, 1993 Search PubMed.
- (a) J. H. Clark and D. J. Macquarrie, Chem. Soc. Rev., 1996, 303–310 RSC; (b) R. A. Sheldon, Chem. Ind., 1997, 12–15 CAS; (c) R. A. Sheldon, J. Mol. Catal. A: Chem., 1996, 107, 75–83 CrossRef CAS; (d) J. M. Thomas and K. I. Zamaraev, Angew. Chem., Int. Ed. Engl., 1994, 33, 308–311 CrossRef.
- (a) Handbook of C–H Transformations, ed. G. Dyker, Wiley-VCH, Weinheim, 2005 Search PubMed; (b) K. Godula and D. Sames, Science, 2006, 312, 67–72 CrossRef CAS.
- R. G. Bergman, Nature, 2007, 446, 391–393 CrossRef CAS.
- R. A. Sheldon, Pure Appl. Chem., 2000, 72, 1233–1246 CrossRef CAS.
- (a) C.-J. Li, Acc. Chem. Res., 2009, 42, 335–344 CrossRef CAS; (b) C. J. Scheuermann, Chem.–Asian J., 2010, 5, 436–451 CrossRef CAS.
- Z. Li and C.-J. Li, J. Am. Chem. Soc., 2005, 127, 3672–3673 CrossRef CAS.
- Y. Shen, M. Li, S. Wang, T. Zhan, Z. Tan and C. Guo, Chem. Commun., 2009, 953–955 RSC.
- Z. Li, R. Yu and H. Li, Angew. Chem., Int. Ed., 2008, 47, 7497–7500 CrossRef CAS.
- Á. Pintér and M. Klussmann, Adv. Synth. Catal., 2012, 354, 701–711 CrossRef.
- Á. Pintér, A. Sud, D. Sureshkumar and M. Klussmann, Angew. Chem., Int. Ed., 2010, 49, 5004–5007 CrossRef.
- B. Zhang, Y. Cui and N. Jiao, Chem. Commun., 2012, 48, 4498–4500 RSC.
- B. Zhang, S.-K. Xiang, L.-H. Zhang, Y. Cui and N. Jiao, Org. Lett., 2011, 13, 5212–5215 CrossRef CAS.
- (a) G. Sartori, A. Armstrong, R. Maggi, A. Mazzacani, R. Sartorio, F. Bigi and B. Dominguez-Fernandez, J. Org. Chem., 2003, 68, 3232–3237 CrossRef CAS; (b) C. G. Piscopo, S. Loebbecke, R. Maggi and G. Sartori, Adv. Synth. Catal., 2010, 352, 1625–1629 CrossRef CAS; (c) F. Gregori, I. Nobili, F. Bigi, R. Maggi, G. Predieri and G. Sartori, J. Mol. Catal. A: Chem., 2008, 286, 124–127 CrossRef CAS; (d) R. Maggi, S. Chitsaz, S. Loebbecke, C. G. Piscopo, G. Sartori and M. Schwarzer, Green Chem., 2011, 13, 1121–1123 RSC.
- O. Kwon, S. Min Park and G. Seo, Chem. Commun., 2007, 4113–4115 RSC.
- R. D. Badley and W. T. Ford, J. Org. Chem., 1989, 54, 5437–5443 CrossRef CAS.
- S. Leveneur, D. Y. Murzin and T. Salmi, J. Mol. Catal. A: Chem., 2009, 303, 148–155 CrossRef CAS.
- S. Brunnauer, P. G. Emmeth and E. Teller, J. Am. Chem. Soc., 1938, 60, 309–319 CrossRef.
- J.-P. Dacquin, H. E. Cross, D. R. Brown, T. Düren, J. J. Williams, A. F. Lee and K. Wilson, Green Chem., 2010, 12, 1383–1391 RSC.
- (a) G. W. Jones, K. T. Chang and H. Shechter, J. Am. Chem. Soc., 1979, 101, 3906–3916 CrossRef CAS; (b) H. Nishino, Bull. Chem. Soc. Jpn., 1986, 59, 1733–1739 CrossRef CAS; (c) Sigma Aldrich, Supplier Catalog Number L342688; (d) Z.-T. Weng, Y. Li and S.-K. Tian, J. Org. Chem., 2011, 76, 8095–8099 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: General details, experimental procedures and characterisation data of new compounds. See DOI: 10.1039/c2cy20428b |
| ‡ Evidence for the formation of hydrogen peroxide was found by addition of MnO2 at the end of the reaction which resulted in gas evolution, indicating catalytic decomposition.12 |
§ Typical procedure: xanthene (1a) (182 mg, 1.0 mmol), methylene active compound (4) (5.0 mmol) and Amberlyst-15 dried at 100 °C overnight (11 mg, 5 mol%) were mixed in a Schlenk tube equipped with a magnetic stirring bar. The tube was flushed with oxygen and then, after stirring at 40 °C for 24 h, the reaction mixture was purified directly by column chromatography (silica gel, eluant hexane–ethyl acetate 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) without further workup, giving the corresponding products 5. Products 5aa, 5ab, 5ad, 5ae, 5ag, 5ah, 5ai and 5bb are known compounds;12,21 products 5ac and 5af are new compounds (see ESI†). 1) without further workup, giving the corresponding products 5. Products 5aa, 5ab, 5ad, 5ae, 5ag, 5ah, 5ai and 5bb are known compounds;12,21 products 5ac and 5af are new compounds (see ESI†). |
| This journal is © The Royal Society of Chemistry 2012 |